Herb-Drug Pharmacokinetic Interactions: Transport and Metabolism of Indinavir in the Presence of Selected Herbal Products
Abstract
:1. Introduction
2. Results and Discussion
2.1. In Vitro Transport Studies
2.1.1. Bi-Directional Transport of Indinavir in the Presence of Linctagon Forte®

| Experimental Group | ER Value | |
|---|---|---|
| Average (n = 3) | * SD | |
| Indinavir alone (negative control) | 1.70 | 0.133 |
| Indinavir with verapamil (positive control) | 1.12 | <1 × 10−3 |
| Indinavir with Linctagon Forte® low concentration | 4.63 | 0.334 |
| Indinavir with Linctagon Forte® medium concentration | 5.70 | 0.379 |
| Indinavir with Linctagon Forte® high concentration | 3.46 | 0.982 |
| Indinavir with Viral Choice® low concentration | 3.95 | 0.391 |
| Indinavir with Viral Choice® medium concentration | 3.41 | 0.286 |
| Indinavir with Viral Choice® high concentration | 2.58 | 0.328 |
| Indinavir with Canova® low concentration | 2.11 | 0.023 |
| Indinavir with Canova® medium concentration | 1.83 | 0.180 |
| Indinavir with Canova® high concentration | 1.67 | 0.120 |
2.1.2. Bi-Directional Transport of Indinavir in the Presence of Viral Choice®
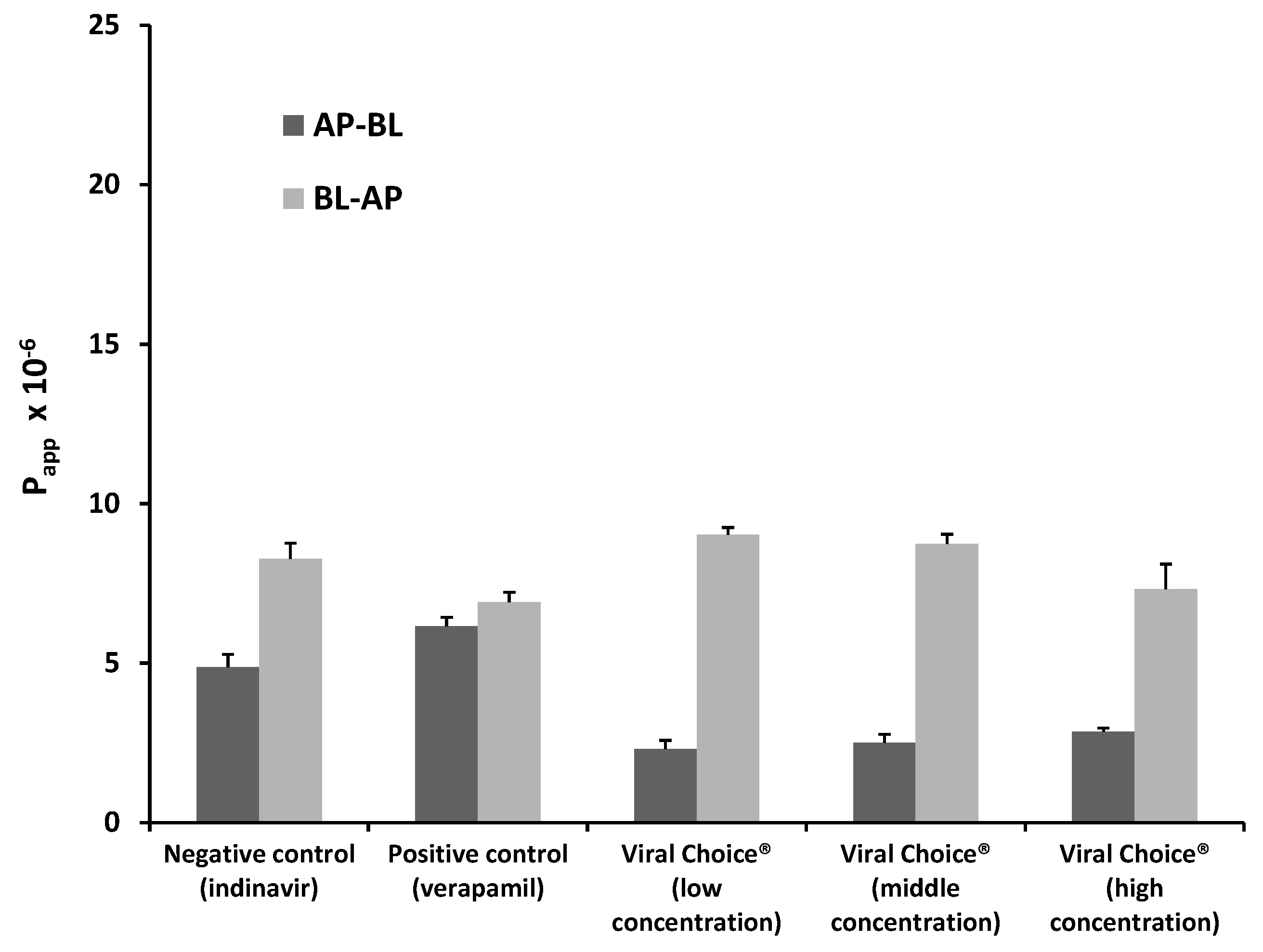
2.1.3. Bi-Directional Transport of Indinavir in the Presence of Canova®
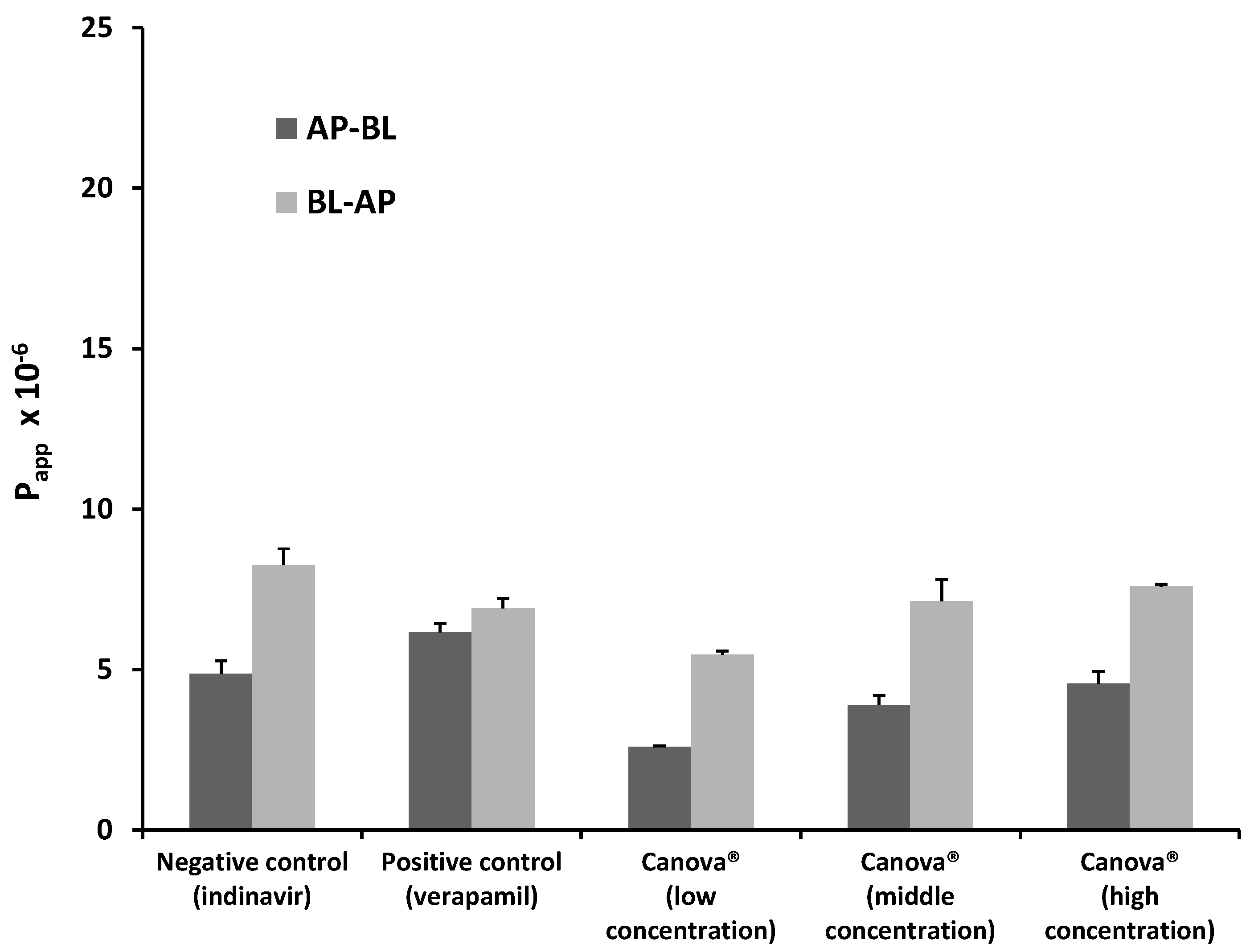
2.2. In Vitro Metabolism Studies
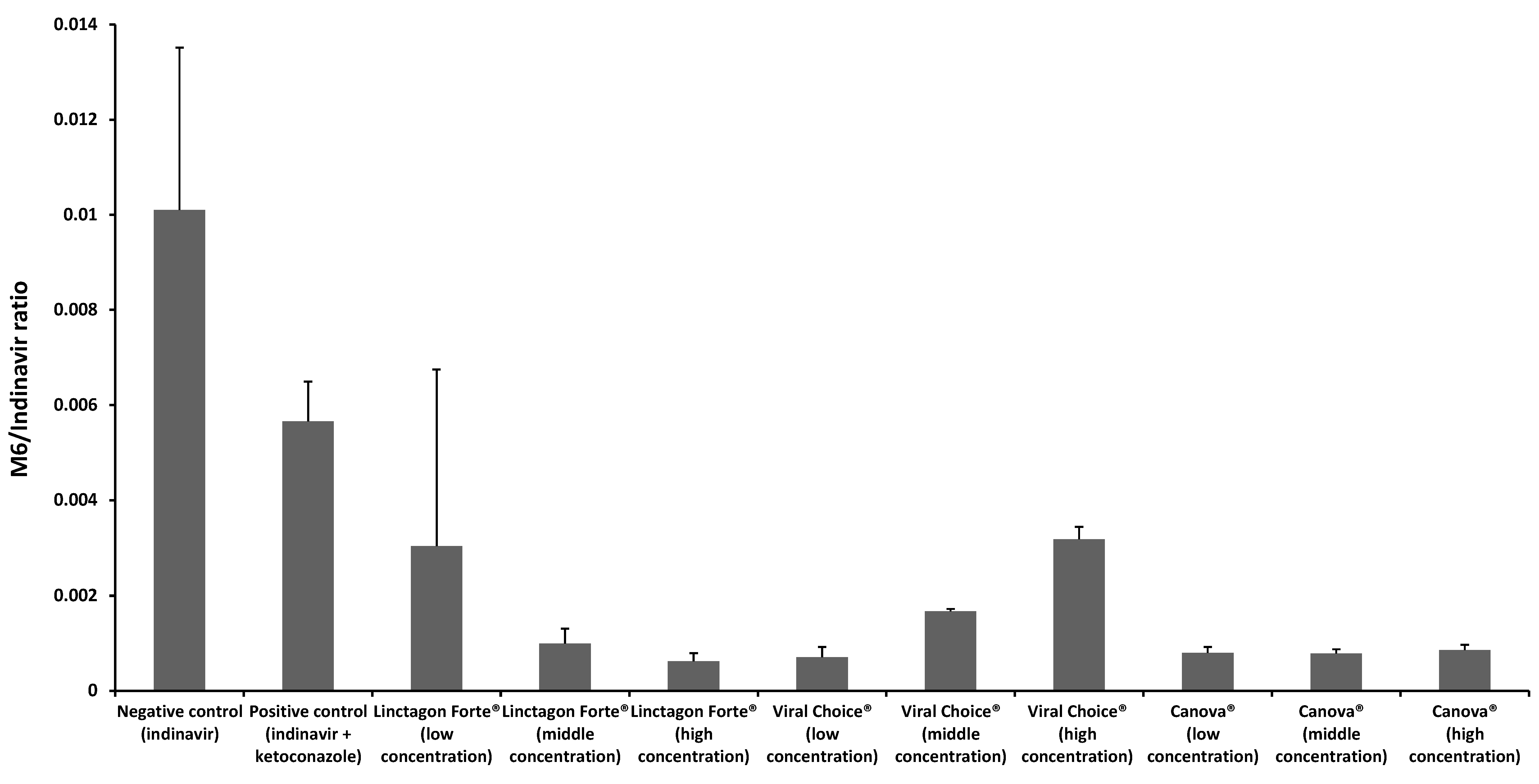
2.2.1. Metabolism of Indinavir in the Presence of Linctagon Forte®
2.2.2. Metabolism of Indinavir in the Presence of Viral Choice®
2.2.3. Metabolism of Indinavir in the Presence of Canova®
3. Experimental Section
3.1. Chemicals and Reagents
| Active Constituent | Quantity per Tablet |
|---|---|
| Pelargonium Sidoides | 250 mg |
| Quercetin | 60 mg |
| Bromelain | 40 mg |
| Active Constituent | Quantity per Capsule |
|---|---|
| Echinacea Extract | 80 mg |
| Phytosterols (Plant sterols & sterolin) | 25 mg |
| l-Arginine | 10 mg |
| l-Methionine | 33.3 mg |
| Absorption enzymes | 5 mg |
