Molecular Interactions of β-(1→3)-Glucans with Their Receptors
Abstract
:1. Introduction
2. Conformation of β-(1→3)-Glucans
3. Interactions of β-(1→3)-Glucans with Human Main Receptors
3.1. Dectin-1
3.2. Langerin
3.3. CR3
3.4. Glycolipids, Scavenger Receptors and other Receptors
3.5. Other Lectins
4. Elicitation of Immune Responses in Invertebrates
4.1. Insects Receptors
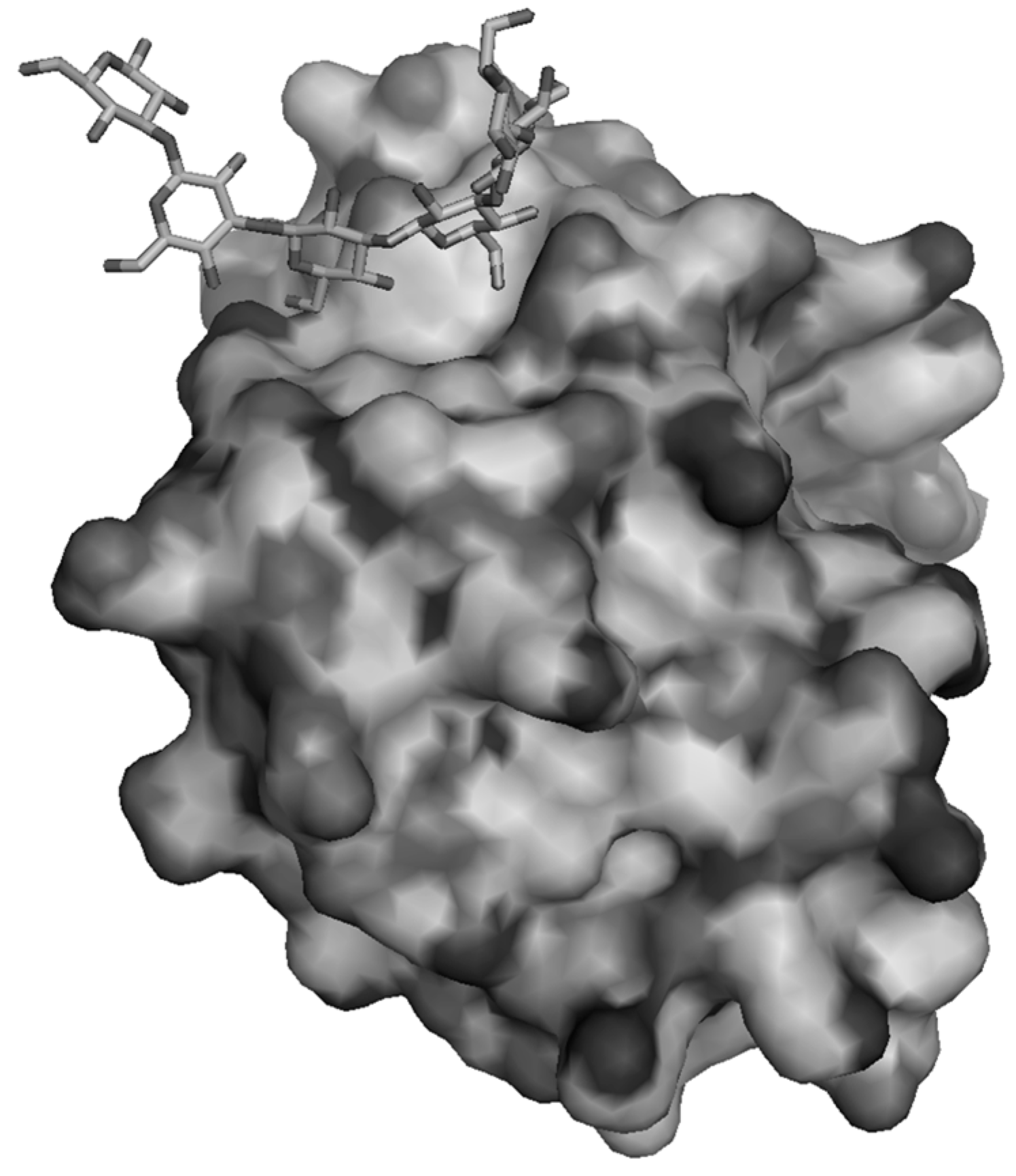
4.2. Crustacean Receptors
5. Recruitment of Glucanases
| CBM Family | Catalytic Activity of the Associated Glucanase | Organisms |
|---|---|---|
| CBM 4 | Endo- β-(1→3)-glucanases | Paenibacillus sp. |
| (E. C. 3.2.1.39/3.2.1.6) | ||
| Lichenase A/Laminarinase | Ruminiclostridium thermocellum a | |
| (E.C. 3.2.1.6/3.2.1.73) | Thermotoga maritime a | |
| Endo- β-(1→3)-glucanase | Thermotoga neapolitana | |
| (E. C. 3.2.1.39) | Thermotoga petrophila | |
| CBM 6 | Bacillus halodurans a | |
| Endo- β-(1→3)-glucanase | Lysobacter enzymogenes | |
| (E. C. 3.2.1.39) | Streptomyces sioyaensis | |
| Zobellia galactanivorans a | ||
| CBM 43 | Aspergillus fumigatus | |
| β-(1→3)-Glucanosyltransglycosylase | Candida albicans | |
| (E.C. 2.4.1.-) | Pichia pastoris | |
| Paracoccidioides brasiliensis | ||
| Saccharomyces cerevisiae a | ||
| Schizosaccharomyces pombe | ||
| Olea europaea a | ||
| Endo-β-(1→3)-glucanase | Pisum sativum | |
| (E.C. 3.2.1.39) | Salix gilgiana | |
| Triticum aestivum | ||
| CBM 52 | Endo-β-(1→3)-glucanase (E.C. 3.2.1.39) | Schizosaccharomyces pombe |
| CBM 56 | Endo-β-(1→3)-glucanase (E.C. 3.2.1.39) | Bacillus circulans |
| Bacillus halodurans | ||
| Paenibacillus sp. |
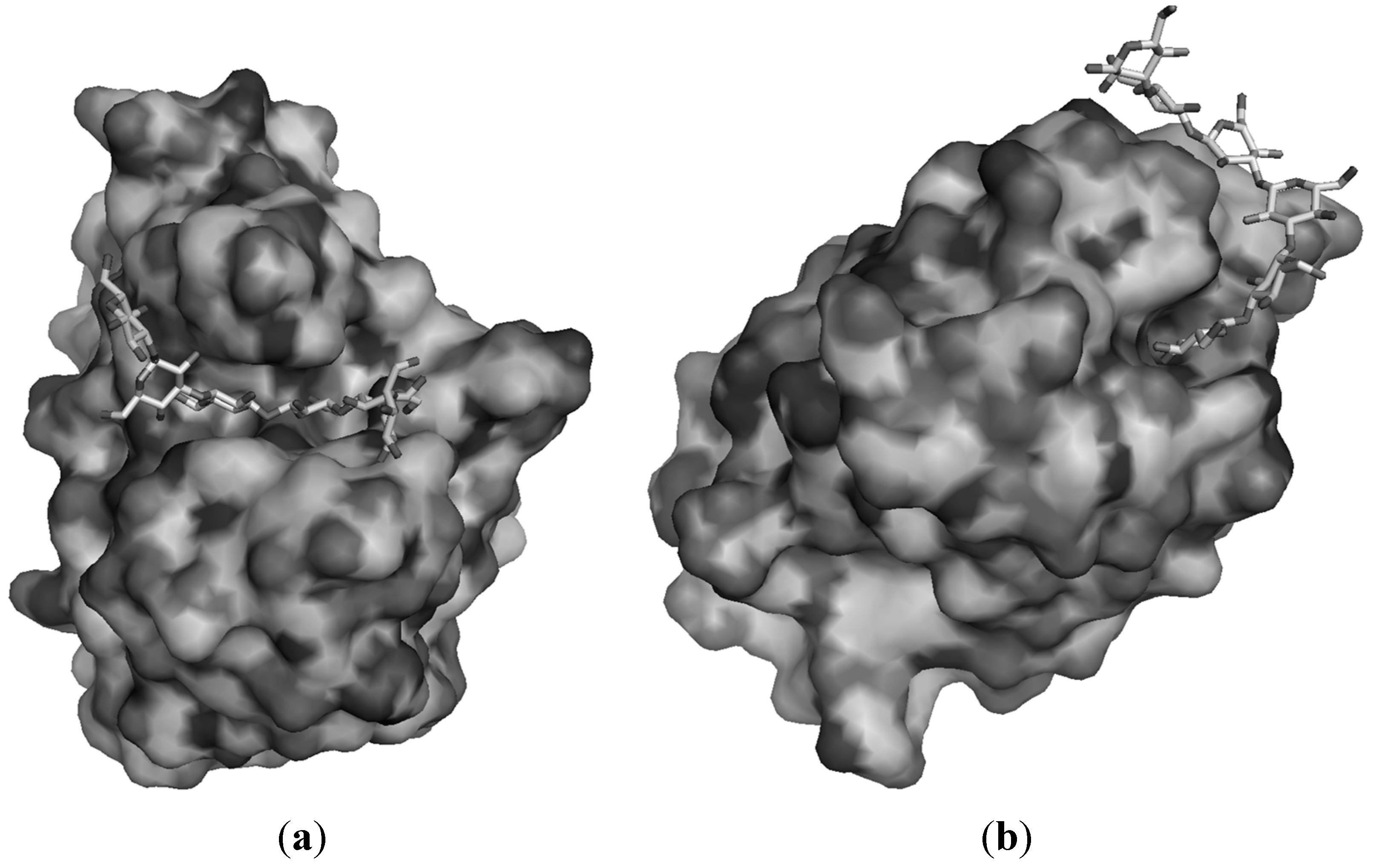
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Clark, A.E.; Kerrigan, A.M.; Brown, G.D. β-Glucan receptors. In Biology and Chemistry of β-Glucans—Mechanisms of Action; Vetvicka, V., Novak, M., Eds.; Bentham Science Publishers: Oak Park, IL, USA, 2011; Volume 1, pp. 39–48. [Google Scholar]
- Yan, J.; Allendorf, D.J.; Brandley, B. Yeast whole glucan particle (WGP) β-glucan in conjunction with antitumor monoclonal antibodies to treat cancer. Expert Opin. Biol. Ther. 2005, 5, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Sima, P. β-Glucan in invertebrates. Invertebr. Surviv. J. 2004, 1, 60–65. [Google Scholar]
- Chuah, C.T.; Sarko, A.; Deslandes, Y.; Marchessault, R.H. Packing analysis of carbohydrates and polysaccharides. Part 14. Triple-helical crystalline structure of curdlan and paramylon hydrates. Macromolecules 1983, 16, 1375–1382. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Uehara, N.; Saito, H. Conformation-Dependent Change in Antitumor Activity of Linear and Branched (1,3)-β-d-Glucans on the Basis of Conformational Elucidation by Carbon-13 Nuclear Magnetic Resonance Spectroscopy. Chem. Pharm. Bull. 1992, 40, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Kulicke, W.M.; Lettau, A.I.; Thielking, H. Correlation between immunological activity, molar mass, and molecular structure of different (1,3)-β-d-glucans. Carbohydr. Res. 1997, 297, 135–143. [Google Scholar] [CrossRef]
- Young, S.H.; Dong, W.J.; Jacobs, R.R. Observation of a partially opened triple-helix conformation in (1,3)-β-glucan by fluorescence resonance energy transfer spectroscopy. J. Biol. Chem. 2000, 275, 11874–11879. [Google Scholar] [CrossRef] [PubMed]
- Okobira, T.; Miyoshi, K.; Uezu, K.; Sakurai, K.; Shinkai, S. Molecular Dynamics Studies of Side Chain Effect on the β-1,3-d-Glucan Triple Helix in Aqueous Solution. Biomacromolecules 2008, 9, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Guo, L.; Ruan, Y.; Zhu, H.; Wang, L.; Zhou, L.; Yun, X.; Gu, J. Laminarin-mediated targeting to Dectin-1 enhances antigen-specific immune responses. Biochem. Biophys. Res. Commun. 2010, 391, 958–962. [Google Scholar] [CrossRef] [PubMed]
