In Silico, Ex Vivo and In Vivo Studies of Roflumilast as a Potential Antidiarrheal and Antispasmodic agent: Inhibition of the PDE-4 Enzyme and Voltage-gated Ca++ ion Channels
Abstract
:1. Introduction
2. Results
2.1. Effect on Castor Oil-Provoked Diarrhea
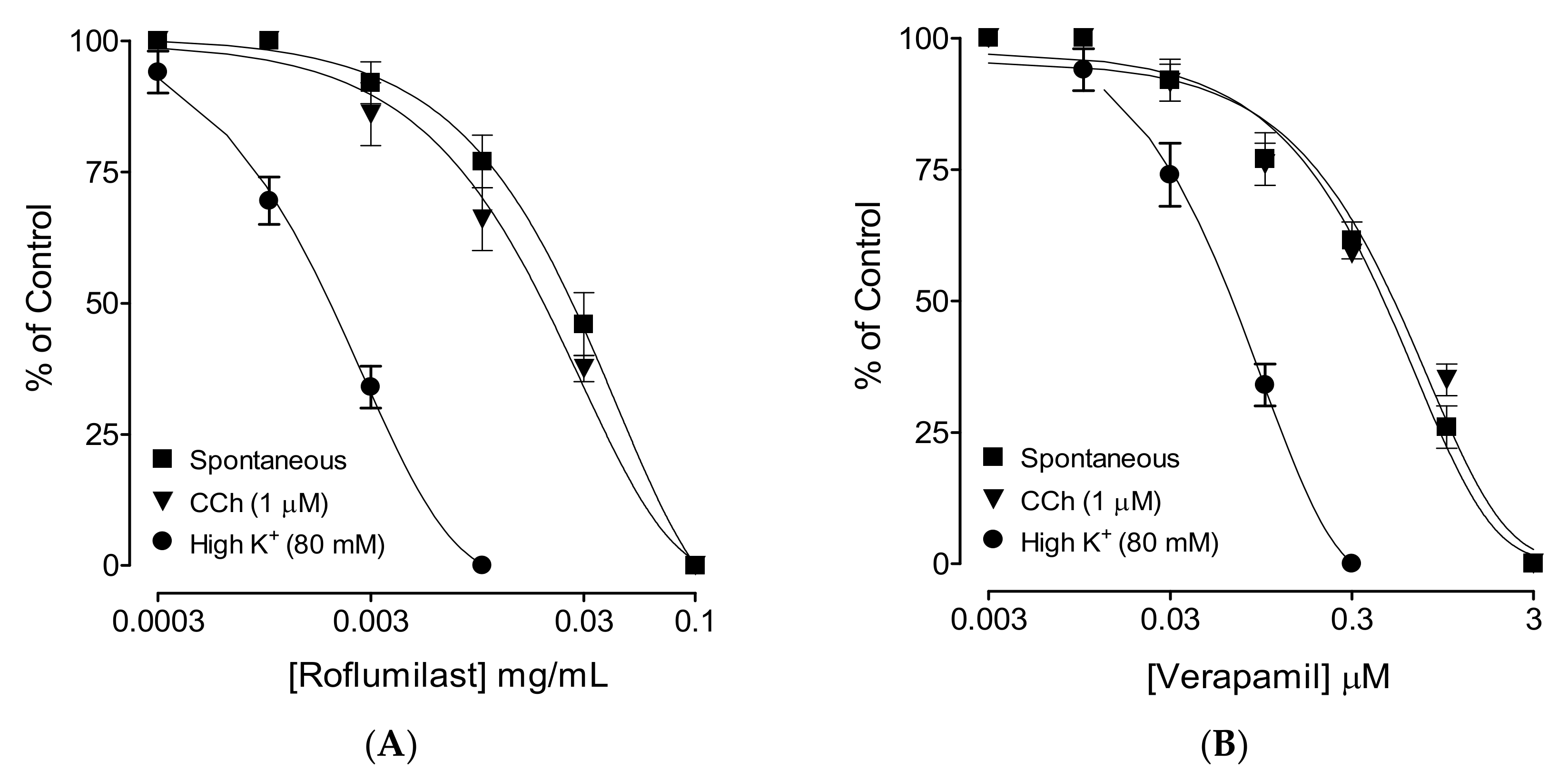
2.2. Effect on Spontaneous Contractions
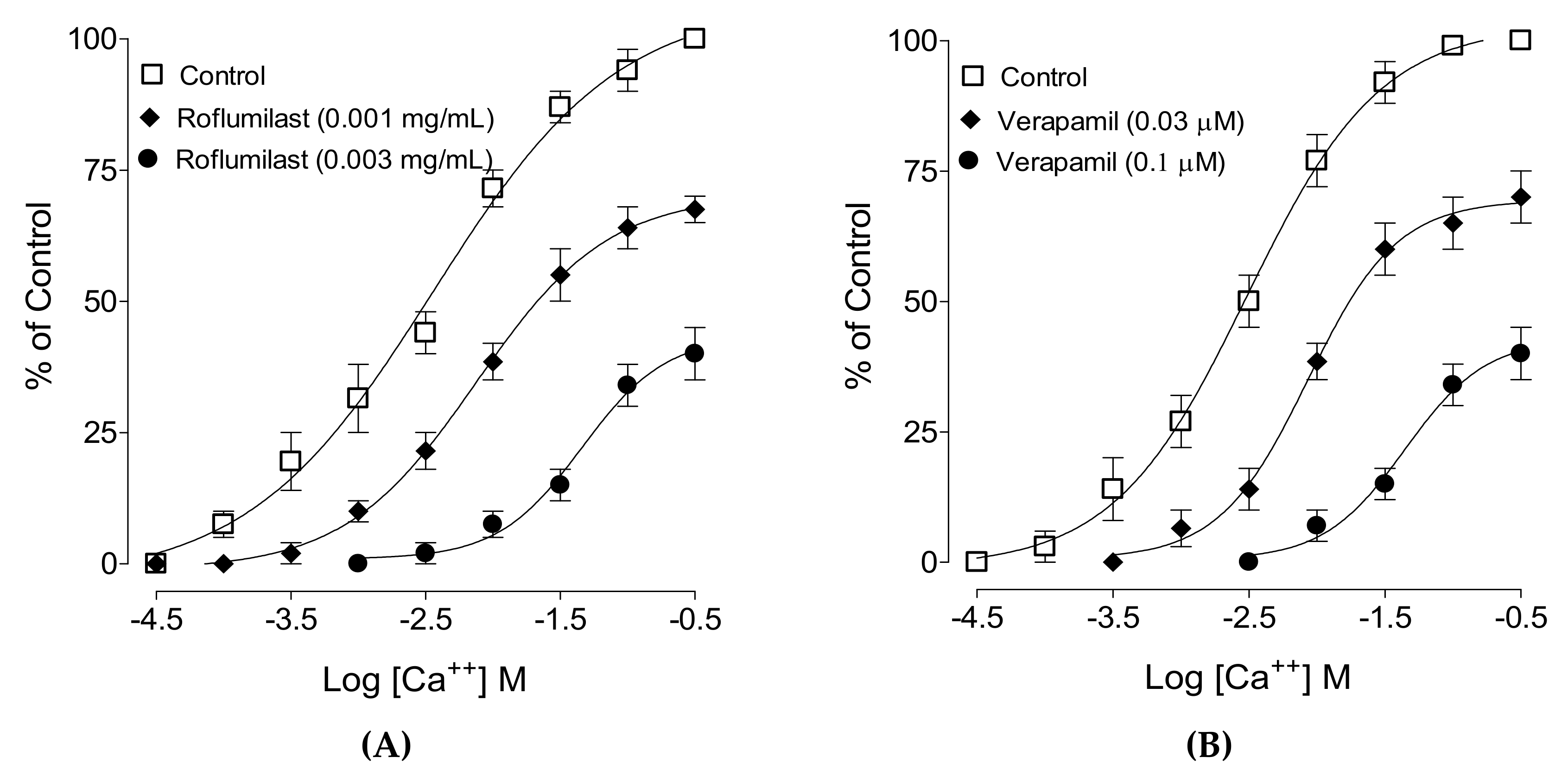
2.3. Effect on Ca++ Curves
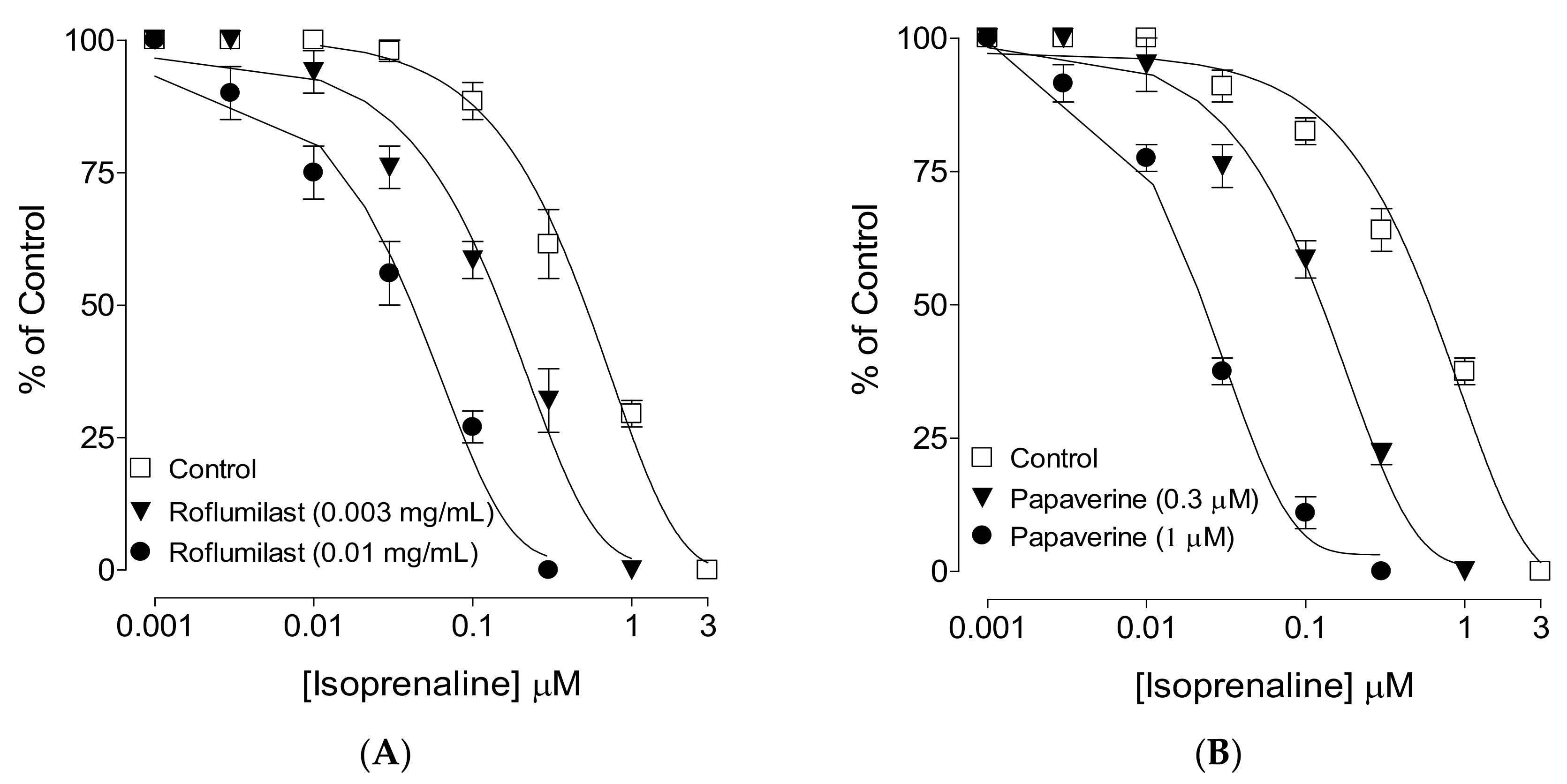
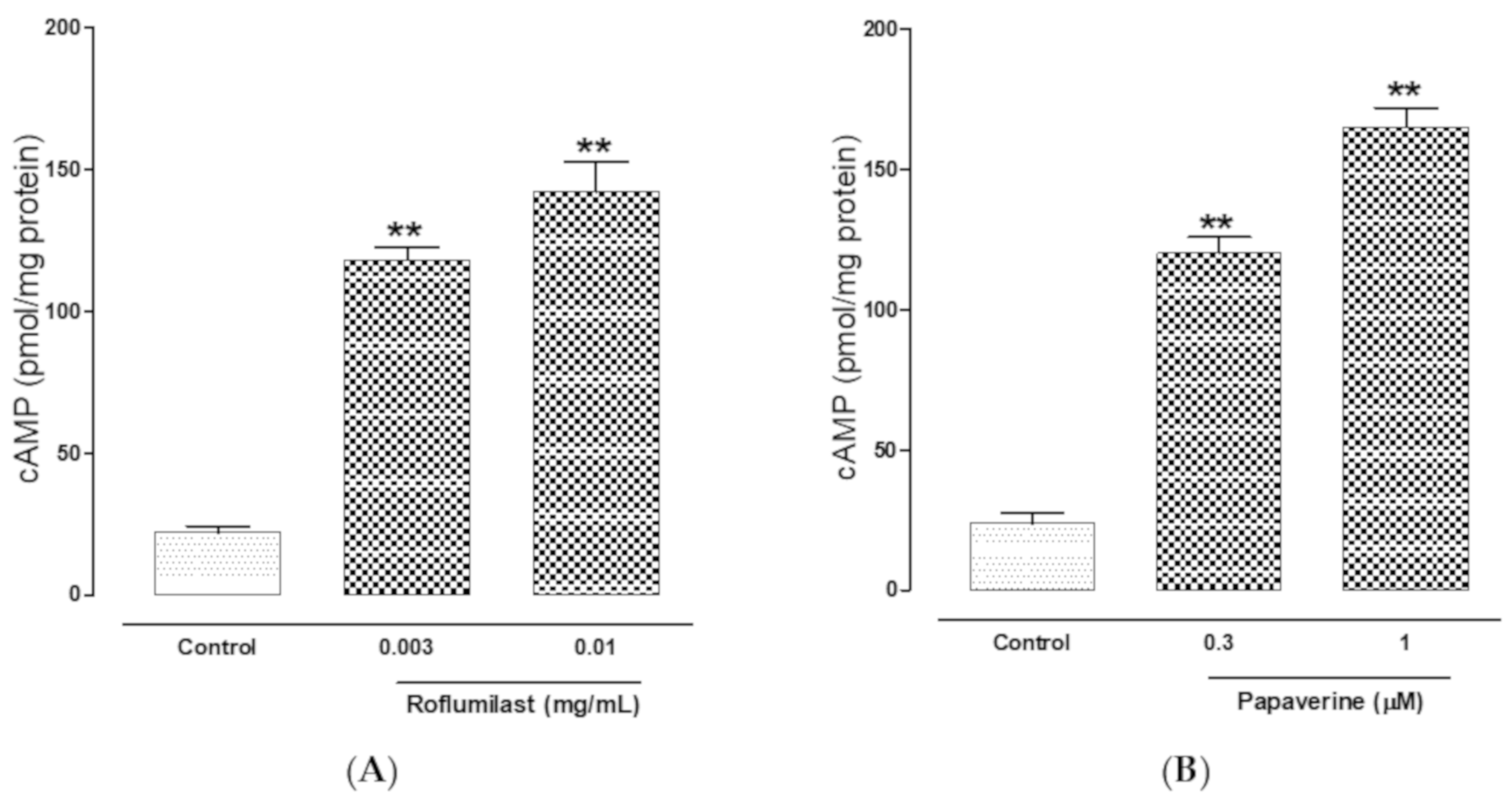
2.4. PDE-Inhibitory Effect
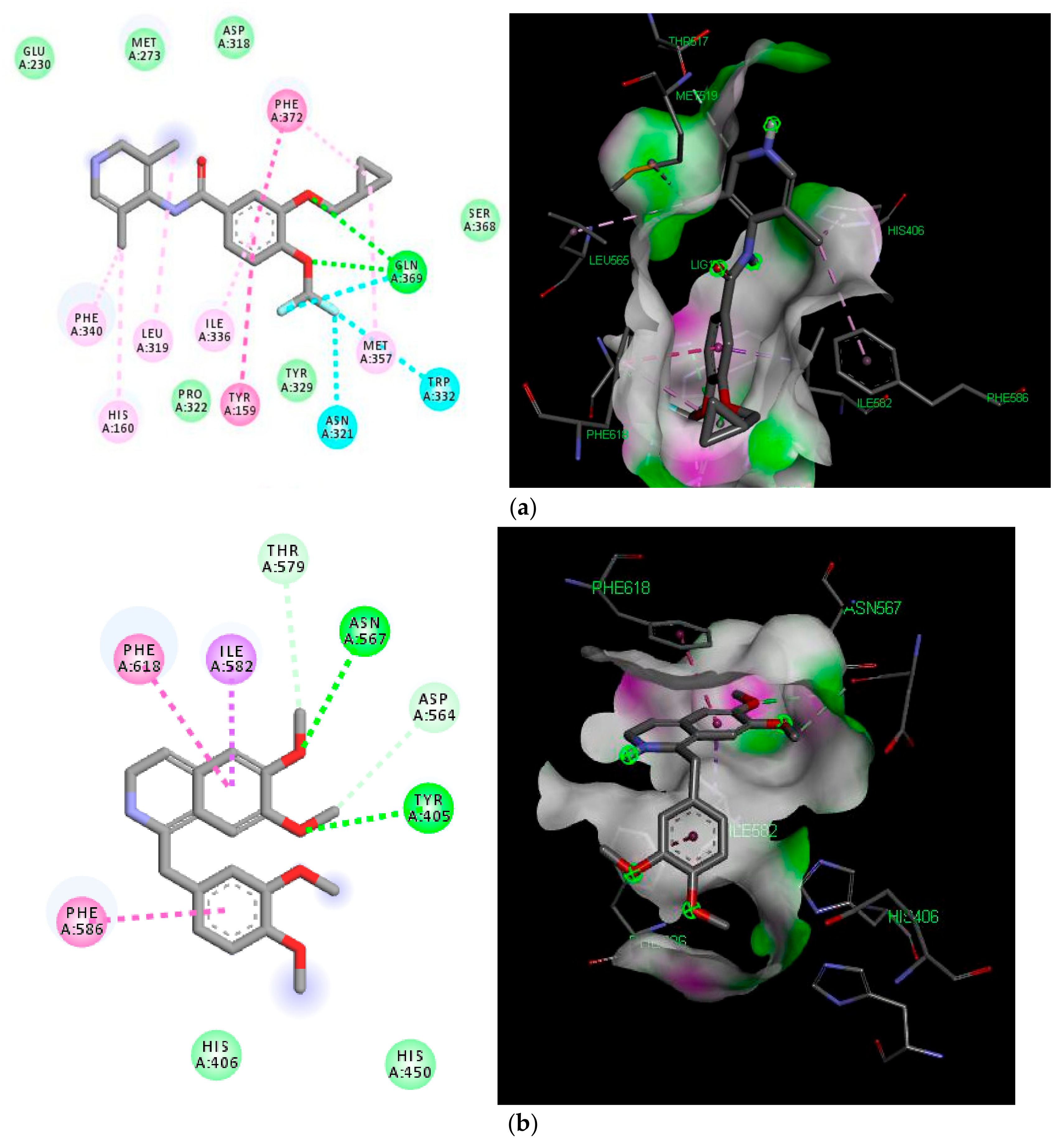
2.5. Molecular Docking Analyses
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals
4.3. In Vivo Experiments
4.4. Ex Vivo Experiments
4.4.1. Antispasmodic Effect
4.4.2. Ca++ Channel-Blocking Activity
4.4.3. PDE-Inhibitory Activity
4.5. In Silico Studies
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Malagelada, J.R. A symptom-based approach to making a positive diagnosis of irritable bowel syndrome with constipation. Intern. J. Clin. Pract. 2006, 60, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, Y.; Zhang, J.; Huang, X.; Wang, Y.; Xu, X.; Zheng, B.; Zhou, X.; Tian, H.; Liu, L.; et al. Antidiarrheal effect of Alpinia oxyphylla Miq. (Zingiberaceae) in experimental mice and its possible mechanism of action. J. Ethnopharmacol. 2015, 168, 182–190. [Google Scholar] [CrossRef]
- Al Jarousha, A.M.; ElJarou, M.A.; El Qouqa, I.A. Bacterial enteropathogens and risk factors associated with childhood diarrhea. Indian J. Pediatr. 