N-Alkylaminoferrocene-Based Prodrugs Targeting Mitochondria of Cancer Cells
Abstract
1. Introduction
2. Results and Discussion
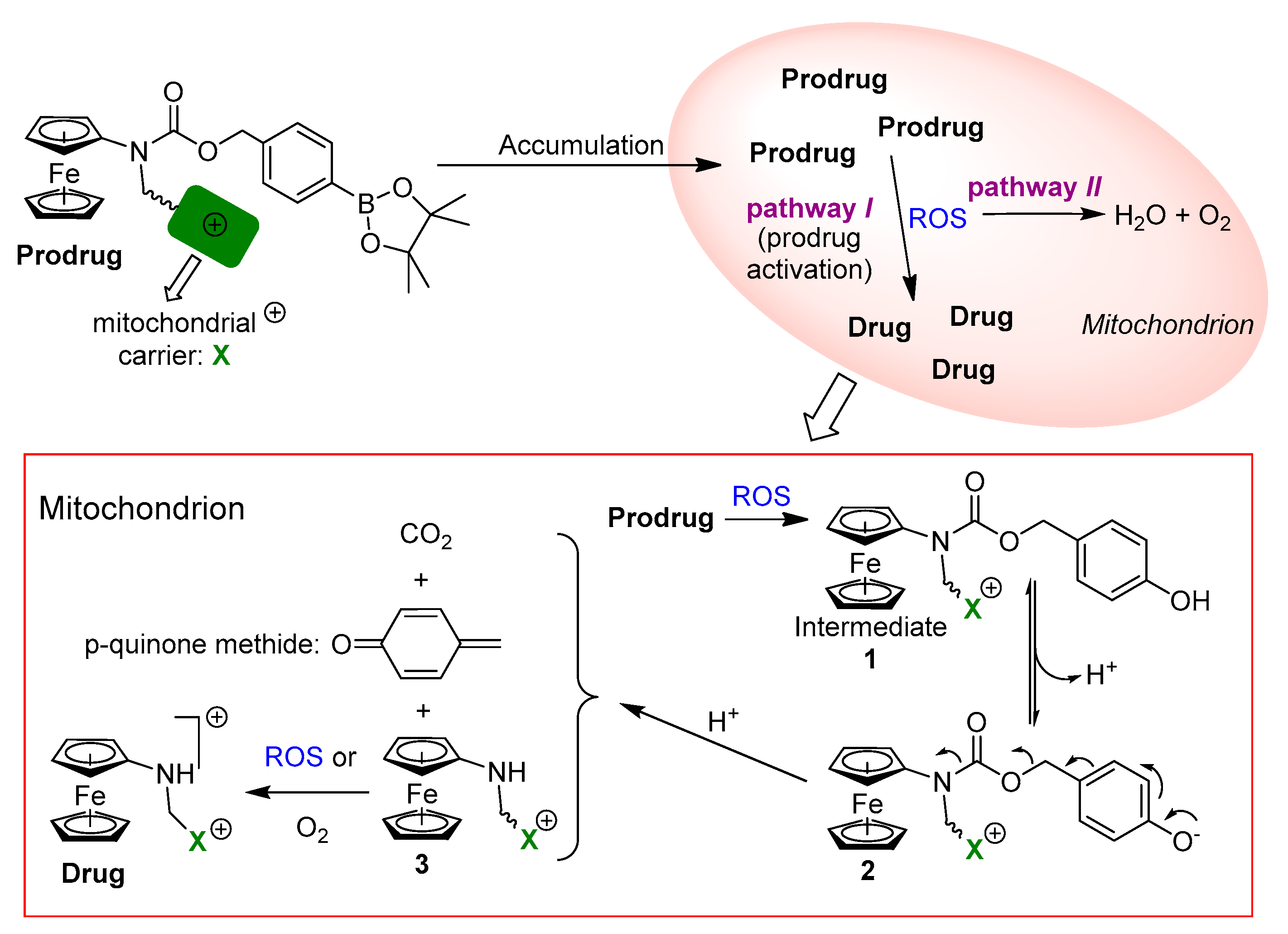
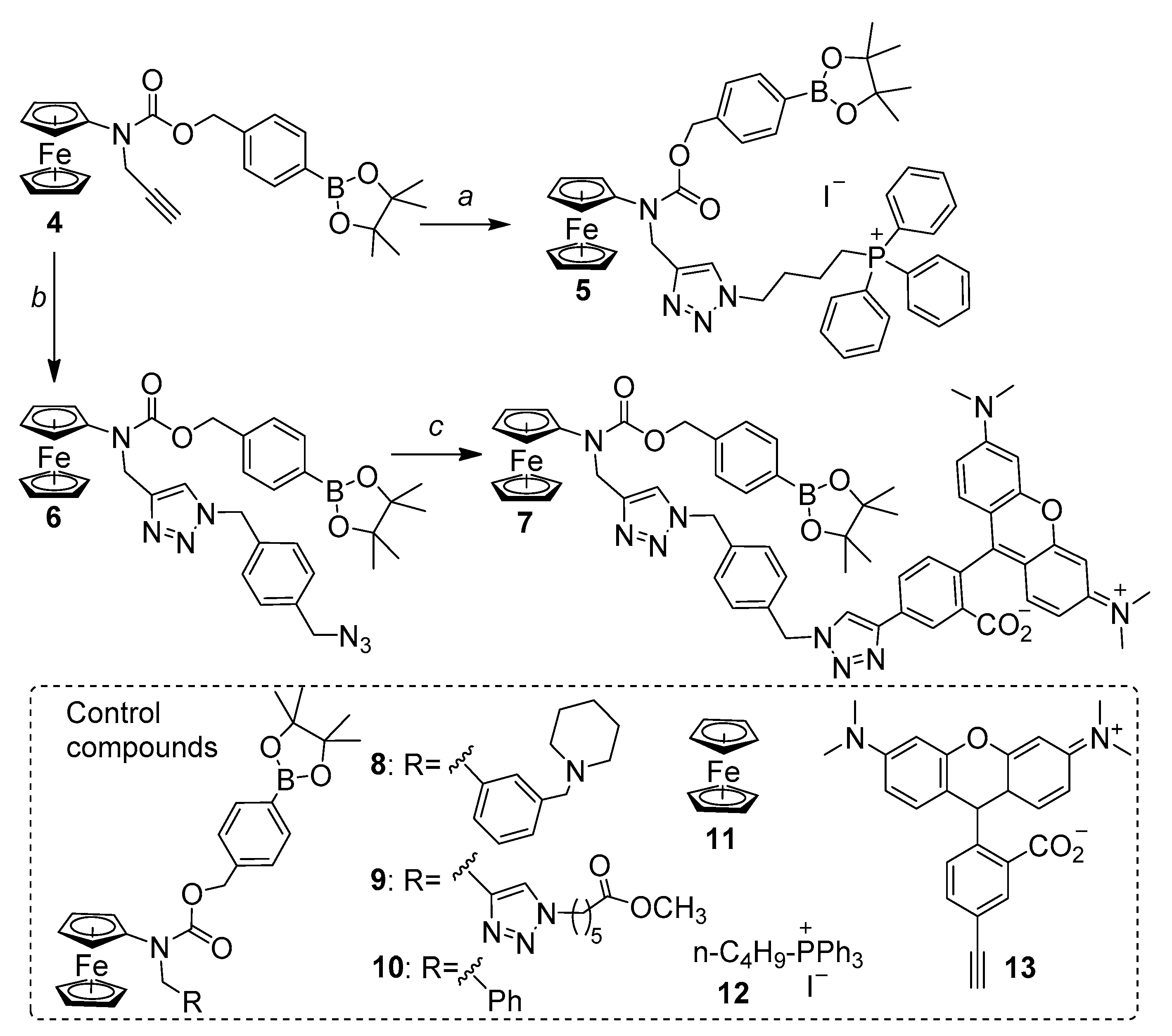
2.1. Design, Synthesis and Basic Properties of Prodrugs in Cell Free Settings
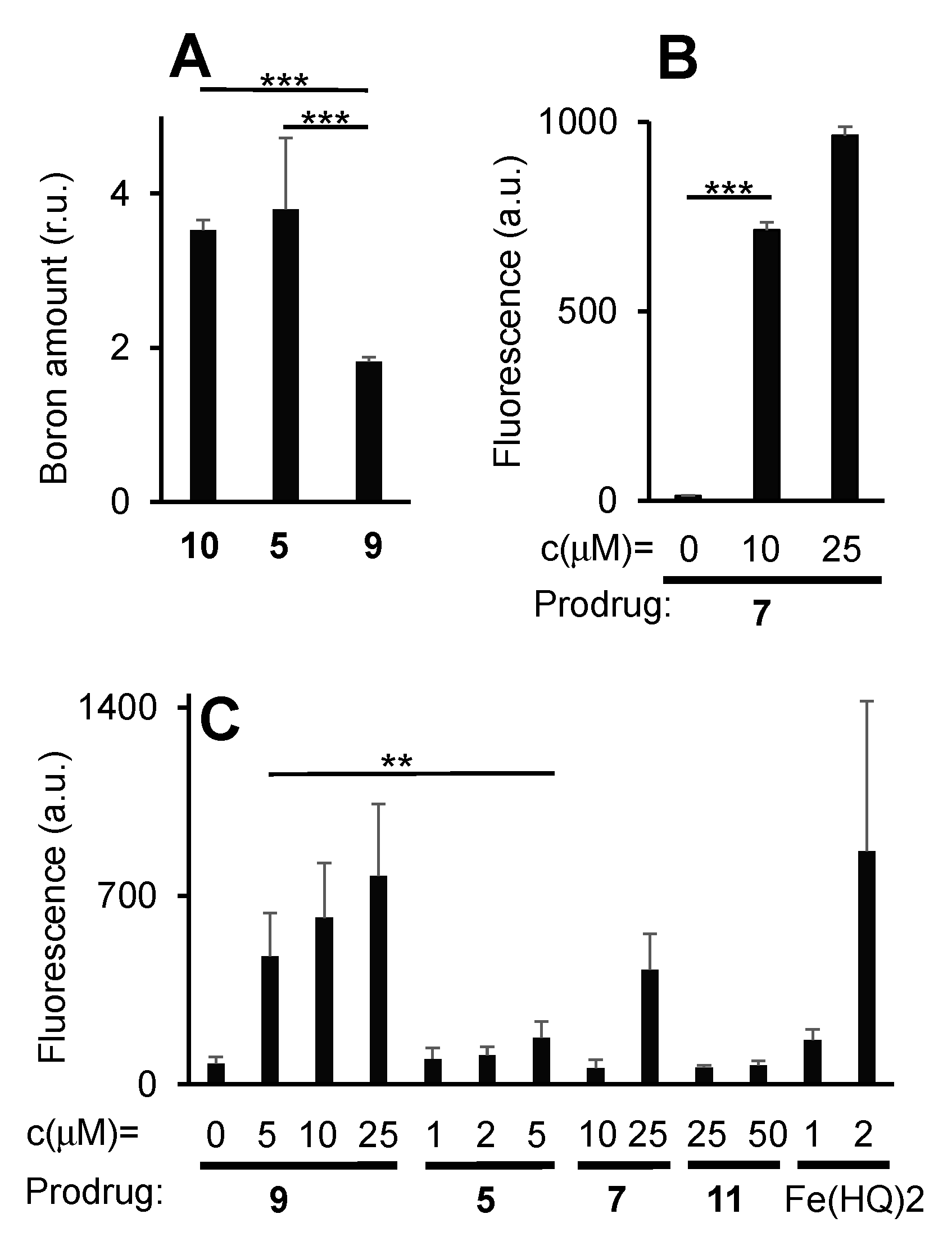
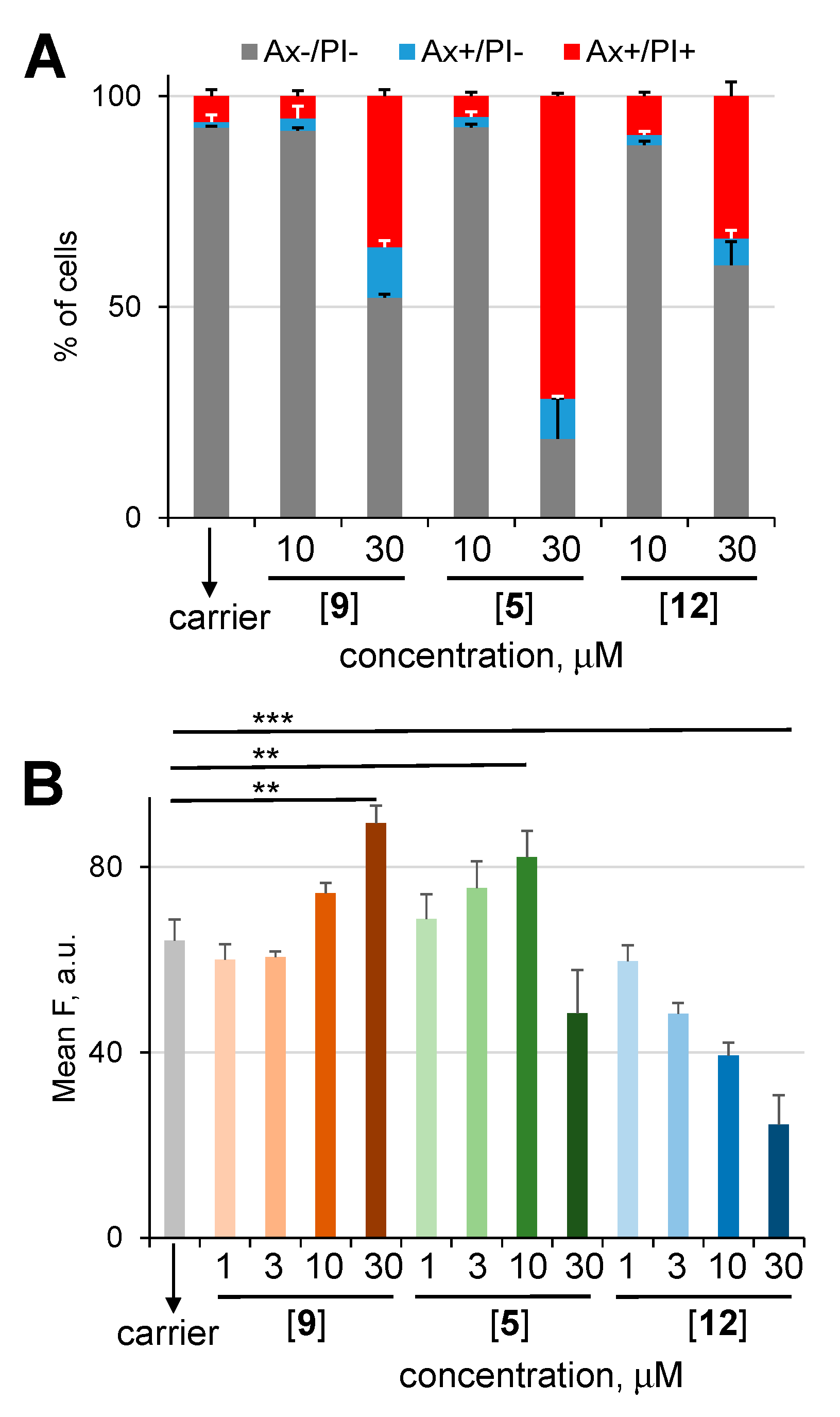
2.2. Study of Cellular Effects of Prodrugs and Control Compounds
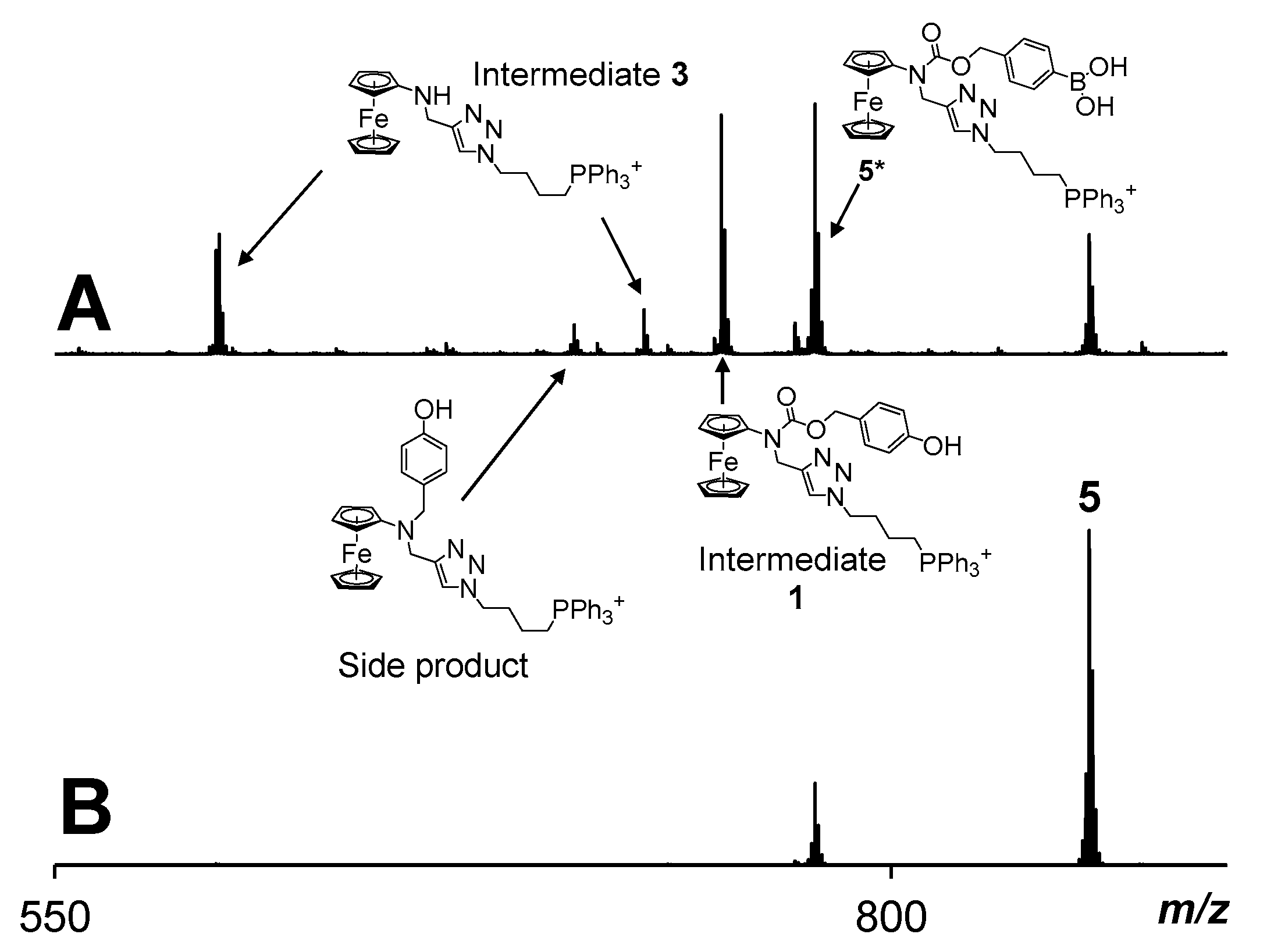
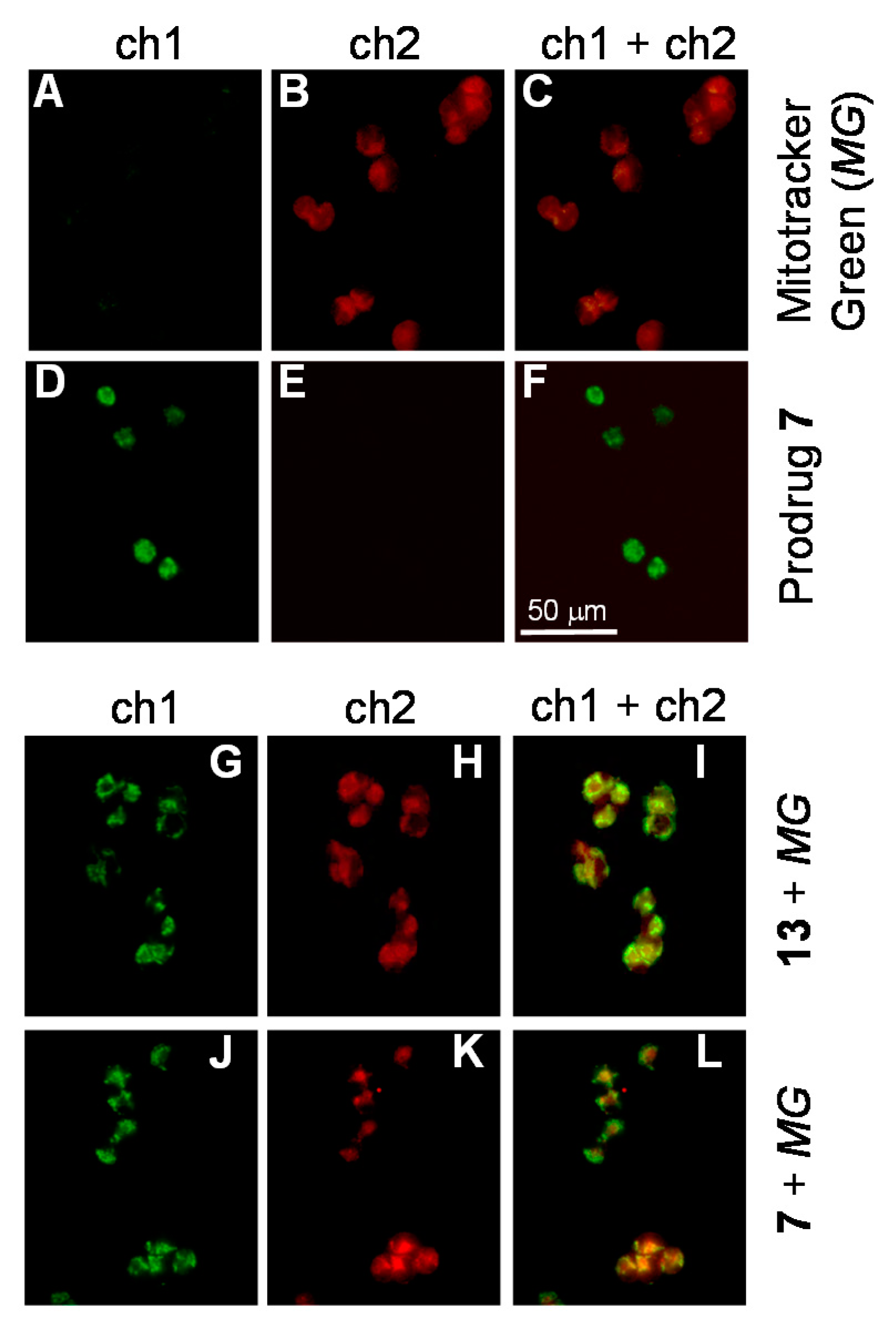
2.3. The Mode of Action of Mitochondria-Targeting NAAF-Prodrug 5
3. Materials and Methods
3.1. Determination of Solubility of Prodrugs 5 and 7 in Aqueous Buffers
3.2. Monitoring Generation of Reactive Oxygen Species (ROS) in the Presence of Either Prodrugs or Control Compounds in Cell Free Settings
3.3. Monitoring Activation of Prodrug 7 in the Presence of H2O2 by Fluorescence Spectroscopy
3.4. Monitoring of H2O2-Induced Activation of Prodrug 5 by Using ESI Mass Spectrometry
3.5. Cells and Cell Cultures
3.6. Determination of the Viability of Adherent Cells (A2780, DU-145 and HDFa)
3.7. Determination of the Viability of Suspension BL-2 Cells