- Tamaru, S.; Tokunaga, D.; Hori, K.; Matsuda, S.; Shinkai, S. Giant amino acids designed on the polysaccharide scaffold and their protein-like structural interconversion. Org. Biomol. Chem. 2014, 12, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, L.; Xu, X. Morphologies and conformation transition of lentinan in aqueous NaOH solution. Biopolymers 2004, 75, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Sletmoen, M.; Christensen, B.E.; Stokke, B.T. Probing macromolecular architectures of nanosized cyclic structures of (1,3)-β-d-glucans by AFM and SEC-MALLS. Carbohydr. Res. 2005, 340, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, D.E.; Goodall, D.G. Terraced self assembled nano-structures from laminarin. Int. J. Biol. Macromol. 2007, 40, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Ishii, T.; Ikeda, Y.; Hoshino, A.; Tamura, H.; Aketagawa, J.; Tanaka, S.; Ohno, N. Characterization of β-glucan recognition site on C-type lectin, dectin 1. Infect. Immun. 2004, 72, 4159–4171. [Google Scholar] [CrossRef] [PubMed]
- Harada, T. Production, properties, and application of curdlan. In Extracellular Microbial Polysaccharides; Sandford, P.A., Laskin, A., Eds.; Am. Chem. Soc. Publishers: Washington, DC, USA, 1977; Volume 45, pp. 265–283. [Google Scholar]
- Ogawa, K.; Tsurugi, J.; Watanabe, T. The dependence of the conformation of a (1,3)-β-d-glucan on chain-length in alkaline solution. Carbohydr. Res. 1973, 29, 397–403. [Google Scholar] [CrossRef]
- Buck, K.W.; Chen, A.W.; Dickerson, A.G.; Chain, E.B. Formation and Structure of Extracellular Glucans Produced by Claviceps Species. J. Gen. Macrobiol. 1968, 51, 337–352. [Google Scholar] [CrossRef]
- Perlin, A.S.; Taber, W.A. A glucan produced by Claviceps purpurea. Can. J. Chem. 1963, 41, 2278–2282. [Google Scholar] [CrossRef]
- Brown, G.D.; Gordon, S. Immune recongnition. A new receptor for β-glucans. Nature 2001, 413, 36–37. [Google Scholar] [CrossRef] [PubMed]
- Batbayar, S.; Lee, D.H.; Kim, H.W. Immunomodulation of fungal β-Glucan in host defense signaling by Dectin-1. Biomol. Ther. 2012, 20, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Gantner, B.N.; Simmons, R.M.; Canavera, S.J.; Akira, S.; Underhill, D.M. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 2003, 197, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D. Dectin-1: A signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 2006, 6, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, K.T.; Carbajal, K.S.; Segal, B.M.; Gigera, R.J. Neuroinflammation triggered by β-glucan/dectin-1 signaling enables CNS axon regeneration. PNAS 2015, 112, 2581–2586. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.K.; Tam, J.M.; Khan, N.S.; Seward, M.; Davids, P.J.; Puranam, S.; Sokolovska, A.; Sykes, D.B.; Dagher, Z.; Becker, C.; et al. Dectin-1 Activation Controls Maturation of β-1,3-Glucan-containing Phagosomes. J. Biol. Chem. 2013, 288, 16043–16054. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.P.; Wang, Y.; Lv, X.F.; Shen, X.K.; Ni, X.; Ding, L. Structure of a β-glucan from Grifola frondosa and its antitumor effects by activating Dectin-1/Syk/NF-κB signaling. Glycoconj. J. 2010, 29, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Willcocks, S.; Offord, V.; Seyfert, H.M.; Coffey, T.J.; Werling, D. Species-specific PAMP recognition by TLR2 and evidence for species-restricted interaction with Dectin-1. J. Leukoc. Biol. 2013, 94, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.; Chan, W.; Sze, D. The effects of β-glucan on human immune and cancer cells. J. Hematol. Oncol. 2009, 2. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y. Role of the 1,3-β-d-glucan receptor dectin-1 in fungal infection and activation of innate and anti-tumor immunity. Trends Glycosci. Glycotechnol. 2007, 19, 195–207. [Google Scholar] [CrossRef]
- Veldhuizen, E.J.A.; van Eijk, M.; Haagsman, H.P. The carbohydrate recognition domain of collectins. FEBS J. 2011, 278, 3930–3941. [Google Scholar] [CrossRef] [PubMed]
- Palma, A.S.; Feizi, T.; Zhang, Y.; Stoll, M.S.; Lawson, A.M.; Dıaz-Rodrıguez, E.; Campanero-Rhodes, M.A.; Costa, J.; Gordon, S.; Brown, G.D.; et al. Ligands for the β-Glucan Receptor, Dectin-1, Assigned Using “Designer” Microarrays of Oligosaccharide Probes (Neoglycolipids) Generated from Glucan Polysaccharides. J. Biol. Chem. 2006, 281, 5771–5779. [Google Scholar] [CrossRef] [PubMed]