2011, 78, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.; Hwang, L.; Melmed, G.Y.; Low, K.; Vasiliauskas, E.; Ippoliti, A.; Yang, J.; Lezcano, S.; Conklin, J.L.; Sahakian, A. New clinical method for distinguishing D-IBS from other gastrointestinal conditions causing diarrhea: The LA/IBS diagnostic strategy. Dig. Dis. Sci. 2010, 55, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Young, V.B.; Schmidt, T.M. Antibiotic-associated diarrhea accompanied by large-scale alterations in the composition of the fecal microbiota. J. Clin. Microbiol. 2004, 42, 1203–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farthing, M.J. Diarrhoea: A significant worldwide problem. Int. J. Antimicrob. Agents 2000, 14, 65–69. [Google Scholar] [CrossRef]
- Semenya, S.S.; Maroyi, A. Medicinal plants used by the Bapedi traditional healers to treat diarrhea in the Limpopo province. S. Afr. J. Ethnopharmacol. 2012, 144, 395–401. [Google Scholar] [CrossRef]
- Brunton, L.L. Agents for Control of Gastric Acidity and Treatment of Peptic Ulcers Goodman Gilman’s The Pharmacological Basis of Therapeutics, 11th ed.; McGaw-Hill: New York, NY, USA, 2008; pp. 623–652. [Google Scholar]
- Jailwala, J.; Imperiale, T.F.; Kroenke, K. Pharmacologic treatment of the irritable bowel syndrome: A systematic review of randomized, controlled trials. Ann. Intern. Med. 2000, 133, 136–147. [Google Scholar] [CrossRef]
- Lesbros-Pantoflickova, D.; Michetti, P.; Fried, M.; Beglinger, C.; Blum, A. Meta-analysis: The treatment of irritable bowel syndrome. Aliment. Pharmacol. Ther. 2004, 20, 1253–1269. [Google Scholar] [CrossRef]
- Castro, A.; Jerez, M.J.; Gil, C.; Martinez, A. Cyclic nucleotide phosphodiesterases and their role in immunomodulatory responses: Advances in the development of specific phosphodiesterase inhibitors. Med. Res. Rev. 2005, 25, 229–244. [Google Scholar] [CrossRef]