| Garlic | 50 mg |
| Vitamin A | 333 RE |
| Vitamin B6 | 3 mg |
| Vitamin B12 | 4 µg |
| Folic acid | 250 µg |
| Vitamin C | 150 mg |
| Vitamin D | 4 µg |
| Vitamin E | 10 mg |
| Biotin | 100 µg |
| Copper | 0.33 mg |
| Iron | 2 mg |
| Selenium | 5 µg |
| Zinc | 3 mg |
| Active Constituent | Quantity per mL |
|---|---|
| Aconitum napellus | DH 20 (0.06 mL) |
| Apis mellifica | DH 19 (0.06 mL) |
| Arsenicum album | DH 17 (0.06 mL) |
| Asafoetida | DH 20 (0.06 mL) |
| Barita carbônica | DH 20 (0.06 mL) |
| Bryonia alba | DH 14 (0.06 mL) |
| Calcarea carbônica | DH 20 (0.06 mL) |
| Conium maculatum | DH 16 (0.06 mL) |
| Ipecacaunha | DH 13 (0.06 mL) |
| Lachesis muta | DH 18 (0.06 mL) |
| Lycopodium clavatum | DH 20 (0.06 mL) |
| Pulsatilla nigricans | DH 13 (0.06 mL) |
| Rhus toxicodendrum | DH 17 (0.06 mL) |
| Ricinus communis | DH 14 (0.06 mL) |
| Silicea | DH 18 (0.06 mL) |
| Thuya occidentalis | DH 16 (0.06 mL) |
| Veratrum album | DH 20 (0.06 mL) |
3.2. In Vitro Transport Studies
3.2.1. Caco-2 Cell Culturing and Seeding on Transwell® 6-Well Membrane Plates
3.2.2. Herbal Product Solution Preparations
3.2.3. In Vitro Bi-Directional Transport Studies
3.3. In Vitro Metabolism Studies
3.3.1. LS180 Cell Culturing and Seeding out in 6-Well Plates for Metabolism Studies
3.3.2. Seeding of LS180 Cells onto 6-Well Plates
3.3.3. Metabolism Inhibition Studies
3.4. Chromatographic Analysis
3.4.1. High Performance Liquid Chromatography Method
3.4.2. Liquid Chromatography Linked to Mass Spectroscopy (LC-MS/MS)
| Electro Spray Ionisation Settings | Value |
|---|---|
| Nebulizer gas (Gas 1) (arbitrary unit) | 30 |
| Turbo gas (Gas 2) (arbitrary unit) | 40 |
| CUR (curtain gas) (arbitrary unit) | 15 |
| CAD (collision gas) (arbitrary unit) | 3 |
| TEM (source temperature) (°C) | 500 |
| IS (Ion Spray Voltage) (V) | 3500 |
| MS/MS Settings | Indinavir | M6 |
|---|---|---|
| Protonated molecular mass (m/z) | 614 | 523 |