- Sylla, B.; Guegan, J.P.; Wieruszeski, J.M.; Nugier-Chauvin, C.; Legentil, L.; Daniellou, R.; Ferrieres, V. Probing β-(1→3)-d-glucans interactions with recombinant human receptors using high-resolution NMR studies. Carbohydr. Res. 2011, 346, 1490–1494. [Google Scholar] [CrossRef] [PubMed]
- Hanashima, S.; Ikeda, A.; Tanaka, H.; Adachi, Y.; Ohno, N.; Takahashi, T.; Yamaguchi, Y. NMR study of short β(1–3)-glucans provides insights into the structure and interaction with Dectin-1. Glycoconj. J. 2014, 31, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Zhou, Z.; Burgula, S.; Liao, J.; Yuan, C.; Wu, Q.; Guo, Z. Synthesis and Immunological Studies of Linear Oligosaccharides of β-Glucan As Antigens for Antifungal Vaccine Development. Bioconj. Chem. 2015, 26, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Donadei, A.; Gallorini, S.; Berti, F.; O’Hagan, D.T.; Adamo, R.; Baudner, B.C. Rational Design of Adjuvant for Skin Delivery: Conjugation of Synthetic β-Glucan Dectin-1 Agonist to Protein Antigen. Mol. Pharm. 2015, 12, 1662–1672. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.L.; Rice, P.J.; Graves, B.; Ensley, H.E.; Yu, H.; Brown, G.D.; Gordon, S.; Monteiro, M.A.; Papp-Szabo, E.; Lowman, D.W.; et al. Differential High-Affinity Interaction of Dectin-1 with Natural or Synthetic Glucans Is Dependent upon Primary Structure and Is Influenced by Polymer Chain Length and Side-Chain Branching. J. Pharmacol. Exp. Ther. 2008, 325, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Tada, R.; Adachi, Y.; Ishibashi, K.I.; Tsukbaki, K.; Ohno, N. Binding Capacity of a Barley β-d-Glucan to the β-Glucan Recognition Molecule Dectin-1. J. Agric. Food Chem. 2008, 56, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Chaung, H.C.; Huang, T.C.; Yu, J.H.; Wu, M.L.; Chung, W.B. Immunomodulatory effects of β-glucans on porcine alveolar macrophages and bone marrow haematopoietic cell-derived dendritic cells. Vet. Immunol. Immunopathol. 2009, 131, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Stambach, N.S.; Taylor, M.E. Characterization of carbohydrate recognition by Langerin, a C-type lectin of Langerhans cells. Glycobiology 2003, 13, 401–410. [Google Scholar] [CrossRef] [PubMed]
- De Jong, M.A.W.P.; Vriend, L.E.M.; Theelen, B.; Taylor, M.E.; Fluitsma, D.; Boekhout, T.; Geijtenbeek, T.B.H. C-type lectin Langerin is a β-glucan receptor on human Langerhans cells that recognizes opportunistic and pathogenic fungi. Mol. Immunol. 2010, 47, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, H.; Taylor, M.E.; Razi, N.; McBride, R.; Knirel, Y.A.; Graham, S.A.; Drickamer, K.; Weis, W.I. Structural Basis for Langerin Recognition of Diverse Pathogen and Mammalian Glycans through a Single Binding Site. J. Mol. Biol. 2011, 405, 1027–1039. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, M.; Ostroff, G.R.; Levitz, S.M.; Bartling, T.R.; Mantis, N.J. A population of langerin-positive dendritic cells in murine Peyer’s patches involved in sampling β-glucan microparticles. PLoS ONE 2014, 9, e91002. [Google Scholar] [CrossRef] [PubMed]
- Ross, G.D.; Vetvicka, V.; Yan, J.; Xia, Y.; Vetvickova, J. Therapeutic intervention with complement and β-glucan in cancer. Immunopharmacol. 1999, 42, 61–74. [Google Scholar] [CrossRef]
- Xia, Y.; Borland, G.; Huang, J.; Mizukami, I.F.; Petty, H.R.; Todd, R.F.; Ross, G.D. Function of the Lectin Domain of Mac-1/Complement Receptor Type 3 (CD11b/CD18) in Regulating Neutrophil Adhesion. J. Immunol. 2002, 169, 6417–6426. [Google Scholar] [CrossRef] [PubMed]
- Tsikitis, V.L.; Morin, N.A.; Harrington, E.O.; Albina, J.E.; Reichner, J.S. The Lectin-Like Domain of Complement Receptor 3 Protects Endothelial Barrier Function from Activated Neutrophils. J. Immunol. 2004, 173, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Harler, M.B.; Wakshull, E.; Filardo, E.J.; Albina, J.E.; Reichner, J.S. Promotion of Neutrophil Chemotaxis Through Differential Regulation of β1 and β2 Integrins. J. Immunol. 1999, 162, 6792–6799. [Google Scholar] [PubMed]