- Moore, A.R.; Willoughby, D.A. The role of cAMP regulation in controlling inflammation. Clin. Exp. Immunol. 1995, 101, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Sawant, O.; Ganpathy, P.; Pitre, S.; Kadam, V.J. Phosphodiesterase inhibitors: Their role and implications. Int. J. Pharm. Tech. Res. 2009, 1, 1148–1160. [Google Scholar]
- Boswell-Smith, V.; Spina, D.; Page, C.P. Phosphodiesterase inhibitors. Br. J. Pharmacol. 2006, 147 (Suppl. 1), S252–S257. [Google Scholar] [CrossRef]
- Schreiber, S.; Keshavarzian, A.; Isaacs, K.L.; Schollenberger, J.; Guzman, J.P.; Orlandi, C.; Hanauer, S.B. A randomized, placebo-controlled, phase II study of tetomilast in active ulcerative colitis. Gastroenterology 2007, 132, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Imam, F.; Al-Harbi, N.O.; Al-Harbi, M.M.; Ansari, M.A.; Almutairi, M.M.; Alshammari, M.; Almukhlafi, T.S.; Ansari, M.N.; Aljerian, K.; Ahmad, S.F. Apremilast reversed carfilzomib-induced cardiotoxicity through inhibition of oxidative stress, NF-κB and MAPK signaling in rats. Toxicol. Mech. Methods 2016, 26, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Bäumer, W.; Hoppmann, J.; Rundfeldt, C.; Kietzmann, M. Highly selective phosphodiesterase 4 inhibitors for the treatment of allergic skin diseases and psoriasis. Inflamm. Allergy Drug Targets 2007, 6, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, A.S., Jr. Series introduction: The transcription factor NF-kappaB and human disease. J. Clin. Invest. 2001, 107, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Erdogan, S.; Celik, S.; Aslantas, O.; Kontas, T.; Ocak, S. Elevated cAMP levels reverse Brucella melitensis-induced lipid peroxidation and stimulate IL-10 transcription in rats. Res. Vet. Sci. 2007, 82, 181–186. [Google Scholar] [CrossRef]