3.8. Determination of Prodrug 5 Uptake by BL-2 Cells by Quantification of Intracellular Boron Amounts Using the Curcumin-Assay
3.9. Determination of Prodrug 7 Uptake by BL-2 Cells by Quantification the Fluorescence Derived from 7 Using Flow Cytometry
3.10. Determination of ROS-Amplification in BL-2 Cells in the Presence of Prodrugs
3.11. Experiments with Jurkat Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arruebo, M.; Vilaboa, N.; Sáez-Gutierres, B.; Lambea, J.; Tres, A.; Valladares, M.; González-Fernández, Á. Assessment of the evolution of cancer treatment therapies. Cancers 2011, 3, 3279–3330. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, L.H.; Donohue, J. Cis-diamminedichloroplatinum, vinblastine, and bleomycin combination chemotherapy in disseminated testicular cancer. Ann. Intern. Med. 1977, 87, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Haugnes, H.S.; Bosl, G.J.; Boer, H.; Gietema, J.A.; Brydøy, M.; Oldenburg, J.; Dahl, A.A.; Bremnes, R.M.; Fosså, S.D. Long-term and late effects of germ cell testicular cancer treatment and implications for follow-up. J. Clin. Oncol. 2012, 30, 3752–3763. [Google Scholar] [CrossRef] [PubMed]