- Van Bruggen, R.; Drewniak, A.; Jansen, M.; van Houdt, M.; Roos, D.; Chapel, H.; Verhoeven, A.J.; Kuijpers, T.W. Complement receptor 3, not Dectin-1, is the major receptor on human neutrophils for β-glucan-bearing particles. Mol. Immunol. 2009, 47, 575–581. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, X.M.; Heflin, K.E.; Lavigne, L.M.; Yu, K.; Kim, M.; Salomon, A.R.; Reichner, J.S. Lectin Site Ligation of CR3 Induces Conformational Changes and Signaling. J. Biol. Chem. 2012, 287, 3337–3348. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Vetvicka, V.; Yun, J.; Hanikyrova, M.; Mayadas, T.; Ross, G.D. The β-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells. J. Immunol. 1999, 162, 2281–2290. [Google Scholar] [PubMed]
- Hong, F.; Yan, J.; Baran, J.T.; Allendorf, D.J.; Hansen, R.D.; Ostroff, G.R.; Xing, P.X.; Cheung, N.K.V.; Ross, G.D. Mechanism by Which Orally Administered β-1,3-Glucans Enhance the Tumoricidal Activity of Antitumor Monoclonal Antibodies in Murine Tumor Models. J. Immunol. 2004, 173, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Thornton, B.P.; Vĕtvicka, V.; Pitman, M.; Goldman, R.C.; Ross, G.D. Analysis of the sugar specificity and molecular location of the β-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18). J. Immunol. 1996, 156, 1235–1246. [Google Scholar] [PubMed]
- Bose, N.; Chan, A.S.H.; Guerrero, F.; Maristany, C.M.; Walsh, R.M.; Ertelt, K.E.; Jonas, A.B.; Gorden, K.B.; Dudney, C.M.; Wurst, L.R.; et al. Binding of soluble yeast β-glucan to human neutrophils and monocytes is complement-dependent. Front. Immunol. 2013, 4, 230. [Google Scholar] [CrossRef] [PubMed]
- Ferry, A.; Malik, G.; Guinchard, X.; Vetvicka, V.; Crich, D. Synthesis and Evaluation of Di- and Trimeric Hydroxylamine-Based β-(1→3)-Glucan Mimetics. J. Am. Chem. Soc. 2014, 136, 14852–14857. [Google Scholar] [CrossRef] [PubMed]
- Jamois, F.; Ferrières, V.; Guégan, J.P.; Yvin, J.C.; Plusquellec, D.; Vetvicka, V. Glucan-like synthetic oligosaccharides: Iterative synthesis of linear oligo-β-(1,3)-glucans and immunostimulatory effects. Glycobiology 2005, 15, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Saraswat-Ohri, S.; Vashishta, A.; Vetvicka, V.; Descroix, K.; Jamois, F.; Yvin, J.C.; Ferrières, V. Biological properties of (1→3)-beta-d-glucan-based synthetic oligosaccharides. J. Med. Food 2011, 14, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Saraswat-Ohri, S.; Vashishta, A.; Descroix, K.; Jamois, F.; Yvin, J.C.; Ferrières, V. New 4-deoxy-(1→3)-β-d-glucan-based oligosaccharides and their immunostimulating potential. Carbohydr. Res. 2011, 346, 2213–2221. [Google Scholar] [CrossRef] [PubMed]
- Descroix, K.; Větvička, V.; Laurent, I.; Jamois, F.; Yvin, J.-C.; Ferrières, V. New oligo-beta-(1,3)-glucan derivatives as immunostimulating agents. Bioorganic Med. Chem. 2010, 18, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Sylla, B.; Legentil, L.; Saraswat-Ohri, S.; Vashishta, A.; Daniellou, R.; Wang, H.W.; Vetvicka, V.; Ferrières, V. Oligo-β-(1→3)-glucans: Impact of thio-bridges on immunostimulating activities and the development of cancer stem cells. J. Med. Chem. 2014, 57, 8280–8292. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, J.W.; Lindermuth, J.; Fish, P.A.; Palace, G.P.; Stevenson, T.T.; DeMong, D.E. A novel carbohydrate-glycosphingolipid interaction between a β-(1–3)-glucan immunomodulator, PGG-glucan, and lactosylceramide of human leukocytes. J. Biol. Chem. 1998, 273, 22014–22020. [Google Scholar] [CrossRef] [PubMed]
- Tsikitis, V.L.; Martin, A.; Albina, J.; Reichner, J. Ligation of the lactosylceramide receptor (CDw17) promotes neutrophil migration. J. Am. Coll. Surg. 2004, 199, 44. [Google Scholar] [CrossRef]
- Wakshull, E.; Brunke-Reese, D.; Lindermuth, J.; Fisette, L.; Nathans, R.S.; Crowley, J.J.; Tufts, J.C.; Zimmerman, J.; Mackin, W.; Adams, D.S. PGG-Glucan, a soluble β-(1,3)-glucan, enhances the oxidative burst response, microbicidal activity, and activates an NF-κB-like factor in human PMN: Evidence for a glycosphingolipid β-(1,3)-glucan receptor. Immunopharmacology 1999, 41, 89–107. [Google Scholar] [CrossRef]