- Ansari, M.N.; Aloliet, R.I.; Ganaie, M.A.; Khan, T.H.; Rehman, N.U.; Imam, F.; Hamad, A.M. Roflumilast, a phosphodiesterase 4 inhibitor, attenuates cadmium-induced renal toxicity via modulation of NF-κB activation and induction of NQO1 in rats. Hum. Exp. Toxicol. 2019, 38, 588–597. [Google Scholar] [CrossRef]
- Ansari, M.N.; Ganaie, M.A.; Rehman, N.U.; Alharthy, K.M.; Khan, T.H.; Imam, F.; Ansari, M.A.; Al-Harbi, N.O.; Jan, B.L.; Sheikh, I.A.; et al. Protective role of Roflumilast against cadmium-induced cardiotoxicity through inhibition of oxidative stress and NF-κB signaling in rats. Saudi Pharm. J. 2019, 27, 673–681. [Google Scholar] [CrossRef]
- Croci, T.; Landi, M.; Emonds-Alt, X.; Le-Fur, G.; Maffrand, J.P.; Manara, L. Role of tachykinins in castor oil diarrhoea in rats. Br. J. Pharmacol. 1997, 121, 375–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, N.U.; Gilani, A.H.; Khan, A.; Nazneen, M.; El Gamal, A.A.; Fawzy, G.A.; Al-Ati, H.Y.; Abdel-kader, M.S. Antidiarrheal and Antispasmodic Activities of Buddleja polystachya are Mediated Through Dual Inhibition of Ca(++) Influx and Phosphodiesterase Enzyme. Phytother. Res. 2015, 29, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Memon, R.; Gilani, A.H. Antispasmodic and antidiarrheal activities of Valeriana hardwickii rhizome are putatively mediated through calcium channel blockade. Evid. Based Complement. Alternat. Med. 2011, 2011, 304960. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Rehman, N.U.; Alkharfy, K.M.; Gilani, A.H. Antidiarrheal and antispasmodic activities of Salvia officinalis (Sage) are mediated through activation of K+ channels. Bangl. J. Pharmacol. 2011, 6, 111–116. [Google Scholar]