| Product ion molecular mass (m/z) | 421 | 273 |
| Dwell time (ms) | 150 | 150 |
| DP (declustering potential) (V) | 45 | 40 |
| EP (entrance potential) (V) | 12 | 9 |
| CEP(collision cell entrance potential) (V) | 35 | 40 |
| CE (collision energy) (eV) | 50 | 45 |
| CXP (collision cell exit potential) (V) | 11 | 11 |
| Scan Type | MRM | MRM |
| Polarity | Positive | Positive |
| Pause time | 5ms | 5ms |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cohen, K.; Andrews, S.; Maartens, G. Drug interactions with antiretroviral therapy: Drug interactions. S. Afr. J. HIV Med. 2002, 3, 42–47. [Google Scholar]
- Littlewood, R.A.; Vanable, P.A. Complementary and alternative medicine use among HIV+ people: Research synthesis and implications for HIV care. AIDS Care 2008, 20, 1002–1018. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Mitra, A.K. MDR- and CYP3A4-mediated drug-herbal interactions. Life Sci. 2006, 78, 2131–2145. [Google Scholar] [CrossRef] [PubMed]
- Tarirai, C.; Viljoen, A.M.; Hamman, J.H. Herb-drug pharmacokinetic interactions reviewed. Expert Opin. Drug Metab. Toxicol. 2010, 6, 1515–1538. [Google Scholar] [CrossRef] [PubMed]
- Sharom, F.J. ABC multidrug transporters: Structure, function and role in chemoresistance. Pharmacogenomics 2008, 9, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Hellum, B.H.; Nilsen, O.D. In vitro inhibition of CYP3A4 metabolism and P-glycoprotein-mediated transport by trade herbal products. Basic Clin. Pharmacol. Toxicol. 2008, 102, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Liska, D.J. The detoxification enzyme system. Altern. Med. Rev. 1998, 3, 187–198. [Google Scholar] [PubMed]
- Brendler, T.; van Wyk, B.E. A historical, scientific and commercial perspective on the medicinal use of Pelargonium sidoides (Geraniaceae). J. Ethnopharmacol. 