The Effects of a Novel Series of KTTKS Analogues on Cytotoxicity and Proteolytic Activity
Abstract
1. Introduction
2. Results
2.1. Peptide Synthesis
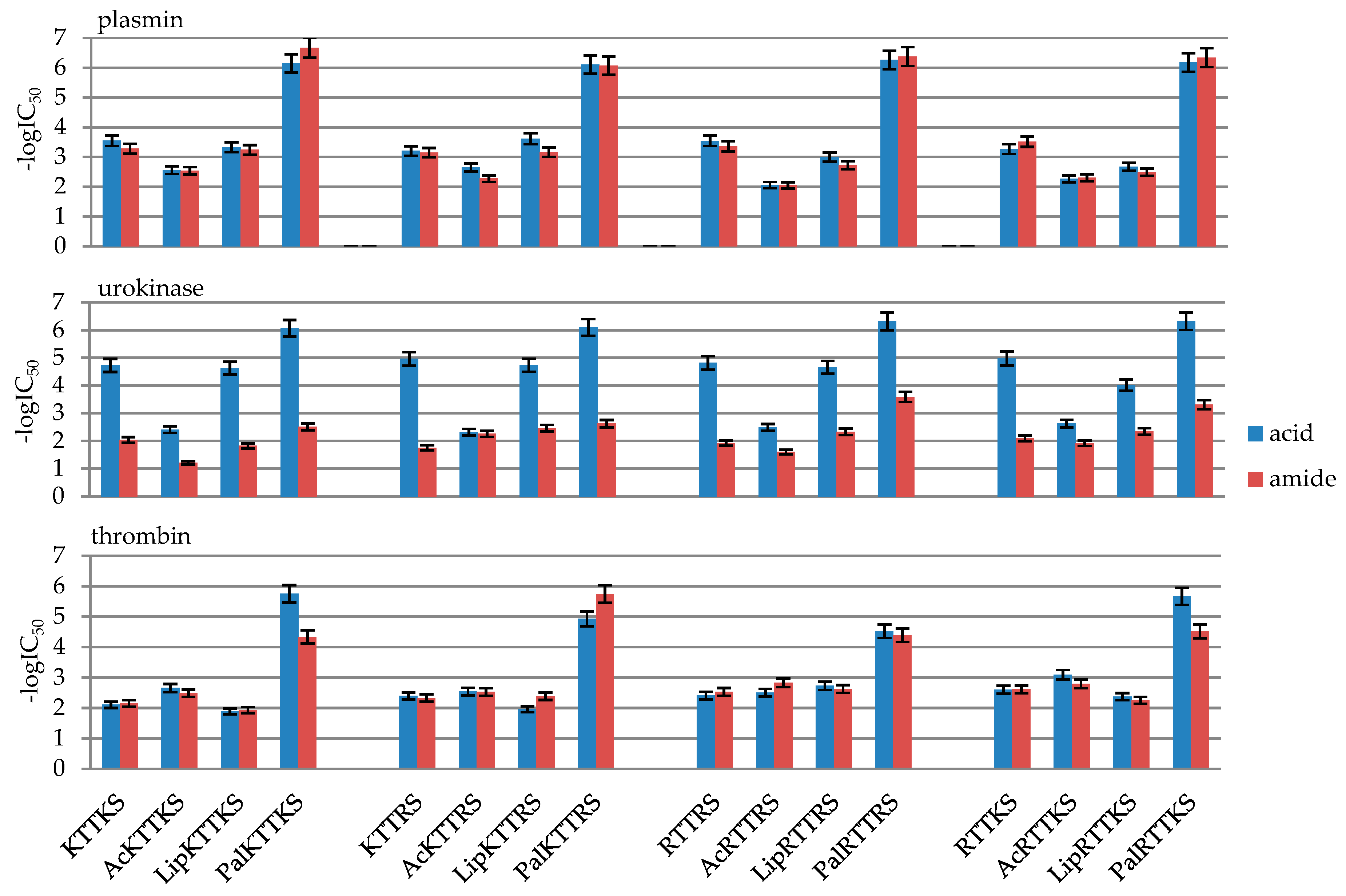
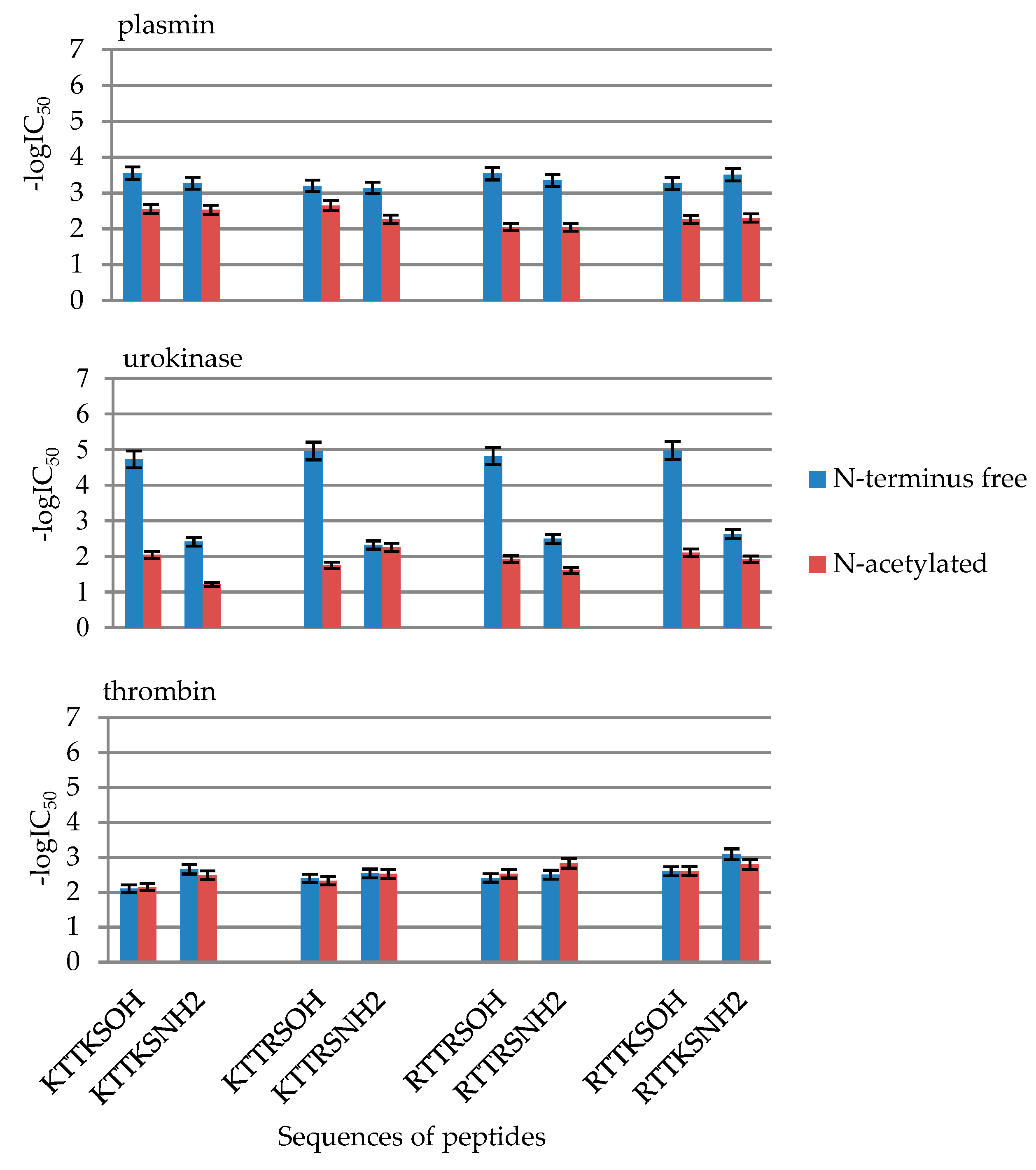
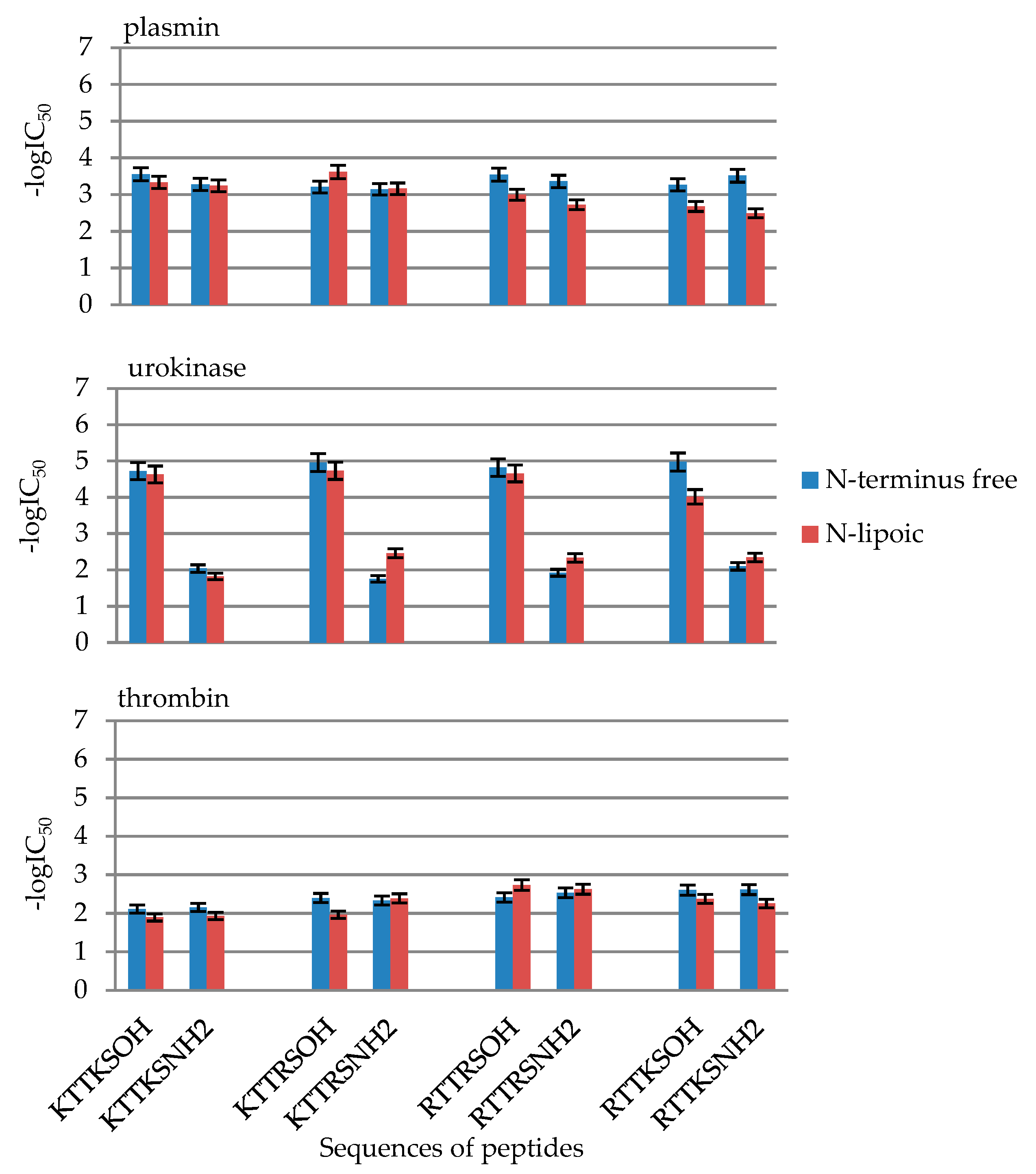
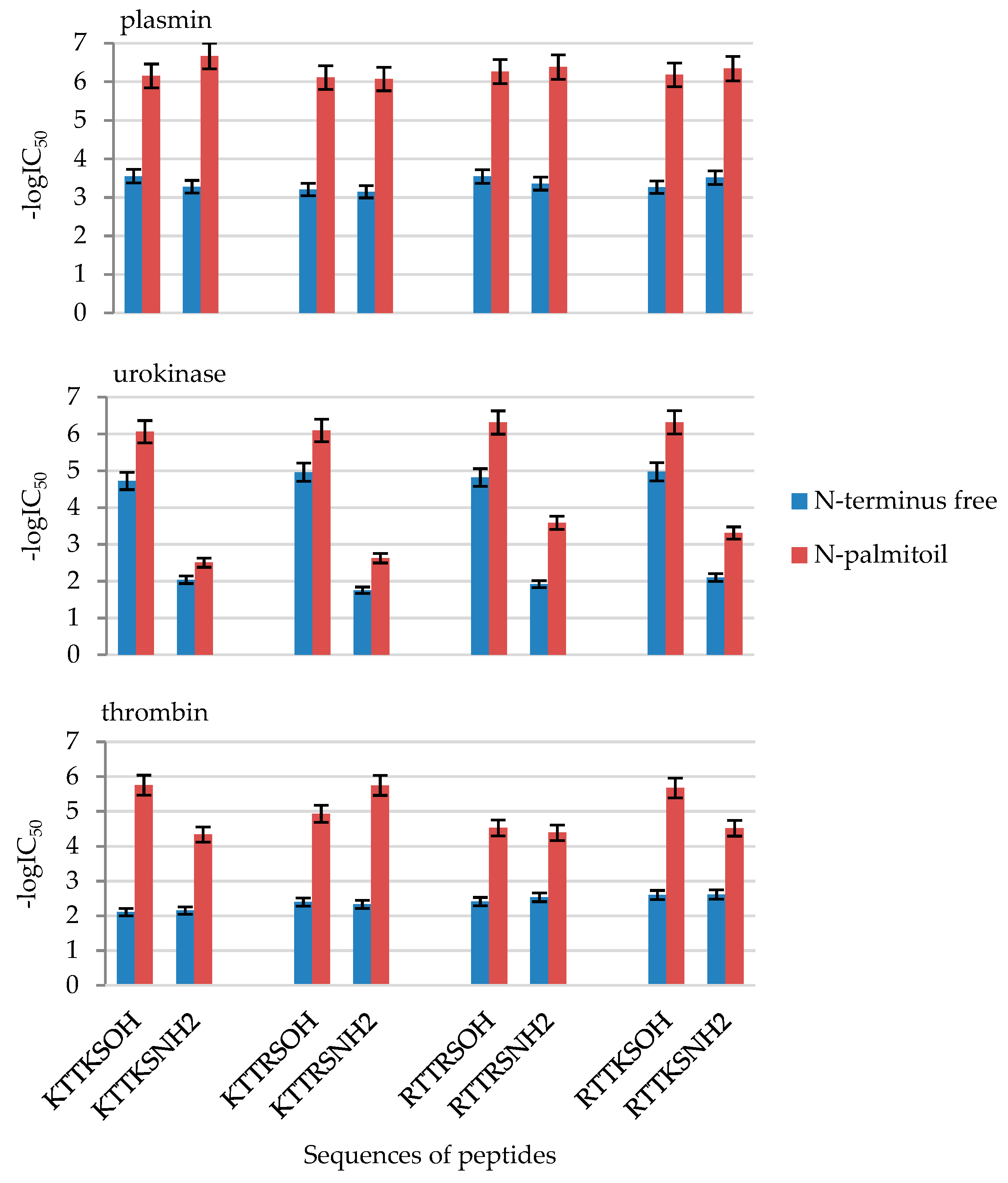
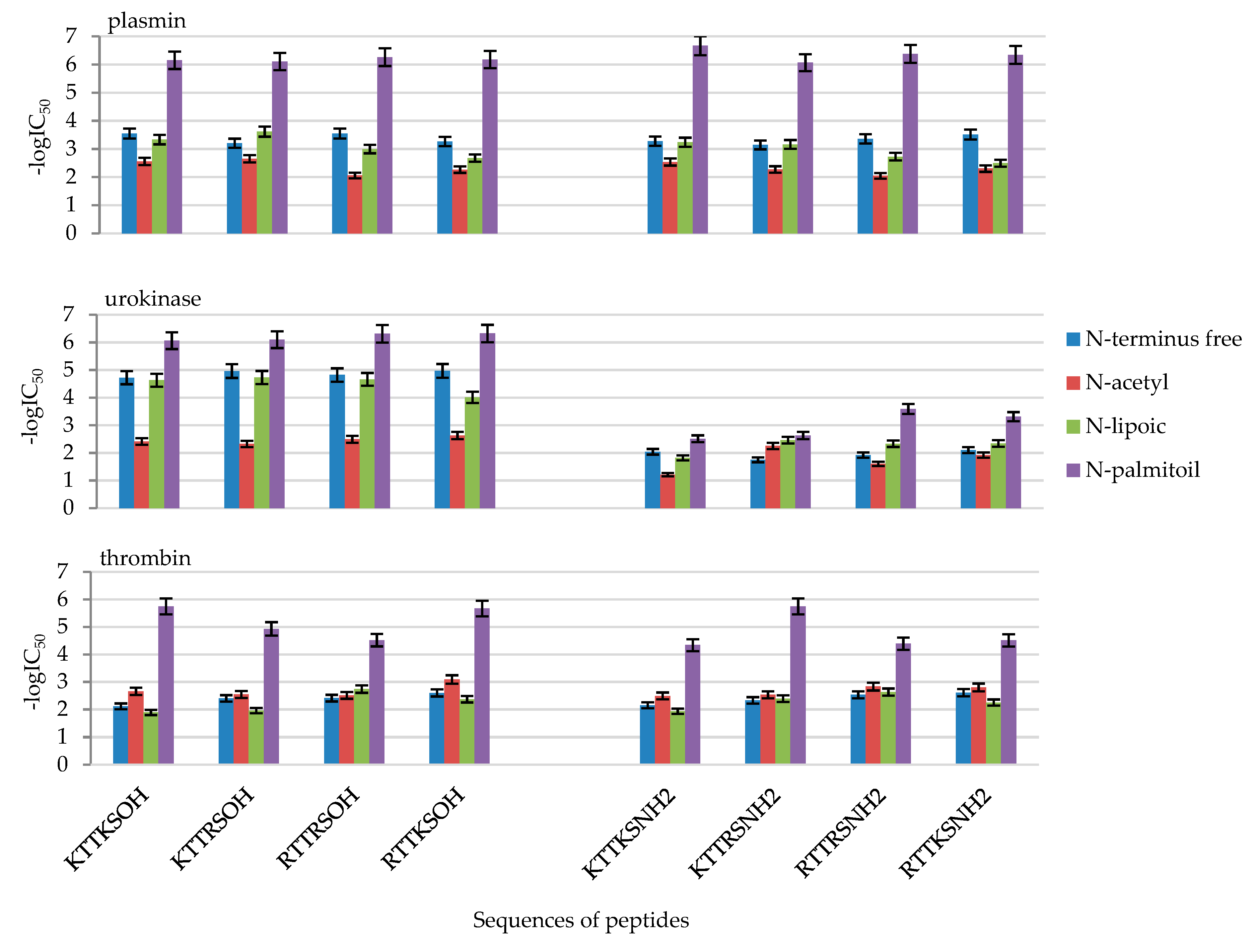
2.2. Enzymatic Investigations
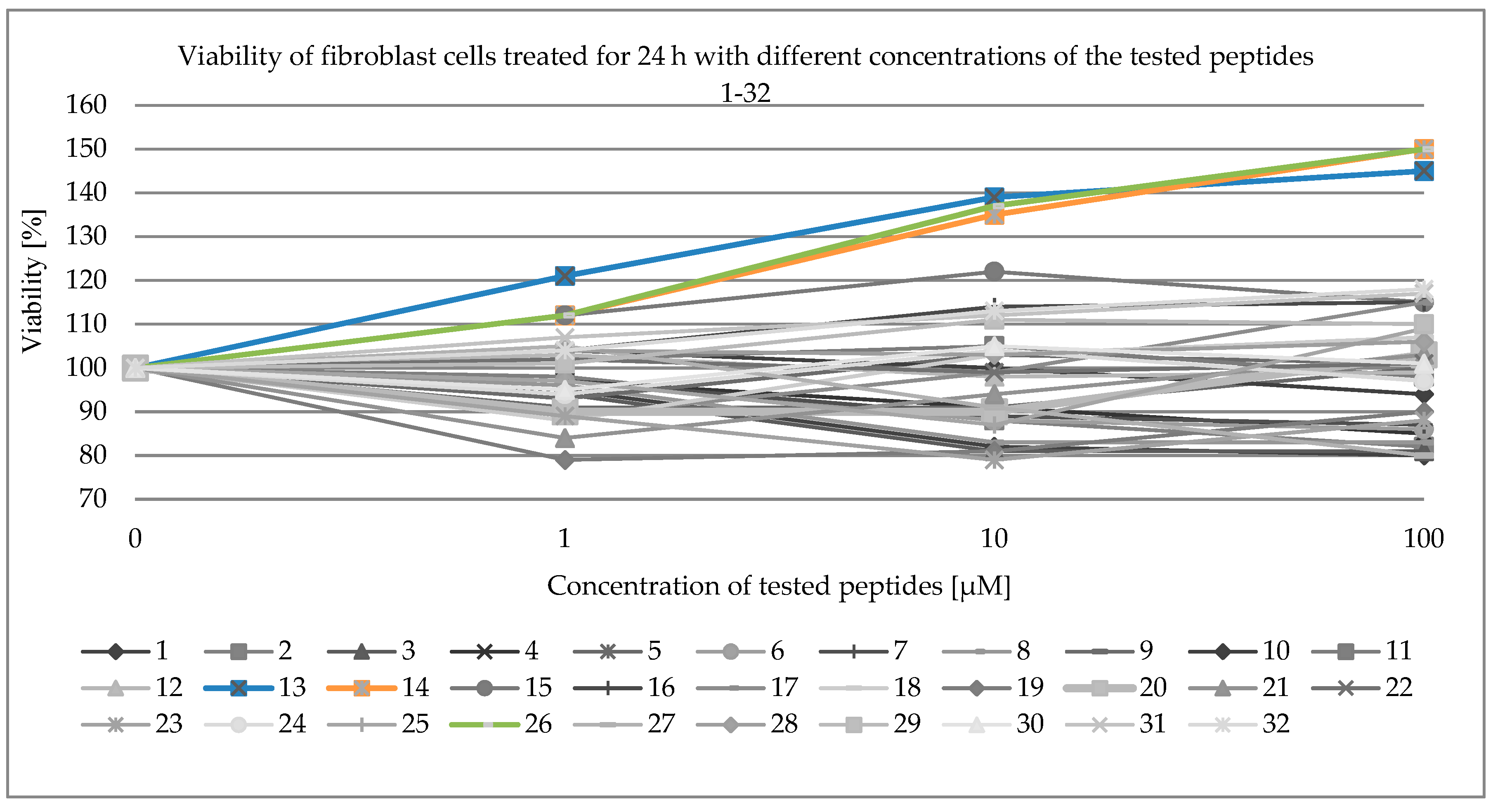
2.3. Viability of Fibroblast Cells
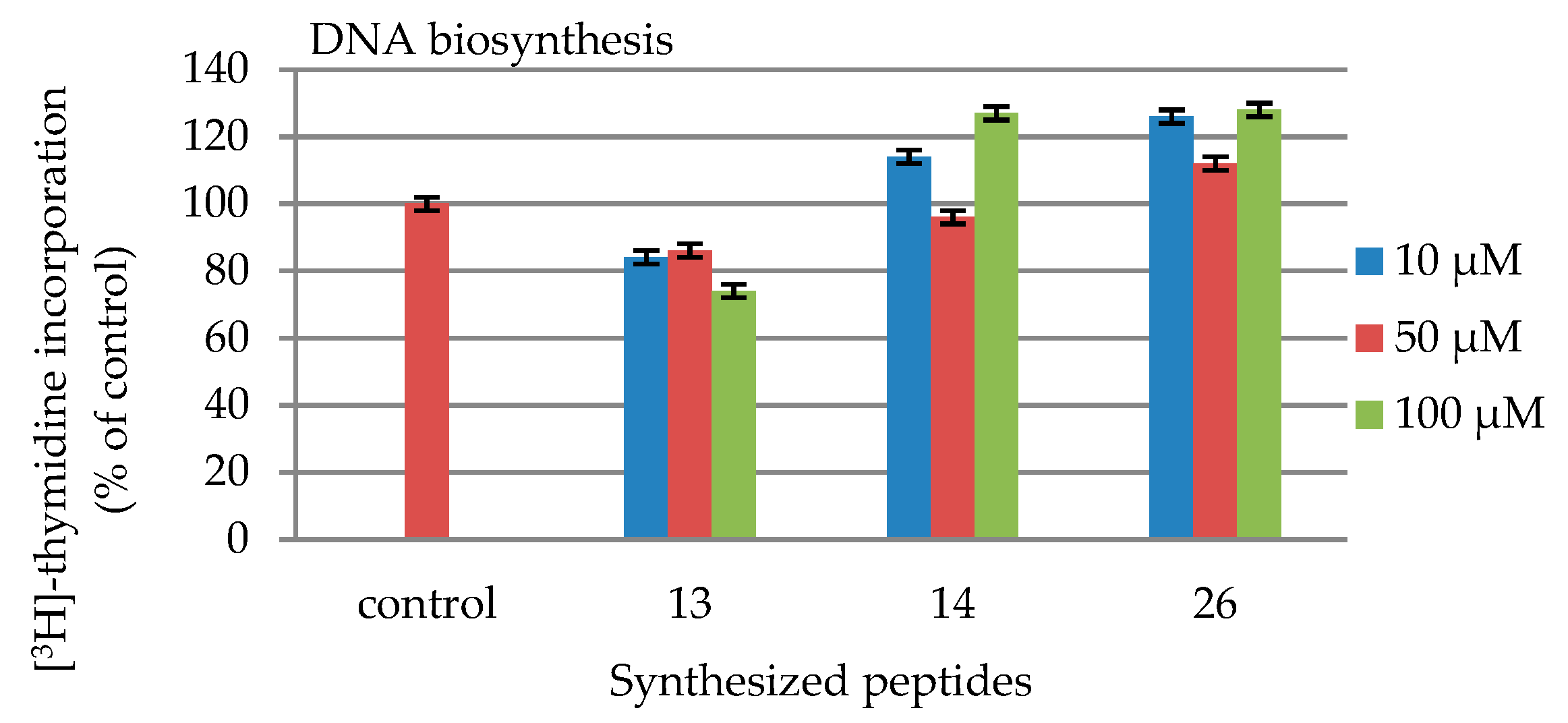
2.4. DNA and Collagen Biosynthesis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Peptide Synthesis
4.3. Enzymatic Investigations
- Tris buffer—0.6 mL (pH 8.8), enzyme: urokinase (50 units/mL), synthetic substrate: pyro-Glu-Gly-Arg-pNA·HCl (0.1 mL, 3 mM);
- Tris buffer—0.5 mL (pH 8.4), enzyme: thrombin (1 units/mL), synthetic substrate: H-D-Phe-Pip-Arg-pNA (0.2 mL, 0.75 mM);
- Tris buffer—0.5 mL (pH 7.4), enzyme: plasmin (0.4 units/mL), synthetic substrate: H-D-Val-Leu-Lys-pNA (0.2 mL, 3 mM);
- Borate buffer—0.5 mL (pH 7.5), enzyme: trypsin (0.4 units/mL), synthetic substrate: Bzl-L-Arg-pNA·HCl (0.2 mL, 8 mM);
- Tris buffer—0.6 mL (pH 9.0), enzyme: kallikrein (3 units/mL), synthetic substrate: H-D-Val-Leu-Arg-pNA·2HCl (0.1 mL, 75 mM);
- Tris buffer—0.6 mL (pH 8.4), enzyme: t-PA (167 mg/mL), synthetic substrate: H-D-Ile-Pro-Arg-pNA (0.1 mL, 10 mM)
- Tris buffer—0.6 mL (pH 9.0), enzyme: chymotrypsin (0.4 units/mL), synthetic substrate: Suc-Phe-pNA (0.2 mL, 8 mM)
4.4. Viability of Fibroblast Cells
4.4.1. Cell Culture
4.4.2. Cell Viability Assay
4.4.3. DNA Biosynthesis
4.4.4. Collagen Biosynthesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Baumann, L. Skin ageing and its treatment. J. Pathol. 2007, 211, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosm. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Maquart, F.X.; Bellon, G.; Pasco, S.; Monboisse, J.C. Matrikines in the regulation of extracellular matrix degradation. Biochimie 2005, 87, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Aldag, C.; Nogueira Teixeira, D.; Leventhal, P.S. Skin rejuvenation using cosmetic products containing growth factors, cytokines, and matrikines: A review of the literature. Clin. Cosmet. Investig. Dermatol. 2016, 9, 411–419. [Google Scholar] [CrossRef]
- Reddy, B.; Jow, T.; Hantash, B.M. Bioactive oligopeptides in dermatology: Part, I. Exp. Dermatol. 2012, 21, 569–575. [Google Scholar] [CrossRef]
- Malerich, S.; Berson, D. Next Generation Cosmeceuticals. Dermatol. Clin. 2014, 32, 13–21. [Google Scholar] [CrossRef]