- Khan, M.; Khan, A.U.; Rehman, N.U.; Gilani, A.H. Pharmacological basis for medicinal use of Lens culinaris in gastrointestinal and respiratory disorders. Phytother. Res. 2014, 28, 1349–1358. [Google Scholar] [CrossRef]
- Gilani, A.H.; Rehman, N.U.; Mehmood, A.H.; Alkharfy, K.M. Species differences in the antidiarrheal and antispasmodic activities of Lepidium sativum and insight into underlying mechanisms. Phytother. Res. 2013, 27, 1086–1094. [Google Scholar] [CrossRef]
- Downie, J.W.; Twiddy, D.A.; Awad, S.A. Antimuscarinic and non-competitive antagonist properties of dicyclomine hydrochloride in isolated human and rabbit bladder muscle. J. Pharmacol. Exp. Ther. 1977, 201, 662–668. [Google Scholar]
- Fleckenstein, A. Specific pharmacology of Ca++ in myocardium, cardiac pacemakers and vascular smooth muscle. Rev. Pharmacol. Toxicol. 1977, 17, 149–166. [Google Scholar] [CrossRef]
- Hamilton, T.C.; Weir, S.W.; Weston, A.H. Comparison of the effects of BRL 34915 and verapamil on electrical and mechanical activity in rat portal vein. Br. J. Pharmacol. 1986, 88, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Kishii, K.; Morimoto, T.; Nakajima, N.; Yamazaki, K.; Tsujitani, M.; Takayanagi, I. Effects of LP-805, a novel vasorelaxant agent, a potassium channel opener, on rat thoracic aorta. Gen. Pharmacol. 1992, 23, 347–353. [Google Scholar] [CrossRef]