- Hahn, P.Y.; Evans, S.E.; Kottom, T.J.; Standing, J.E.; Pagano, R.E.; Limper, A.H. Pneumocystis carinii cell wall β-glucan induces release of macrophage inflammatory protein-2 from alveolar epithelial cells via a lactosylceramide-mediated mechanism. J. Biol. Chem. 2003, 278, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Akramiene, D.; Kondrotas, A.; Didziapetriene, J.; Kevelaitis, E. Effects of β-glucans on the immune system. Medicina (Kaunas) 2007, 43, 597–606. [Google Scholar] [PubMed]
- Dzik, J.M. The ancestry and cumulative evolution of immune reactions. Acta Biochim. Pol. 2010, 57, 443–466. [Google Scholar] [PubMed]
- Rice, P.J.; Kelley, J.L.; Kogan, G.; Ensley, H.E.; Kalbfleisch, J.H.; Browder, I.W.; Williams, D.L. Human monocyte scavenger receptors are pattern recognition receptors for (1→3)-β-d-glucans. J. Leukoc. Biol. 2002, 72, 140–146. [Google Scholar] [PubMed]
- Vera, J.; Fenutria, R.; Canadas, O.; Figueras, M.; Mota, R.; Sarrias, M.R.; Williams, D.L.; Casals, C.; Yelamos, J.; Lozano, F. The CD5 ectodomain interacts with conserved fungal cell wall components and protects from zymosan-induced septic shock-like syndrome. Proc. Natl. Acad. Sci. USA 2009, 106, 1506–1511. [Google Scholar] [CrossRef] [PubMed]
- Van de Veerdonk, F.L.; Joosten, L.A.B.; Devesa, I.; Mora-Montes, H.M.; Kanneganti, T.D.; Dinarello, C.A.; van der Meer, J.W.M.; Gow, N.A.R.; Kullberg, B.J.; Netea, M.G. Bypassing Pathogen-Induced Inflammasome Activation for the Regulation of Interleukin-1β Production by the Fungal Pathogen Candida albicans. J. Infect. Dis. 2009, 199, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Kankkunen, P.; Teirilä, L.; Rintahaka, J.; Alenius, H.; Wolff, H.; Matikainen, S. (1,3)-β-Glucans Activate Both Dectin-1 and NLRP3 Inflammasome in Human Macrophages. J. Immunol. 2010, 184, 6335–6342. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, M.; Fujita, T. Ficolins and the lectin complement pathway. Immunol. Rev. 2001, 180, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Lynch, N.J.; Roscher, S.; Hartung, T.; Morath, S.; Matsushita, M.; Maennel, D.N.; Kuraya, M.; Fujita, T.; Schwaeble, W.J. L-Ficolin Specifically Binds to Lipoteichoic Acid, a Cell Wall Constituent of Gram-Positive Bacteria, and Activates the Lectin Pathway of Complement. J. Immunol. 2004, 172, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Krarup, A.; Sørensen, U.B.S.; Matsushita, M.; Jensenius, J.C.; Thiel, S. Effect of Capsulation of Opportunistic Pathogenic Bacteria on Binding of the Pattern Recognition Molecules Mannan-Binding Lectin, L-Ficolin, and H-Ficolin. Infect. Immun. 2005, 73, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.G.; Cho, M.Y.; Zhao, M.; Park, J.W.; Matsushita, M.; Fujita, T.; Lee, B.L. Human Mannose-binding Lectin and l-Ficolin Function as Specific Pattern Recognition Proteins in the Lectin Activation Pathway of Complement. J. Biol. Chem. 2004, 279, 25307–25312. [Google Scholar] [CrossRef] [PubMed]
- Garlatti, V.; Belloy, N.; Martin, L.; Lacroix, M.; Matsushita, M.; Endo, Y.; Fujita, T.; Fontecilla-Camps, J.C.; Arlaud, G.J.; Thielens, N.M.; et al. Structural insights into the innate immune recognition specificities of L- and H-ficolins. EMBO J. 2007, 26, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Roh, K.B.; Kim, C.H.; Lee, H.; Kwon, H.M.; Park, J.W.; Ryu, J.H.; Kurokawa, K.; Ha, N.C.; Lee, W.J.; Lemaitre, B.; et al. Proteolytic Cascade for the Activation of the Insect Toll Pathway Induced by the Fungal Cell Wall Component. J. Biol. Chem. 2009, 284, 19474–19481. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Osaki, T.; Lee, J.Y.; Baek, M.J.; Zhang, R.; Park, J.W.; Kawabata, S.; Söderhäll, K.; Lee, B.L. Peptidoglycan Recognition Proteins Involved in 1,3-β-d-Glucan-dependent Prophenoloxidase Activation System of Insect. J. Biol. Chem. 2004, 279, 3218–3227. [Google Scholar] [CrossRef] [PubMed]