2008, 119, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, P.; Schneider, S.; Stintzing, F.C.; Carle, R.; Reichling, J. Efficacy of an aqueous Pelargonium sidoides extract against herpesvirus. Phytomedicine 2008, 15, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Tahan, F.; Yaman, M. Can the Pelargonium sidoides root extract EPs® 7630 prevent asthma attacks during viral infections of the upper respiratory tract in children? Phytomedicine 2012, 20, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Barrett, B. Medicinal properties of Echinacea: A critical review. Phytomedcine 2003, 10, 66–86. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.S.; Nilsen, O.D. Echinacea purpurea and P-Glycoprotein drug transport in Caco-2 cells. Phytother. Res. 2009, 23, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Gorski, J.C.; Huang, S.; Pinto, A.; Hamman, M.A.; Hilligoss, J.K.; Narjis, A.Z.; Desai, M.; Miller, M.; Hall, S.D. The effect of Echinacea (Echinacea purpurea root) on cytochrome P450 activity in vivo. Clin. Pharmacol. Ther. 2004, 75, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Tattelman, E. Health effects of garlic. Am. Fam. Physician 2005, 72, 103–106. [Google Scholar] [PubMed]
- Banerjee, S.K.; Mukherjee, P.K.; Maulik, S.K. Garlic as an antioxidant: The good, the bad, and the ugly. Phytother. Res. 2002, 17, 97–106. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.C.; Cabrini, D.A.; Santos, E.P.; Marques, M.C.A.; Buchi, D.F. Canova medication and medicinal plants in south of Brazil. In Trends and Developments in Ethnopharmacolgy; Pretorius, R., Ed.; Research Signpost: Kerala, India, 2008; pp. 1–17. [Google Scholar]
- Takahachi, G.; Maluf, M.L.F.; Svidzinski, T.I.E.; Dalalio, M.M.O.; Bersani-Amado, C.A.; Cuman, R.K.N. In vivo and in vitro effects of the Canova medicine on experimental infection with Paracoccidioides brasiliensis in mice. Indian J. Pharmacol. 2006, 38, 350–354. [Google Scholar]
- Staines, S.S. Herbal medicines: Adverse effects and drug-herb interactions. J. Malta Coll. Pharm. Pract. 2011, 17, 38–41. [Google Scholar]