- Smith, E.J.; Thompson, A.P.; O’Driscoll, A.; Clarke, D.J. Pathogenesis of adherent-invasive Escherichia coli. Future Microbiol. 2013, 8, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, T.; Watanabe, A.; Shimizu, K.; Urakawa, N.; Nakajyo, S. Effects of various selective phosphodiesterase inhibitors on carbachol-induced contraction and cyclic nucleotide contents in the guinea pig gall bladder. J. Vet. Med. Sci. 2005, 67, 659–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choo, L.K.; Mitchelson, F. Antagonism of cholinomimetics by troxypyrrolidinium in guinea-pig atria and longitudinal ileal muscle: Comparison with hemicholinium-3. Eur. J. Pharmacol. 1978, 52, 313–322. [Google Scholar] [CrossRef]
- Sato, Y.; Akao, T.; He, J.X.; Nojima, H.; Kuraishi, Y.; Morota, T.; Asano, T.; Tani, T. Glycycoumarin from Glycyrrhizae Radix acts as a potent antispasmodic through inhibition of phosphodiesterase 3. J. Ethnopharmacol. 2006, 105, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.Y.; MacKerell, A.D., Jr. Do Halogen-Hydrogen Bond Donor Interactions Dominate the Favorable Contribution of Halogens to Ligand-Protein Binding? J. Phys. Chem. B. 2017, 121, 6813–6821. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Guide for the Care and Use of Laboratory Animals; National Academy Press: Washington, DC, USA, 1996; pp. 1–7. [Google Scholar]
- Jebunnessa Uddin, S.B.; Mahabub-Uz-Zaman, M.; Akhtar, R.; Ahmed, N.U. Antidiarrheal activity of ethanolic bark extract of Mitragyna diversifolia. Bangl. J. Pharmacol. 2009, 4, 144–146. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.J.; Begum, S.; Hassan, S.I.; Ali, S.N.; Siddiqui, B.S.; Gilani, A.H. Pharmacological basis for the medicinal use of Psidium guajava leave in hyperactive gut disorders. Bangl. J. Pharmacol. 2011, 6, 100–105. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Rehman, N.U.; Gilani, A.H.; Ahmed, Z.; Al-Massarani, S.; El-Gamal, A.; Farag, M. Possible Mechanism(s) Underlying the Antidiarrheal, Antispasmodic and Bronchodilatory Activities of the Pericarp of Albizia lebbeck. Int. J. Pharmacol. 2018, 15, 56–65. [Google Scholar]