- Sukhithasri, V.; Nisha, N.; Biswas, L.; Anil Kumar, V.; Biswas, R. Innate immune recognition of microbial cell wall components and microbial strategies to evade such recognitions. Microbiol. Res. 2013, 168, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Gordon, S. Immune recognition of fungal β-glucans. Cell. Microbiol. 2005, 7, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.J.; Zhong, X.; Lin, X.Y.; Huang, X.H.; Yu, X.Q. Characterization of a novel Manduca sexta beta-1, 3-glucan recognition protein (βGRP3) with multiple functions. Insect Biochem. Mol. Biol. 2014, 52, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.Z.; Yu, X.Q. The extended loop of the C-terminal carbohydrate-recognition domain of Manduca sexta immulectin-2 is important for ligand binding and functions. Amino Acids 2012, 42, 2383–2391. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, H. Binding properties of the regulatory domains in Manduca sexta hemolymph proteinase-14, an initiation enzyme of the prophenoloxidase activation system. Dev. Comp. Immunol. 2010, 34, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A. Evolution of the βGRP/GNBP/β-1,3-glucanase family of insects. Immunogenetics 2012, 64, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, M.; Ashida, M. A Pattern-recognition Protein for β-1,3-Glucan: The binding domain and the cDNA cloning of the β-1,3-glucan recognition protein from the skillworm, Bombyx mori. J. Biol. Chem. 2000, 275, 4995–5002. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Kanost, M.R. A β1,3-Glucan Recognition Protein from an Insect, Manduca sexta, Agglutinates Microorganisms and Activates the Phenoloxidase Cascade. J. Biol. Chem. 2000, 275, 7505–7514. [Google Scholar] [CrossRef] [PubMed]
- Fabrick, J.A.; Baker, J.E.; Kanost, M.R. Innate Immunity in a Pyralid Moth: Functional of domains from a β-1,3-glucan recognition protein. J. Biol. Chem. 2004, 279, 26605–26611. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, D.; Dai, H.; Hiromasa, Y.; Krishnamoorthi, R.; Kanost, M.R. Self-association of an insect β-1,3-glucan recognition protein upon binding laminarin stimulates prophenoloxidase activation as an innate immune response. J. Biol. Chem. 2014, 289, 28399–28410. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Hiromasa, Y.; Takahashi, D.; VanderVelde, D.; Fabrick, J.A.; Kanost, M.R.; Krishnamoorthi, R. An Initial Event in the Insect Innate Immune Response: Structural and Biological Studies of Interactions between β-1,3-Glucan and the N-Terminal Domain of β-1,3-Glucan Recognition Protein. Biochemistry 2013, 52, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Kanagawa, M.; Satoh, T.; Ikeda, A.; Adachi, Y.; Ohno, N.; Yamaguchi, Y. Structural Insights into Recognition of Triple-helical β-Glucans by an Insect Fungal Receptor. J. Biol. Chem. 2011, 286, 29158–29165. [Google Scholar] [CrossRef] [PubMed]
- Takahasi, K.; Ochiai, M.; Horiuchi, M.; Kumeta, H.; Ogura, K.; Ashida, M.; Inagaki, F. Solution structure of the silkworm βGRP/GNBP3 N-terminal domain reveals the mechanism for β-1,3-glucan-specific recognition. PNAS 2009, 106, 11679–11684. [Google Scholar] [CrossRef] [PubMed]
- Mishima, Y.; Quintin, J.; Aimanianda, V.; Kellenberger, C.; Coste, F.; Clavaud, C.; Hetru, C.; Hoffmann, J.A.; Latgé, J.P.; Ferrandon, D.; et al. The N-terminal Domain of Drosophila Gram-negative Binding Protein 3 (GNBP3) Defines a Novel Family of Fungal Pattern Recognition Receptors. J. Biol. Chem. 2009, 284, 28687–28697. [Google Scholar] [CrossRef] [PubMed]
- Chamy, L.E.; Leclerc, V.; Caldelari, I.; Reichhart, J.M. Sensing of ‘danger signals’ and pathogen-associated molecular patterns defines binary signaling pathways ‘upstream’ of Toll. Nat. Immunol. 2008, 9, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Weis, W.I.; Drickamer, K. Structural Basis of Lectin-Carbohydrate Recognition. Ann. Rev. Biochem. 1996, 65, 441–473. [Google Scholar] [CrossRef] [PubMed]
- Rini, J.M. Lectin Structure. Ann. Rev. Biophys. Biomol. Struct. 1995, 24, 551–577. [Google Scholar] [CrossRef] [PubMed]
- Fabrick, J.A.; Baker, J.E.; Kanost, M.R. cDNA cloning, purification, properties, and function of a β-1,3-glucan recognition protein from a pyralid moth, Plodia interpunctella. Insect Biochem. Mol. Biol. 2003, 33, 579–594. [Google Scholar] [CrossRef]