- Antunes, F.; Cadenas, R. Estimation of H2O2 gradients across biomembranes. FEBS Lett. 2000, 475, 121–126. [Google Scholar] [CrossRef]
- Szatrowski, T.P.; Nathan, C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991, 51, 794–798. [Google Scholar] [PubMed]
- Halliwell, B. Oxidative stress and cancer: have we moved forward? Biochem. J. 2007, 401, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hagen, H.; Marzenell, P.; Jentzsch, E.; Wenz, F.; Veldwijk, M.R.; Mokhir, A. Aminoferrocene-based prodrugs activated by reactive oxygen species. J. Med. Chem. 2012, 55, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Marzenell, P.; Hagen, H.; Sellner, L.; Zenz, T.; Grinyte, R.; Pavlov, V.; Daum, S.; Mokhir, A. Aminoferrocene-based prodrugs and their effects on human normal and cancer cells as well as bacterial cells. J. Med. Chem. 2013, 56, 6935–6944. [Google Scholar] [CrossRef] [PubMed]
- Schikora, M.; Reznikov, A.; Chaykovskaya, L.; Sachinska, O.; Polyakova, L.; Mokhir, A. Activity of aminoferrocene-based prodrugs against prostate cancer. Bioorg. Med. Chem. Lett. 2015, 25, 3447–3450. [Google Scholar] [CrossRef]