- Choi, J.; Piao, Y.; Kang, W. Effects of quercetin on the bioavailability of doxorubicin in rats: Role of CYP3A4 and P-gp inhibition by quercetin. Arch. Pharmacol. Toxicol. 2011, 34, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Borska, S.; Chmielewska, M.; Wysocka, T.; Drag-Zalesinka, M.; Zabel, M.; Dziegiel, P. In vitro effect of quercetin on human gastric carcinoma: Targeting cancer cells death and MDR. Food Chem. Toxicol. 2012, 50, 3375–3383. [Google Scholar] [CrossRef] [PubMed]
- Bock, U.; Kolac, C.; Borchard, G.; Koch, K.; Fuchs, R.; Streichhan, P.; Lehr, C. Transport of proteolytic enzymes across caco-2 cell monolayers. Pharm. Res. 1998, 15, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Pavan, R.; Jain, S.; Kumar, A. Properties and therapeutical application of bromelain: A review. Biotechnol. Res. Int. 2012, 2012, 976203. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, C.; Abrams, T.R.; Conquer, J.; Costa, D.; Serrano, J.M.G.; Iovin, R.; Isaac, R.; Nguyen, Y.; Rusie, E.; Tran, D.; et al. An evidence-based systematic review of Umckaloabo (Pelargonium sidoides) by the natural standard research collaboration. J. Diet. Suppl. 2010, 7, 283–302. [Google Scholar] [CrossRef] [PubMed]
- Budzinski, J.W.; Foster, B.C.; Vandenhoek, S.; Arnason, J.T. An in vitro evaluation of human cytochrome P450 3A4 inhibition by selected commercial herbal extracts and tinctures. Phytomedicine 2000, 7, 273–282. [Google Scholar] [CrossRef]
- Colalto, C. Herbal interactions on absorption of drugs: Mechanisms of action and clinical risk assessment. Pharm. Res. 2010, 62, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Ye, L.; Lv, C.; Zheng, Z.; Gong, Y.; Liu, Z. Involvement of CYP3A4/5 and CYP2D6 in the metabolism of aconite using human liver microsomes and recombinant CYP450 enzymes. Toxicol. Lett. 2011, 202, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Tam, T.W.; Liu, R.; Arnason, J.T.; Krantis, A.; Staines, W.A.; Haddad, P.S.; Foster, B.C. Cree antidiabetic plant extracts display mechanism-based inactivation of CYP3A4. Can. J. Physiol. Pharmacol. 