- Maquart, F.X.; Pasco, S.; Ramont, L.; Hornebeck, W.; Monboisse, J.C. An introduction to matrikines: Extracellular matrix-derived peptides which regulate cell activity: Implication in tumor invasion. Crit. Rev. Oncol. Hematol. 2004, 49, 199–202. [Google Scholar] [CrossRef]
- Tran, K.T.; Lamb, P.; Deng, J.S. Matrikines and matricryptins: Implications for cutaneous cancers and skin repair. J. Dermatol. Sci 2005, 40, 11–20. [Google Scholar] [CrossRef]
- Wells, J.M.; Gaggar, A.; Blalock, J.E. MMP generated matrikines. Matrix Biol. 2015, 44–46, 122–129. [Google Scholar] [CrossRef]
- Ricard-Blum, S.; Salza, R. Matricryptins and matrikines: Biologically active fragments of the extracellular matrix. Exp. Derm. 2014, 23, 457–463. [Google Scholar] [CrossRef]
- Senyürek, I.; Klein, G.; Kalbacher, H.; Deeg, M.; Schittek, B. Peptides derived from the human laminin α4 and α5 chains exhibit antimicrobial activity. Peptides 2010, 31, 468–1472. [Google Scholar]
- Wang, H.; Chen, Y.; Lu, X.; Liu, G.; Fu, Y.; Luo, Y. Endostatin Prevents Dietary-Induced Obesity by Inhibiting Adipogenesis and Angiogenesis. Diabetes 2015, 64, 2442–2456. [Google Scholar] [CrossRef]
- Blaise, S.; Romier, B.; Kawecki, C.; Ghirardi, M.; Rabenoelina, F.; Baud, S.; Duca, L.; Maurice, P.; Heinz, A.; Schmelzer, C.E.H.; et al. Elastin-derived peptides are new regulators of insulin resistance development in mice diabetes. Diabetes 2013, 62, 3807–3816. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gu, X.; Zhang, N.; Kolonin, M.G.; An, Z.; Sun, K. Divergent functions of endotrophin on different cell populations in adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E952–E963. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Park, J.; Kim, M.; Scherer, P.E. Endotrophin, a multifaceted player in metabolic dysregulation and cancer progression, is a predictive biomarker for the response to PPARγ agonist treatment. Diabetologia 2017, 60, 24–29. [Google Scholar] [CrossRef]
- Briknarová, K.; Åkerman, M.E.; Hoyt, D.W.; Ruoslahti, E.; Ely, K.R. Anastellin, an FN3 fragment with fibronectin polymerization activity, resembles amyloid fibril precursors. J. Mol. Biol. 2003, 332, 205–215. [Google Scholar] [CrossRef]
- You, R.; Klein, R.M.; McKeown-Longo, P.J. Regulation of p38 MAP kinase by anastellin is independent of anastellin’s effect on matrix fibronectin. Matrix Biol. 2009, 28, 101–109. [Google Scholar] [CrossRef][Green Version]
- Pickart, L.; Margolina, A. Regenerative and protective actions of the GHK-Cu peptide in the light of the new gene data. Int. J. Mol. Sci. 2018, 19, 1987. [Google Scholar] [CrossRef]