- Daum, S.; Chekhun, V.; Todor, I.; Lukianova, N.; Shvets, Y.; Sellner, L.; Putzker, K.; Lewis, J.; Zenz, T.; Graaf, I.; et al. Improved synthesis of N-benzylaminoferrocene-based prodrugs and evaluation of their toxicity and antileukemic activity. J. Med. Chem. 2015, 58, 2015–2024. [Google Scholar] [CrossRef]
- Daum, S.; Babiy, S.; Konovalova, H.; Hofer, W.; Shtemenko, A.; Shtemenko, N.; Janko, C.; Alexiou, C.; Mokhir, A. Tuning the structure of aminoferrocene-based anticancer prodrugs to prevent their aggregation in aqueous solution. J. Inorg. Biochem. 2018, 178, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Jaouen, J.; Vessières, A.; Top, S. Ferrocifen type anticancer drugs. Chem. Soc. Rev. 2015, 44, 8802–8817. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pigeon, P.; Top, S.; García, J.S.; Troufflard, C.; Ciofini, I.; McGlinchey, M.J.; Jaouen, J. Atypical lone pair-pi interaction with quinone methides in a series of imido-ferrociphenol anticancer drug candidates. Angew. Chem. Int. Ed. 2019, 58, 8421–8425. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, Y.; Peng, X. ROS-inducible DNA cross-linking agent as a new anticancer prodrug building block. Chem. Eur. J. 2012, 18, 3850–3854. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, H.; Balakrishnan, K.; Lin, Z.; Cao, S.; Chen, W.; Fan, Y.; Guthrie, Q.A.; Sun, H.; Teske, K.A.; et al. Hydrogen peroxide activated quinone methide precursors with enhanced DNA cross-linking capability and cytotoxicity towards cancer cells. Eur. J. Med. Chem. 2017, 133, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Doering, M.; Ba, L.A.; Lilienthal, N.; Nicco, C.; Scherer, C.; Abbas, M.; Peer Zada, A.A.; Coriat, R.; Burkholz, T.; Wessjohann, L.; et al. Synthesis and selective anticancer activity of organochalcogen based redox catalysts. J. Med. Chem. 2010, 53, 6954–6963. [Google Scholar] [CrossRef]
- Kim, E.J.; Bhuniya, S.; Lee, H.; Kim, H.M.; Cheong, C.; Maiti, S.; Hong, K.S.; Kim, J.S. An activatable prodrug for the treatment of metastatic tumors. J. Am. Chem. Soc. 2014, 136, 13888. [Google Scholar] [CrossRef]
- Daum, S.; Reshetnikov, V.; Sisa, M.; Dumych, T.; Lootsik, M.D.; Bilyy, R.; Bila, E.; Janko, C.; Alexiou, C.; Herrmann, M.; et al. Lysosome-targeting amplifiers of reactive oxygen species as anticancer prodrugs. Angew. Chem. Int. Ed. 2017, 56, 15545–15549. [Google Scholar] [CrossRef]