- Godfraind, T.; Miller, R.; Wibo, M. Calcium antagonism and calcium entry blockade. Pharmacol. Rev. 1986, 38, 321–416. [Google Scholar]
- Gilani, A.H.; Shaheen, F.; Christopoulos, A.; Mitchelson, F. Interaction of ebeinone, an alkaloid from Fritillaria imperialis, at two muscarinic acetylcholine receptor subtypes. Life Sci. 1997, 60, 535–544. [Google Scholar] [CrossRef]
- Gilani, A.H.; Khan, A.; Subhan, F.; Khan, M. Antispasmodic and bronchodilator activities of St. John’s wort are putatively mediated through dual inhibition of calcium influx and phosphodiesterase. Fundam. Clin. Pharmacol. 2005, 19, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Blattner, R.; Classen, H.G.; Dehnert, H.; Doring, H.J. Experiments on Isolated Smooth Muscle Preparations; Hugo Sachs Elektronik KG: March, Germany, 1978; pp. 53–71. [Google Scholar]
- Rang, H.P.; Dale, M.M.; Ritter, J.M. Pharmacology, 4th ed.; Churchill Livingstone: New York, NY, USA, 1999; pp. 289–290. [Google Scholar]
- Dallakyan, S.; Olson, A.J. Small-Molecule Library Screening by Docking with PyRx. Methods Mol. Bio. 2015, 1263, 243–250. [Google Scholar]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comp. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef] [Green Version]
- Dassault Systèmes BIOVIA, Discovery Studio Modeling Environment. Release 2017; Dassault Systèmes: San Diego, CA, USA, 2016. [Google Scholar]
Sample Availability: Samples of the roflumilast are available from the authors. |