- Pickart, L. The human tri-peptide GHK and tissue remodeling. J. Biomater. Sci. Polym. Edn. 2008, 19, 969–988. [Google Scholar] [CrossRef]
- Baud, S.; Duca, L.; Bochicchio, B.; Brassart, B.; Belloy, N.; Pepe, A.; Dauchez, M.; Martiny, L.; Debelle, L. Elastin peptides in aging and pathological conditions. Biomol. Concepts 2013, 4, 65–76. [Google Scholar] [CrossRef]
- Rousselle, P.; Carulli, S.; Chajra, H.; Dayan, G.; Pin, D.; Herbage, B. The syndecan binding sequence KKLRIKSKEK in laminin α3 LG4 domain promotes epidermal repair. Eur. J. Dermatol. 2013. [Google Scholar] [CrossRef]
- Malinda, K.M.; Wysocki, A.B.; Koblinski, J.E.; Kleinman, H.K.; Ponce, M.L. Angiogenic laminin-derived peptides stimulate wound healing. Int. J. Biochem. Cell Biol. 2008, 40, 2771–2780. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, K.; Deng-Pichon, U.; Warnet, J.M.; Rat, P. Hyaluronan fragments improve wound healing on in vitro cutaneous model through P2 × 7 purinoreceptor basal activation: Role of molecular weight. PLoS ONE 2012, 7, e48351. [Google Scholar] [CrossRef]
- D’Agostino, A.; Stellavato, A.; Busico, T.; Papa, A.; Tirino, V.; Papaccio, G.; La Gatta, A.; De Rosa, M.; Schiraldi, C. In vitro analysis of the effects on wound healing of high- and low-molecular weight chains of hyaluronan and their hybrid H-HA/L-HA complexes. BMC Cell Biol. 2015, 16, 19–34. [Google Scholar]
- Gorouhi, F.; Maibach, H.I. Role of topical peptides in preventing or treating aged skin. Int. J. Cosmet. Sci. 2009, 31, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Abu Samah, N.H.; Heard, C.M. Topically applied KTTKS: A review. Int. J. Cosmet. Sci. 2011, 33, 483–490. [Google Scholar] [CrossRef]
- Jones, R.R.; Castelletto, V.; Connon, C.J.; Hamley, I.W. Collagen stimulating effect of peptide amphiphile C16–KTTKS on human fibroblasts. Mol. Pharm. 2013, 10, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte—Fibroblast interaction in wound healing. J. Investig. Dermatol. 2007, 172, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, A.V. The stratum corneum and aging. In Textbook of Aging Skin; Farage, M.A., Miller, K.W., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Suzuki, Y.; Nomura, J.; Koyama, J.; Horii, I. The role of proteases in stratum corneum: Involvement in stratum corneum desquamation. Arch. Dermatol. Res. 1994, 286, 249–253. [Google Scholar] [CrossRef]
- Harding, C.R.; Watkinson, A.; Rawlings, A.V.; Scott, I.R. Dry skin, moisturization and corneodesmolysis. Int. J. Cosmet. Sci. 2000, 22, 21–52. [Google Scholar] [CrossRef] [PubMed]