- Tong, Y.; Kanost, M.R. Manduca sexta Serpin-4 and Serpin-5 Inhibit the Prophenol Oxidase Activation Pathway: cDNA cloning, protein expression, and characterization. J. Biol. Chem. 2005, 280, 14923–14931. [Google Scholar] [CrossRef] [PubMed]
- Duvic, B.; Söderhäll, K. Purification and characterization of a beta-1,3-glucan binding protein from plasma of the crayfish Pacifastacus leniusculus. J. Biol. Chem. 1990, 265, 9327–9332. [Google Scholar] [PubMed]
- Wang, X.-W.; Wang, J.-X. Pattern recognition receptors acting in innate immune system of shrimp against pathogen infections. Fish Shellfish Immunol. 2013, 34, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qiu, L.; Zhou, Z.; Song, L. Research progress on the mollusc immunity in China. Dev. Comp. Immunol. 2013, 39, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Du, X.J.; Zhao, X.F.; Wang, J.X. Molecular cloning and characterization of a lipopolysaccharide and β-1,3-glucan binding protein from fleshy prawn (Fenneropenaeus chinensis). Mol. Immunol. 2007, 44, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Dziarski, R. Peptidoglycan recognition proteins (PGRPs). Mol. Immunol. 2004, 40, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Söderhäll, K. Early events in crustacean innate immunity. Fish Shellfish Immunol. 2002, 12, 421–437. [Google Scholar] [PubMed]
- Cerenius, L.; Liang, Z.; Duvic, B.; Keyser, P.; Hellman, U.; Palva, E.T.; Iwanaga, S.; Söderhäll, K. Structure and biological activity of a 1,3-beta-d-glucan-binding protein in crustacean blood. J. Biol. Chem. 1994, 269, 29462–29467. [Google Scholar] [PubMed]
- Hou, L.; Liu, X.; Jing, Y.; Zhang, J.; Ding, Z.; Huang, Y.; Gu, W.; Meng, Q.; Wang, W. Molecular cloning and characterization of the lipopolysaccharide and β-1,3-glucan binding protein from red claw crayfish, Cherax quadricarinatus. Aquaculture 2015, 441, 1–7. [Google Scholar] [CrossRef]
- Sivakamavalli, J.; Vaseeharan, B. Bifunctional role of a pattern recognition molecule β-1,3 glucan binding protein purified from mangrove crab Episesarma tetragonum. J. Invertebr. Pathol. 2014, 119, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Sivakamavalli, J.; Selvaraj, C.; Singh, S.K.; Vaseeharan, B. Interaction investigations of crustacean β-GBP recognition toward pathogenic microbial cell membrane and stimulate upon prophenoloxidase activation. J. Mol. Recognit. 2014, 27, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Sivakamavalli, J.; Tripathi, S.K.; Singh, S.K.; Vaseeharan, B. Homology modeling, molecular dynamics, and docking studies of pattern-recognition transmembrane protein-lipopolysaccharide and β-1,3 glucan-binding protein from Fenneropenaeus indicus. J. Biomol. Struct. Dyn. 2015, 33, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- Muta, T.; Seki, N.; Takaki, Y.; Hashimoto, R.; Oda, T.; Iwanaga, A.; Tokunaga, F.; Iwanaga, S. Purified Horseshoe Crab Factor G: Recontitution and characterization of the (1,3)-β-d-glucan-sensitive serine protease cascade. J. Biol. Chem. 1995, 270, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Seki, N.; Muta, T.; Oda, T.; Iwaki, D.; Kuma, K.; Miyata, T.; Iwanaga, S. Horseshoe crab (1,3)-beta-d-glucan-sensitive coagulation factor G. A serine protease zymogen heterodimer with similarities to beta-glucan-binding proteins. J. Biol. Chem. 1994, 269, 1370–1374. [Google Scholar] [PubMed]
- Takaki, Y.; Seki, N.; Kawabata, S.; Iwanaga, S.; Muta, T. Duplicated Binding Sites for (1→3)-β-d-Glucan in the Horseshoe Crab Coagulation Factor G: Implications for a molecular basis of thepattern recognition in innate immunity. J. Biol. Chem. 2002, 277, 14281–14287. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.J.; Henrissat, B. Structural enzymology of carbohydrate-active enzymes: Implications for the post-genomic era. Biochem. Soc. Trans. 2002, 30, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Bolam, D.N.; Ciruela, A.; Mcqueen-Mason, S.; Simpson, P.; Williamson, M.P.; Rixon, J.E.; Boraston, A.; Hazlewood, G.P.; Gilbert, H.J. Pseudomonas cellulose-binding domains mediate their effects by increasing enzyme substrate proximity. Biochem. J. 1998, 331, 775–781. [Google Scholar] [PubMed]