| Treatment (p.o.) | No. of Mice with Diarrhea | % Protection |
|---|---|---|
| Saline (10 mL/kg) + Castor oil | 5/5 | 0 |
| Roflumilast (0.5 mg/kg) + Castor oil | 3*/5 | 40 |
| Roflumilast (1.5 mg/kg) + Castor oil | 1*/5 | 80 |
| Loperamide (10 mg/kg) + Castor oil | 0**/5 | 100 |
| Name of Molecule | Binding Energy (Kcal/mol) | Types of Bonding | ||
|---|---|---|---|---|
| 5WH5 | 5LAQ | 5WH5 | 5LAQ | |
| Roflumilast | −9.4 | −9.3 | H-bond, Halogen bond, Van der Waals, Pi-sigma, Pi-Pi. | H-bond, Halogen bond, Van der Waals, Pi-sigma, Pi-Pi, |
| Papaverine | −8.3 | −8.2 | Van der Waals, Pi-sigma, Pi-Pi | H-bond, Van der Waals, Pi-sigma, Pi-Pi, |
| Co-crystallized ligand | −10.6 | −8.6 | H-bond, Halogen bond, Pi-sigma, Pi-Pi | H-bond, Pi-Cation, Pi-sigma, Pi-Pi, |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, N.U.; Ansari, M.N.; Samad, A. In Silico, Ex Vivo and In Vivo Studies of Roflumilast as a Potential Antidiarrheal and Antispasmodic agent: Inhibition of the PDE-4 Enzyme and Voltage-gated Ca++ ion Channels. Molecules 2020, 25, 1008. https://doi.org/10.3390/molecules25041008
Rehman NU, Ansari MN, Samad A. In Silico, Ex Vivo and In Vivo Studies of Roflumilast as a Potential Antidiarrheal and Antispasmodic agent: Inhibition of the PDE-4 Enzyme and Voltage-gated Ca++ ion Channels. Molecules. 2020; 25(4):1008. https://doi.org/10.3390/molecules25041008
Chicago/Turabian StyleRehman, Najeeb Ur, Mohd Nazam Ansari, and Abdul Samad. 2020. "In Silico, Ex Vivo and In Vivo Studies of Roflumilast as a Potential Antidiarrheal and Antispasmodic agent: Inhibition of the PDE-4 Enzyme and Voltage-gated Ca++ ion Channels" Molecules 25, no. 4: 1008. https://doi.org/10.3390/molecules25041008