- Amano, S. Characterization and mechanisms of photoageing- related changes in skin. Damages of basement membrane and dermal structures. Exp. Dermatol. 2016, 25 (Suppl. 3), 14–19. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, A.V.; Voegeli, R. Stratum corneum proteases and dry skin conditions. Cell Tissue Res. 2013, 351, 217–235. [Google Scholar] [CrossRef] [PubMed]
- Voegeli, R.; Rawlings, A.V.; Doppler, S.; Heiland, J.; Schreier, T. Profiling of serine protease activities in human stratum corneum and detection of a stratum corneum tryptase-like enzyme. Int. J. Cosmet. Sci. 2007, 29, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Katsuta, Y.; Yoshida, Y.; Kawai, E.; Kohno, Y.; Kitamura, K. Urokinase-type plasminogen activator is activated in stratum corneum after barrier disruption. J. Dermatol. Sci. 2003, 32, 55–57. [Google Scholar] [CrossRef]
- Tagami, H. Location-related differences in structure and function of the stratum corneum with special emphasis on those of the facial skin. Int. J. Cosmet. Sci. 2008, 30, 435–442. [Google Scholar] [CrossRef]
- Voegeli, R.; Rawlings, A.V.; Doppler, S.; Schreier, T. Increased basal transepidermal water loss leads to elevation of some but not all stratum corneum serine proteases. Int. J. Cosmet. Sci. 2008, 30, 435–442. [Google Scholar] [CrossRef]
- Poojary, S.; Minni, K. Tranexamic Acid in Melasma: A Review. Pigment. Disord. 2015, 2, 228–232. [Google Scholar] [CrossRef]
- Taraz, M.; Niknam, S.; Ehsani, A.H. Tranexamic acid in treatment of melasma: A comprehensive review of clinical studies. Dermatol. Ther. 2017, 30, e12465. [Google Scholar] [CrossRef]
- Denda, M.; Kitamura, K.; Elias, P.M.; Feingold, K.R. Trans-4-(Aminomethyl)cyclohexane Carboxylic Acid (T-AMCHA), an Anti-Fibrinolytic Agent, Accelerates Barrier Recovery and Prevents the Epidermal Hyperplasia Induced by Epidermal Injury in Hairless Mice and Humans. J. Investig. Dermatol. 1997, 109, 84–90. [Google Scholar] [CrossRef]
- Vávrová, K.; Hrabálek, A.; Doležal, P.; Holas, T.; Klimentová, J. Biodegradable derivatives of tranexamic acid as transdermal permeation enhancers. J. Control. Release 2005, 104, 41–49. [Google Scholar] [CrossRef]
- Voegeli, R.; Wikstroem, P.; Campiche, R.; Steinmetzer, T.; Jackson, E.; Gempeler, M.; Imfeld, D.; Rawlings, A.V. The effects of benzylsulfonyl-D-Ser-homoPhe-(4-amidino-benzylamide), a dual plasmin and urokinase inhibitor, on facial skin barrier function in subjects with sensitive skin. Int. J. Cosmet. Sci. 2017, 39, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Aisina, R.B.; Mukhametova, L.I. Structure and function of plasminogen/plasmin system. Russ. J. Bioorg. Chem. 2014, 40, 590–605. [Google Scholar] [CrossRef]