2011, 89, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, P.; Bisht, S. Ferula asafoetida: Traditional use and pharmacological activity. Pharmacogn. Rev. 2012, 6, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Al-Jenoobi, F.I.; Al-Thukair, A.A.; Alam, M.A.; Abbas, F.A.; Al-Mohizea, A.M.; Alkharfy, K.H.; Al-Suwayeh, S.A. Modulation of CYP2D6 and CYP3A4 metabolic activities by Ferula asafoetida resin. Saudi Pharm. J. 2014, 22, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Calitz, C.; Steenekamp, J.H.; Steyn, J.D.; Gouws, C.; Viljoen, J.M.; Hamman, J.H. Impact of traditional African medicine on drug metabolism and transport. Expert Opin. Drug Metab. Toxicol. 2014, 7, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Kushida, H.; Sadakane, C.; Ishihara, K.; Wakui, Y.; Yanagisawa, T.; Kimura, M.; Kamei, H.; Yoshida, T. Metabolism of ipecac alkaloids cephaeline and emetine by human hepatic microsomal cytochrome P450s, and their inhibitory effects on P450 enzyme activities. Biol. Pharm. Bull. 2001, 24, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Pelkonen, O.; Abass, K.; Wiesner, J. Thujone and thujone-containing herbal medicinal and botanical products: Toxicological assessment. Regul. Toxicol. Pharmacol. 2013, 65, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Hellum, B.H.; Hu, Z.; Nilsen, O.G. The induction of CYP1A2, CYP2D6 and CYP3A4 by six trade herbal products in cultured primary human hepatocytes. Basic Clin. Pharmacol. Toxicol. 2007, 100, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Brandon, E.F.A.; Bosch, T.M.; Deenen, M.J.; Levink, R.; van der Wal, E.; van Meerveld, J.B.M.; Bijl, M.; Beijnen, J.H.; Schellens, J.H.M.; Meijermen, I. Validation of in vitro models used in drug metabolism and transport studies; genotyping of cytochrome P450, phase II enzymes and drug transport polymorphisms in human hepatoma (HepG2), ovarian carcinoma, (IGROV-1) and colon carcinoma (caco-2, LS180) cell lines. Toxicol. Appl. Pharmacol. 2006, 211, 1–10. [Google Scholar] [PubMed]