- Reshetnikov, V.; Daum, S.; Janko, C.; Karawacka, W.; Tietze, R.; Alexiou, C.; Paryzhak, S.; Dumych, T.; Bilyy, R.; Tripal, P.; et al. ROS-responsive N-alkylaminoferrocenes for cancer cell specific targeting of mitochondria. Angew. Chem. Int. Ed. 2018, 57, 11943–11946. [Google Scholar] [CrossRef]
- Chen, X.; Khairallah, G.N.; O’Hair, R.A.J.; Williams, S.J. Fixed-charge labels for simplified reaction analysis: 5-Hydroxy-1,2,3-triazoles as byproducts of a copper(I)-catalyzed click reaction. Tetrahedron Lett. 2011, 52, 2750–2753. [Google Scholar] [CrossRef]
- Reshetnikov, V.; Daum, S.; Mokhir, A. Cancer specific, intracellular, reductive activation of anticancer Pt(IV)-prodrugs. Chem. Eur. J. 2017, 23, 5678–5681. [Google Scholar] [CrossRef] [PubMed]
- Modica-Napolitano, J.; Aprille, J.R. Delocalized lipophilic cations selectively target the mitochondria of carcinoma cells. Adv. Drug Deliv. Rev. 2001, 49, 63–70. [Google Scholar] [CrossRef]