- Markowska, A.; Bruzgo, I.; Midura-Nowaczek, K. Effects of tripeptides on the amidolytic activities of urokinase, thrombin, plasmin and trypsin. Int. J. Pept. Res. 2008, 14, 215–218. [Google Scholar] [CrossRef]
- GuanK, L.; Xiong, Y. Regulation of intermediary metabolism by protein acetylation. Trends Biochem. Sci. 2011, 36, 108–116. [Google Scholar] [CrossRef]
- Lim, S.H.; Sun, Y.; Madanagopal, T.T.; Rosa, V.; Kang, L. Enhanced Skin Permeation of anti-wrinkle peptides via molecular modification. Sci. Rep. 2018, 8, 1596. [Google Scholar] [CrossRef]
- Rochette, L.; Ghibu, S.; Muresan, A.; Vergely, C. Alpha-lipoic acid: Molecular mechanisms and therapeutic potential in diabetes. Can. J. Physiol. Pharm. 2015, 93, 1021–1027. [Google Scholar] [CrossRef]
- Ho, Y.S.; Lai, C.S.; Liu, H.I.; Hoc, S.Y.; Tai, C.; Pan, M.H.; Wang, Y.J. Dihydrolipoic acid inhibits skin tumor promotion through anti-inflammation and anti-oxidation. Biochem. Pharm. 2007, 73, 1786–1795. [Google Scholar] [CrossRef]
- Moini, H.; Packer, L.; Saris, N.E.L. Antioxidant and prooxidant activities of α-lipoic acid and dihydrolipoic acid. Toxicol. Appl. Pharm. 2002, 182, 84–90. [Google Scholar] [CrossRef]
- Chichong, L.; Kim, B.M.; Lee, D.; Lee, M.H.; Kim, J.H.; Pyo, H.B.; Chai, K.Y. Synthesis of lipoic acid–peptide conjugates and their effect on collagen and melanogenesis. Eur. J. Med. Chem. 2013, 69, 449–454. [Google Scholar]
- Zhang, L.; Bulaj, G. Converting peptides into drug leads by lipidation. Curr. Med. Chem. 2012, 19, 1602–1618. [Google Scholar] [CrossRef]
- Safety Assessment of Palmitoyl Oligopeptides as Used in Cosmetics, Cosmetic Ingredient Review. Available online: https://www.cir-safety.org/sites/default/files/palmit072012slr.pdf (accessed on 11 October 2019).
- Schagen, S.K. Topical Peptide Treatments with Effective Anti-Aging Results. Cosmetics 2017, 4, 16. [Google Scholar] [CrossRef]
- Albaugh, V.L.; Barbul, A. Arginine. Ref. Modul. Life Sci. 2017. [Google Scholar] [CrossRef]
- Singh, M.; Rao, D.M.; Pande, S.; Battu, S.; Mahalakshmi, K.; Dutt, K.R.; Ramesh, M. Medicinal uses of l-lysine: Past and future. Int. J. Res. Pharm. Sci. 2011, 2, 637–642. [Google Scholar]
- Markowska, A.; Bruzgo, I.; Miltyk, W.; Midura-Nowaczek, K. Tripeptides with C-terminal arginine as potential inhibitors of urokinase. Int. J. Pept. Res. Ther. 2011, 17, 47–52. [Google Scholar] [CrossRef]