- Gill, J.; Rixon, J.E.; Bolam, D.N.; Mcqueen-Mason, S.; Simpson, P.J.; Williamson, M.P.; Hazlewood, G.P.; Gilbert, H.J. The type II and X cellulose-binding domains of Pseudomonas xylanase A potentiate catalytic activity against complex substrates by a common mechanism. Biochem. J. 1999, 342, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, P.M.; Henrissat, B. Recent Advances in Carbohydrate Bioengineering; Royal Society of Chemistry: Cambridge, UK, 1999. [Google Scholar]
- Boraston, A.B.; Bolam, D.N.; Gilbert, H.J.; Davies, G.J. Carbohydrate-binding modules: Fine tuning polysaccharide recognition. Biochem. J. 2004, 382, 769–772. [Google Scholar] [PubMed]
- Boraston, A.B.; Warren, R.A.J.; Kilburn, D.G. β-1,3-Glucan Binding by a Thermostable Carbohydrate-Binding Module from Thermotoga maritima. Biochemistry 2001, 40, 14679–14685. [Google Scholar] [CrossRef] [PubMed]
- Tomme, P.; Creagh, A.L.; Kilburn, D.G.; Haynes, C.A. Interaction of Polysaccharides with the N-Terminal Cellulose-Binding Domain of Cellulomonas fimi CenC. 1. Binding Specificity and Calorimetric Analysis. Biochemistry 1996, 35, 13885–13894. [Google Scholar] [CrossRef] [PubMed]
- Abou-Hachem, M.; Nordberg-Karlsson, E.; BartonekRoxa, E.; Raghothama, S.; Simpson, P.J.; Gilbert, H.J.; Williamson, M.P.; Holst, O. Carbohydrate-binding modules from a thermostable Rhodothermus marinus xylanase: Cloning, expression and binding studies. Biochem. J. 2000, 345, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Boraston, A.B.; Nurizzo, D.; Notenboom, V.; Ducros, V.; Rose, D.R.; Kilburn, D.G.; Davies, G.J. Differential Oligosaccharide Recognition by Evolutionarily-related β-1,4 and β-1,3 Glucan-binding Modules. J. Mol. Biol. 2002, 319, 1143–1156. [Google Scholar] [CrossRef]
- Van Bueren, A.L.; Morland, C.; Gilbert, H.J.; Boraston, A.B. Family 6 Carbohydrate Binding Modules Recognize the Non-reducing End of β-1,3-Linked Glucans by Presenting a Unique Ligand Binding Surface. J. Biol. Chem. 2005, 280, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.A.S.; Pires, V.M.R.; Gilbert, H.J.; Bolam, D.N.; Fernandes, V.O.; Alves, V.D.; Prates, J.A.M.; Ferreira, L.M.A.; Fontes, C.M.G.A. Family 6 carbohydrate-binding modules display multiple β1,3-linked glucan-specific binding interfaces. FEMS Microbiol. Lett. 2009, 300, 48–57. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tamashiro, T.; Tanabe, Y.; Ikura, T.; Ito, N.; Oda, M. Critical roles of Asp270 and Trp273 in the α-repeat of the carbohydrate-binding module of endo-1,3-β-glucanase for laminarin-binding avidity. Glycoconj. J. 2012, 29, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Guerrero, R.; Schüttelkopf, A.W.; Mouyna, I.; Ibrahim, A.F.M.; Shepherd, S.; Fontaine, T.; Latgé, J.P.; van Aalten, D.M.F. Molecular Mechanisms of Yeast Cell Wall Glucan Remodeling. J. Biol. Chem. 2009, 284, 8461–8469. [Google Scholar] [CrossRef] [PubMed]
- Treviño, M.Á.; Palomares, O.; Castrillo, I.; Villalba, M.; Rodríguez, R.; Rico, M.; Santoro, J.; Bruix, M. Solution structure of the C-terminal domain of Ole e 9, a major allergen of olive pollen. Prot. Sci. 2008, 17, 371–376. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legentil, L.; Paris, F.; Ballet, C.; Trouvelot, S.; Daire, X.; Vetvicka, V.; Ferrières, V. Molecular Interactions of β-(1→3)-Glucans with Their Receptors. Molecules 2015, 20, 9745-9766. https://doi.org/10.3390/molecules20069745
Legentil L, Paris F, Ballet C, Trouvelot S, Daire X, Vetvicka V, Ferrières V. Molecular Interactions of β-(1→3)-Glucans with Their Receptors. Molecules. 2015; 20(6):9745-9766. https://doi.org/10.3390/molecules20069745
Chicago/Turabian StyleLegentil, Laurent, Franck Paris, Caroline Ballet, Sophie Trouvelot, Xavier Daire, Vaclav Vetvicka, and Vincent Ferrières. 2015. "Molecular Interactions of β-(1→3)-Glucans with Their Receptors" Molecules 20, no. 6: 9745-9766. https://doi.org/10.3390/molecules20069745
APA StyleLegentil, L., Paris, F., Ballet, C., Trouvelot, S., Daire, X., Vetvicka, V., & Ferrières, V. (2015). Molecular Interactions of β-(1→3)-Glucans with Their Receptors. Molecules, 20(6), 9745-9766. https://doi.org/10.3390/molecules20069745