- Daum, S.; Toms, J.; Reshetnikov, V.; Özkan, H.G.; Hampel, F.; Maschauer, S.; Hakimioun, A.; Beierlein, F.; Sellner, L.; Schmitt, M.; et al. Active Form of N-Alkylaminoferrocene-Based Anticancer Prodrugs and Their Radiolabeling with 18F. Bioconj. Chem. 2019, 30, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |


| Prodrug or Control | IC50/BL-2 Cells i (μM) | ||
|---|---|---|---|
| 24 h ii | 48 h ii | 96 h ii | |
| 5 | 5 ± 1 | 5 ± 1 | 8 ± 1 |
| 12 | 2 ± 1 | 0.5 ± 0.2 | 0.3 ± 0.1 |
| 9 | 37 ± 2 | 35 ± 2 | 33 ± 2 |
| 7 | − | >25 | − |
| 13 | − | 35 ± 6 | − |
| 11 | >50 | >50 | >50 |
| Fe(HQ)2 iii | 3 ± 1 | 3 ± 1 | 3 ± 1 |
| IC50/A2780 cells i (μM) | |||
| 24 h ii | 48 h ii | 96 h ii | |
| 5 | 10 ± 3 | 5 ± 2 | 5 ± 2 |
| 9 | 42 ± 3 | 32 ± 3 | 30 ± 5 |
| 7 | − | >25 | − |
| 13 | − | >50 | − |
| 11 | >50 | >50 | >50 |
| Fe(HQ)2 | 3 ± 1 | 3 ± 1 | 3 ± 1 |
| IC50/DU-145 cells i (μM) | |||
| 24 h ii | 48 h ii | 96 h ii | |
| 5 | 20 ± 3 | 10 ± 2 | 10 ± 2 |
| 9 | >50 | 47 ± 2 | 35 ± 3 |
| 11 | >50 | >50 | >50 |
| Fe(HQ)2 | 3 ± 1 | 3 ± 1 | 3 ± 1 |
| IC50 (μM) | |||
| Jurkat cells i | HDFa cells i48 h ii | ||
| 24 h ii | 48 h ii | ||
| 5 | 20 ± 3 | 20 ± 2 | 18 ± 5 |
| 12 | − | − | <1 |
| 9 | 30 ± 3 | 30 ± 4 | >50 |