- Markowska, A.; Bruzgo, M.; Surażyński, A.; Midura-Nowaczek, K. Tripeptides with non-code amino acids as potential serine proteases inhibitors. J. Enzym. Inhib. Med. Chem. 2013, 28, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.L.; Park, E.J.; Kim, E.; Na, D.H.; Shin, Y.H. Dermal stability and in vitro skin permeation of collagen pentapeptides (KTTKS and palmitoyl-KTTKS). Biomol. Ther. 2014, 22, 321–327. [Google Scholar] [CrossRef]
- Choi, H.I.; Kim, H.J.; Park, J.I.; Shin, E.H.; Kim, D.W.; Kim, S.S. Design and efficient synthesis of novel ascorbyl conjugated peptide with high collagen biosynthesis stimulating effects. Bioorg. Med. Chem. Lett. 2009, 19, 2079–2082. [Google Scholar] [CrossRef]
- Chan, W.C.; White, P.D. Fmoc Solid Phase Peptide Synthesis: A Practical Approach, 1st ed.; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Okada, Y.; Tsuda, Y.; Teno, N.; Wanaka, K.; Bohgaki, M.; Hijikata-Okunomiya, A.; Naito, T.; Okamoto, S. Synthesis of active center-directed peptide inhibitors of plasmin. Chem. Pharm. Bull. 1988, 36, 1289–1297. [Google Scholar] [CrossRef]
- Peterkofsky, B.; Diegelmann, R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry 1971, 10, 988–994. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

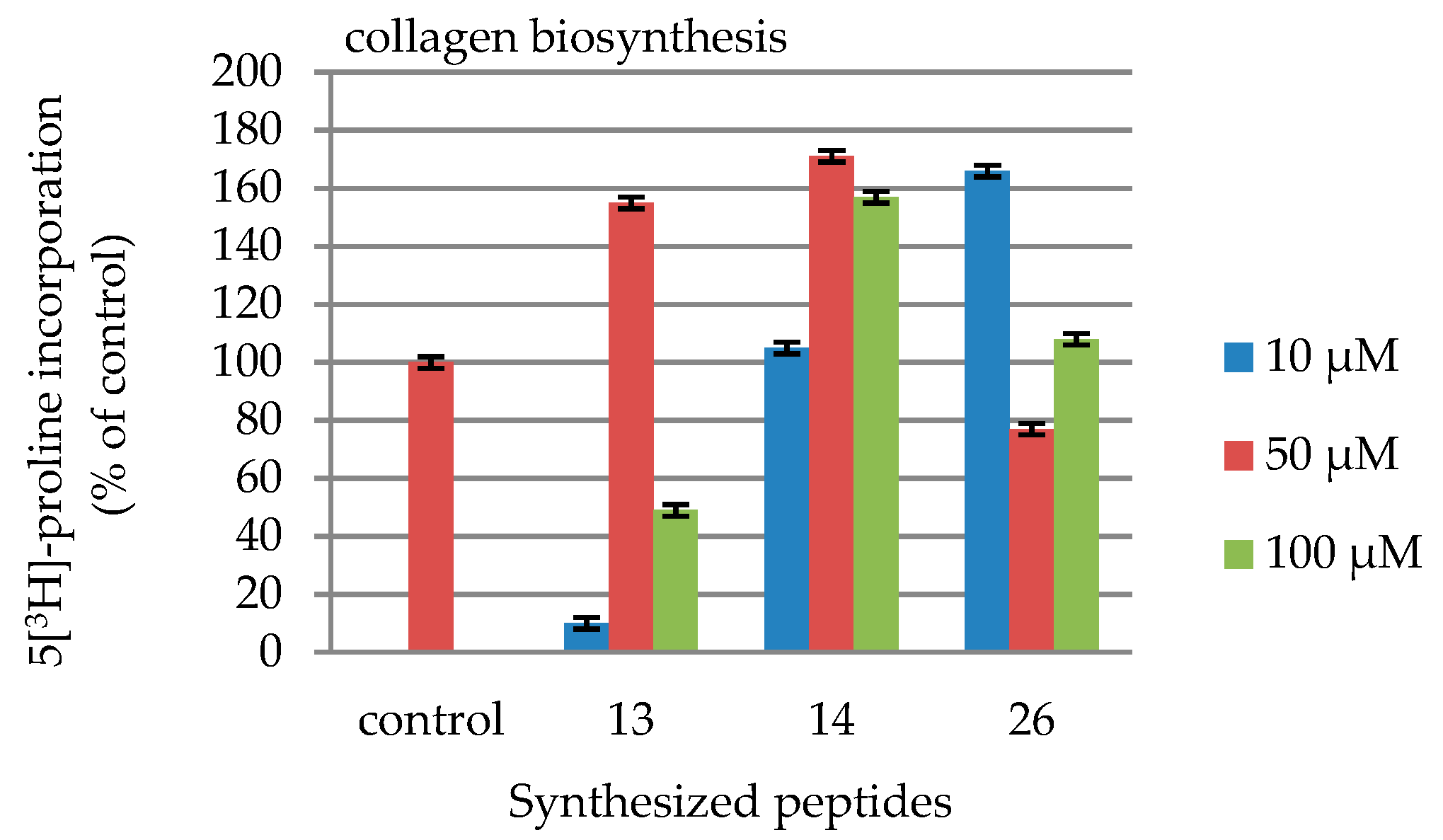
| No | Peptide | IC50 [mM] | ||
|---|---|---|---|---|
| Plasmin | Urokinase | Thrombin | ||
| 1 | KTTKSOH | 0.28 ± 0.014 | 0.019 ± 0.00094 | 7.81 ± 0.39 |
| 2 | AcKTTKSOH | 2.78 ± 0.14 | 3.88 ± 0.19 | 2.20 ± 0.11 |
| 3 | LipKTTKSOH | 0.47 ± 0.023 | 0.024 ± 0.0012 | 12.83 ± 0.64 |
| 4 | PalKTTKSOH | 0.00071 ± 0.000035 | 0.00086 ± 0.000043 | 0.0018 ± 0.00009 |
| 5 | KTTKSNH2 | 0.53 ± 0.026 | 9.15 ± 0.46 | 7.04 ± 0.35 |
| 6 | AcKTTKSNH2 | 2.92 ± 0.15 | 61.76 ± 3.09 | 3.25 ± 0.16 |
| 7 | LipKTTKSNH2 | 0.58 ± 0.029 | 15.07 ± 0.75 | 11.70 ± 0.58 |
| 8 | PalKTTKSNH2 | 0.00021 ± 0.000010 | 3.12 ± 0.16 | 0.046 ± 0.0023 |
| 9 | KTTRSOH | 0.63 ± 0.031 | 0.011 ± 0.00054 | 4.01 ± 0.20 |
| 10 | AcKTTRSOH | 2.24 ± 0.11 | 4.82 ± 0.041 | 2.89 ± 0.14 |
| 11 | LipKTTRSOH | 0.24 ± 0.012 | 0.019 ± 0.00093 | 10.94 ± 0.54 |
| 12 | PalKTTRSOH | 0.00078 ± 0.000039 | 0.00080 ± 0.00004 | 0.012 ± 0.00058 |
| 13 | KTTRSNH2 | 0.72 ± 0.036 | 17.62 ± 0.88 | 4.65 ± 0.23265 |
| 14 | AcKTTRSNH2 | 5.34 ± 0.27 | 5.56 ± 0.28 | 2.95 ± 0.15 |
| 15 | LipKTTRSNH2 | 0.69 ± 0.035 | 3.47 ± 0.17 | 4.11 ± 0.21 |
| 16 | PalKTTRSNH2 | 0.00086 ± 0.000043 | 2.37 ± 0.12 | 0.0018 ± 0.00009 |
| 17 | RTTRSOH | 0.28 ± 0.014 | 0.015 ± 0.00076 | 3.89 ± 0.19 |
| 18 | AcRTTRSOH | 8.81 ± 0.44 | 3.24 ± 0.16 | 3.12 ± 0.16 |
| 19 | LipRTTRSOH | 1.014 ± 0.051 | 0.022 ± 0.0011 | 1.84 ± 0.092 |