- Gupta, A.; Mugundu, G.M.; Desai, P.B.; Thummel, K.E.; Unadkat, J.D. Intestinal human colon adenocarcinoma cell line LS180 is an excellent model to study pregnane X receptor, but not constitutive andostane receptor, mediated CYP3A4 and multidrug resistance transporter 1 induction: Studies with anti-human immunodeficiency virus protease inhibitors. Drug Metab. Dispos. 2008, 36, 1172–1180. [Google Scholar] [PubMed]
- Van der Kerkhof, E.G.; de Graaf, I.A.M.; Groothuis, G.M.M. In vitro methods to study intestinal drug metabolism. Curr. Drug Metab. 2007, 8, 658–675. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calitz, C.; Gouws, C.; Viljoen, J.; Steenekamp, J.; Wiesner, L.; Abay, E.; Hamman, J. Herb-Drug Pharmacokinetic Interactions: Transport and Metabolism of Indinavir in the Presence of Selected Herbal Products. Molecules 2015, 20, 22113-22127. https://doi.org/10.3390/molecules201219838
Calitz C, Gouws C, Viljoen J, Steenekamp J, Wiesner L, Abay E, Hamman J. Herb-Drug Pharmacokinetic Interactions: Transport and Metabolism of Indinavir in the Presence of Selected Herbal Products. Molecules. 2015; 20(12):22113-22127. https://doi.org/10.3390/molecules201219838
Chicago/Turabian StyleCalitz, Carlemi, Chrisna Gouws, Joe Viljoen, Jan Steenekamp, Lubbe Wiesner, Efrem Abay, and Josias Hamman. 2015. "Herb-Drug Pharmacokinetic Interactions: Transport and Metabolism of Indinavir in the Presence of Selected Herbal Products" Molecules 20, no. 12: 22113-22127. https://doi.org/10.3390/molecules201219838
APA StyleCalitz, C., Gouws, C., Viljoen, J., Steenekamp, J., Wiesner, L., Abay, E., & Hamman, J. (2015). Herb-Drug Pharmacokinetic Interactions: Transport and Metabolism of Indinavir in the Presence of Selected Herbal Products. Molecules, 20(12), 22113-22127. https://doi.org/10.3390/molecules201219838