| 11 | >50 | >50 | − |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reshetnikov, V.; Özkan, H.G.; Daum, S.; Janko, C.; Alexiou, C.; Sauer, C.; Heinrich, M.R.; Mokhir, A. N-Alkylaminoferrocene-Based Prodrugs Targeting Mitochondria of Cancer Cells. Molecules 2020, 25, 2545. https://doi.org/10.3390/molecules25112545
Reshetnikov V, Özkan HG, Daum S, Janko C, Alexiou C, Sauer C, Heinrich MR, Mokhir A. N-Alkylaminoferrocene-Based Prodrugs Targeting Mitochondria of Cancer Cells. Molecules. 2020; 25(11):2545. https://doi.org/10.3390/molecules25112545
Chicago/Turabian StyleReshetnikov, Viktor, Hülya Gizem Özkan, Steffen Daum, Christina Janko, Christoph Alexiou, Caroline Sauer, Markus R. Heinrich, and Andriy Mokhir. 2020. "N-Alkylaminoferrocene-Based Prodrugs Targeting Mitochondria of Cancer Cells" Molecules 25, no. 11: 2545. https://doi.org/10.3390/molecules25112545
APA StyleReshetnikov, V., Özkan, H. G., Daum, S., Janko, C., Alexiou, C., Sauer, C., Heinrich, M. R., & Mokhir, A. (2020). N-Alkylaminoferrocene-Based Prodrugs Targeting Mitochondria of Cancer Cells. Molecules, 25(11), 2545. https://doi.org/10.3390/molecules25112545