| 20 | PalRTTRSOH | 0.00055 ± 0.000027 | 0.00049 ± 0.000024 | 0.03004 ± 0.0015 |
| 21 | RTTRSNH2 | 0.44 ± 0.022 | 11.97 ± 0.59 | 2.93 ± 0.14 |
| 22 | AcRTTRSNH2 | 9.08 ± 0.45 | 24.96 ± 1.25 | 1.47 ± 0.074 |
| 23 | LipRTTRSNH2 | 1.89 ± 0.094 | 4.67 ± 0.23 | 2.36 ± 0.12 |
| 24 | PalRTTRSNH2 | 0.00042 ± 0.000021 | 0.26 ± 0.013 | 0.041 ± 0.0020 |
| 25 | RTTKSOH | 0.54 ± 0.027 | 0.0106 ± 0.00053 | 2.51 ± 0.13 |
| 26 | AcRTTKSOH | 5.45 ± 0.27 | 2.36 ± 0.12 | 0.81 ± 0.041 |
| 27 | LipRTTKSOH | 2.12 ± 0.11 | 0.097 ± 0.0049 | 4.23 ± 0.12 |
| 28 | PalRTTKSOH | 0.00067 ± 0.000033 | 0.00048 ± 0.000024 | 0.0021 ± 0.00011 |
| 29 | RTTKSNH2 | 0.30 ± 0.015 | 7.95 ± 0.40 | 2.43 ± 0.12 |
| 30 | AcRTTKSNH2 | 5.00 ± 0.25 | 12.07 ± 0.60 | 1.59 ± 0.080 |
| 31 | LipRTTKSNH2 | 3.23 ± 0.16 | 4.57 ± 0.23 | 5.59 ± 0.28 |
| 32 | PalRTTKSNH2 | 0.00045 ± 0.000023 | 0.49 ± 0.024 | 0.0306 ± 0.0015 |
| No | Compound | -logIC50 | ||
|---|---|---|---|---|
| Plasmin | Urokinase | Thrombin | ||
| 1 | KTTKSOH | 3.55 ± 0.18 | 4.72 ± 0.24 | 2.11 ± 0.11 |
| 2 | AcKTTKSOH | 2.56 ± 0.13 | 2.41 ± 0.12 | 2.66 ± 0.13 |
| 3 | LipKTTKSOH | 3.33 ± 0.17 | 4.63 ± 0.23 | 1.89 ± 0.09 |
| 4 | PalKTTKSOH | 6.15 ± 0.31 | 6.06 ± 0.30 | 5.75 ± 0.29 |
| 5 | KTTKSNH2 | 3.28 ± 0.16 | 2.04 ± 0.10 | 2.15 ± 0.11 |
| 6 | AcKTTKSNH2 | 2.53 ± 0.13 | 1.21 ± 0.06 | 2.49 ± 0.12 |
| 7 | LipKTTKSNH2 | 3.24 ± 0.16 | 1.82 ± 0.09 | 1.93 ± 0.10 |
| 8 | PalKTTKSNH2 | 6.67 ± 0.33 | 2.51 ± 0.13 | 4.34 ± 0.22 |
| 9 | KTTRSOH | 3.20 ± 0.16 | 4.96 ± 0.25 | 2.40 ± 0.12 |
| 10 | AcKTTRSOH | 2.65 ± 0.13 | 2.32 ± 0.12 | 2.54 ± 0.13 |
| 11 | LipKTTRSOH | 3.61 ± 0.18 | 4.73 ± 0.24 | 1.96 ± 0.10 |
| 12 | PalKTTRSOH | 6.11 ± 0.31 | 6.10 ± 0.30 | 4.93 ± 0.25 |
| 13 | KTTRSNH2 | 3.15 ± 0.16 | 1.75 ± 0.09 | 2.33 ± 0.12 |
| 14 | AcKTTRSNH2 | 2.27 ± 0.11 | 2.25 ± 0.11 | 2.53 ± 0.13 |
| 15 | LipKTTRSNH2 | 3.16 ± 0.16 | 2.46 ± 0.12 | 2.39 ± 0.12 |
| 16 | PalKTTRSNH2 | 6.07 ± 0.30 | 2.63 ± 0.13 | 5.75 ± 0.29 |
| 17 | RTTRSOH | 3.54 ± 0.18 | 4.82 ± 0.24 | 2.41 ± 0.12 |
| 18 | AcRTTRSOH | 2.05 ± 0.10 | 2.49 ± 0.12 | 2.51 ± 0.13 |
| 19 | LipRTTRSOH | 2.99 ± 0.15 | 4.66 ± 0.23 | 2.74 ± 0.14 |
| 20 | PalRTTRSOH | 6.26 ± 0.31 | 6.31 ± 0.32 | 4.52 ± 0.23 |
| 21 | RTTRSNH2 | 3.36 ± 0.17 | 1.92 ± 0.10 | 2.53 ± 0.13 |
| 22 | AcRTTRSNH2 | 2.04 ± 0.10 | 1.60 ± 0.08 | 2.83 ± 0.14 |
| 23 | LipRTTRSNH2 | 2.72 ± 0.14 | 2.33 ± 0.12 | 2.63 ± 0.13 |
| 24 | PalRTTRSNH2 | 6.38 ± 0.32 | 3.59 ± 0.18 | 4.39 ± 0.22 |
| 25 | RTTKSOH | 3.27 ± 0.16 | 4.97 ± 0.25 | 2.60 ± 0.13 |
| 26 | AcRTTKSOH | 2.26 ± 0.11 | 2.63 ± 0.13 | 3.09 ± 0.15 |
| 27 | LipRTTKSOH | 2.67 ± 0.13 | 4.01 ± 0.20 | 2.37 ± 0.12 |
| 28 | PalRTTKSOH | 6.18 ± 0.31 | 6.32 ± 0.32 | 5.67 ± 0.28 |
| 29 | RTTKSNH2 | 3.51 ± 0.18 | 2.10 ± 0.10 | 2.61 ± 0.13 |
| 30 | AcRTTKSNH2 | 2.30 ± 0.12 | 1.92 ± 0.10 | 2.80 ± 0.14 |
| 31 | LipRTTKSNH2 | 2.49 ± 0.12 | 2.34 ± 0.12 | 2.25 ± 0.11 |
| 32 | PalRTTKSNH2 | 6.34 ± 0.32 | 3.31 ± 0.17 | 4.51 ± 0.23 |
| Synthesized Peptides | ||||||||||||||||
| Concentration [µmol/L] | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| 1 | 95 | 98 | 94 | 97 | 91 | 97 | 97 | 96 | 93 | 104 | 102 | 103 | 121 | 112 | 112 | 104 |
| 10 | 82 | 88 | 81 | 91 | 91 | 88 | 89 | 83 | 103 | 100 | 105 | 98 | 139 | 135 | 122 | 114 |
| 100 | 80 | 82 | 81 | 85 | 100 | 86 | 87 | 83 | 101 | 94 | 98 | 99 | 145 | 150 | 115 | 115 |
| Synthesized Peptides | ||||||||||||||||
| Concentration [µmol/L] | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 |
| 1 | 89 | 88 | 79 | 90 | 84 | 102 | 89 | 94 | 97 | 112 | 105 | 104 | 101 | 95 | 107 | 104 |
| 10 | 99 | 103 | 81 | 90 | 94 | 99 | 79 | 104 | 87 | 137 | 91 | 103 | 111 | 105 | 112 | 113 |
| 100 | 115 | 107 | 90 | 103 | 103 | 101 | 88 | 97 | 109 | 150 | 80 | 106 | 110 | 101 | 117 | 118 |
| Peptides | |||
|---|---|---|---|
| Concentration [µmol/L] | 13 | 14 | 26 |
| 10 | 84 | 114 | 126 |
| 50 | 86 | 96 | 112 |
| 100 | 74 | 127 | 128 |
| Peptides | |||
|---|---|---|---|
| Concentration [µmol/L] | 13 | 14 | 26 |
| 10 | 10 | 105 | 166 |
| 50 | 155 | 171 | 77 |
| 100 | 49 | 157 | 108 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tałałaj, U.; Uścinowicz, P.; Bruzgo, I.; Surażyński, A.; Zaręba, I.; Markowska, A. The Effects of a Novel Series of KTTKS Analogues on Cytotoxicity and Proteolytic Activity. Molecules 2019, 24, 3698. https://doi.org/10.3390/molecules24203698
Tałałaj U, Uścinowicz P, Bruzgo I, Surażyński A, Zaręba I, Markowska A. The Effects of a Novel Series of KTTKS Analogues on Cytotoxicity and Proteolytic Activity. Molecules. 2019; 24(20):3698. https://doi.org/10.3390/molecules24203698
Chicago/Turabian StyleTałałaj, Urszula, Paulina Uścinowicz, Irena Bruzgo, Arkadiusz Surażyński, Ilona Zaręba, and Agnieszka Markowska. 2019. "The Effects of a Novel Series of KTTKS Analogues on Cytotoxicity and Proteolytic Activity" Molecules 24, no. 20: 3698. https://doi.org/10.3390/molecules24203698
APA StyleTałałaj, U., Uścinowicz, P., Bruzgo, I., Surażyński, A., Zaręba, I., & Markowska, A. (2019). The Effects of a Novel Series of KTTKS Analogues on Cytotoxicity and Proteolytic Activity. Molecules, 24(20), 3698. https://doi.org/10.3390/molecules24203698





