Comparative Study of the Proteins Involved in the Fermentation-Derived Compounds in Two Strains of Saccharomyces cerevisiae during Sparkling Wine Second Fermentation
Abstract
1. Introduction
2. Materials and Methods
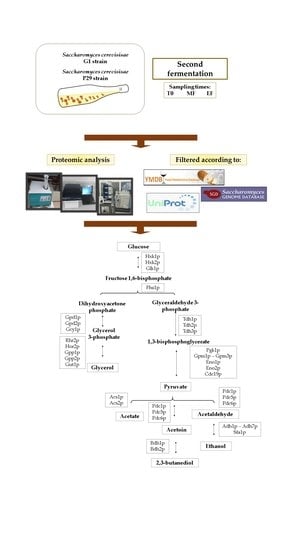
2.1. Microorganism and Experimental Conditions
2.2. Proteome Analysis
2.3. Statistical Analysis
3. Results and Discussion
3.1. Glycolysis/Gluconeogenesis Proteome
3.2. Proteins Related to the Metabolism of Pyruvate to Ethanol and Acetic Acid
3.3. Proteins Related to the Metabolism of Pyruvate-Acetoin-2,3-Butanediol
3.4. Proteins Related to the Metabolism of Dihydroxyacetone Phosphate-Glycerol.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pozo-Bayón, M.Á.; Martínez-Rodríguez, A.; Pueyo, E.; Moreno-Arribas, M.V. Chemical and biochemical features involved in sparkling wine production: From a traditional to an improved winemaking technology. Trends Food Sci. Technol. 2009, 20, 289–299. [Google Scholar] [CrossRef]
- Buxaderas, S.; López-Tamames, E. Sparkling wines: Features and trends from tradition. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2012; Volume 66, pp. 1–45. [Google Scholar]
- Penacho, V.; Valero, E.; González, R. Transcription profiling of sparkling wine second fermentation. Int. J. Food Microbiol. 2012, 153, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Arena, P.M.; Laddomada, B.; Cappello, S.M.; Bleve, G.; Grieco, F.; Beneduce, L.; Berbegal, C.; Spano, G.; Capozzi, V. Starter cultures for sparkling wine. Fermentation 2016, 2, 21. [Google Scholar] [CrossRef]
- Alexandre, H.; Guilloux–Nematier, M. Yeast autolysis in sparkling wine—A review. Aust. J. Grape Wine Res. 2006, 12, 119–127. [Google Scholar] [CrossRef]
- Sartor, S.; Toaldo, I.M.; Panceri, C.P.; Caliari, V.; Luna, A.S.; de Gois, J.S.; Bordignon-Luiz, M.T. Changes in organic acids, polyphenolic and elemental composition of rosé sparkling wines treated with mannoproteins during over-lees aging. Food Res. Int. 2019, 124, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Ortega, N.; Martín-Santamaría, M.; Acedo, A.; Marquina, D.; Pascual, O.; Rozès, N.; Zamora, F.; Santos, A.; Belda, I. Occurrence and enological properties of two new non-conventional yeasts (Nakazawaea ishiwadae and Lodderomyces elongisporus) in wine fermentations. Int. J. Food Microbiol. 2019, 305, 108255. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.; Moreno-García, J.; López-Muñoz, B.; Mauricio, J.C.; García-Martínez, T. Use of a flor velum yeast for modulating colour, ethanol and major aroma compound contents in red wine. Food Chem. 2016, 213, 90–97. [Google Scholar] [CrossRef]
- Quincozes, L.; Santos, P.; Vieira, L.; Gabbardo, M.; Eckhardt, D.P.; Cunha, W.; Costa, V.; Zigiotto, L.; Schumacher, R. Influence of yeasts of the genus Saccharomyces and not Saccharomyces in elaboration of white wines. BIO Web Conf. 2019, 12, 02014. [Google Scholar] [CrossRef]
- Alonso-del-Real, J.; Lairón-Peris, M.; Barrio, E.; Querol, A. Effect of temperature on the prevalence of Saccharomyces non cerevisiae species against a S. cerevisiae wine strain in wine fermentation: Competition, physiological fitness, and influence in final wine composition. Front. Microbiol. 2017, 8, 150. [Google Scholar] [CrossRef]
- Ciani, M.; Comitini, F. Non-Saccharomyces wine yeasts have a promising role in biotechnological approaches to winemaking. Ann. Microbiol. 2011, 61, 25–32. [Google Scholar] [CrossRef]
- Nuñez-Guerrero, M.A.; Paez-Lerma, J.B.; Rutiaga-Quiñones, O.M.; González-Herrera, S.M.; Soto-Cruz, N.O. Performance of mixtures of Saccharomyces and non-Saccharomyces native yeasts during alcoholic fermentation of Agave duranguensis juice. Food Microbiol. 2016, 54, 91–97. [Google Scholar] [CrossRef]
- Suárez-Lepe, J.A.; Morata, A. New trends in yeast selection for winemaking. Trends Food Sci. Technol. 2012, 23, 39–50. [Google Scholar] [CrossRef]
- Ciani, M.; Morales, P.; Comitini, F.; Tronchoni, J.; Canonico, L.; Curiel, J.A.; Oro, L.; Rodrigues, A.J.; Gonzalez, R. Non-conventional yeast species for lowering ethanol content of wines. Front. Microbiol. 2016, 7, 642. [Google Scholar] [CrossRef] [PubMed]
- Medina-Trujillo, L.; González-Royo, E.; Sieczkowski, N.; Heras, J.; Canals, J.M.; Zamora, F. Effect of sequential inoculation (Torulaspora delbrueckii/Saccharomyces cerevisiae) in the first fermentation on the foaming properties of sparkling wine. Eur. Food Res. Technol. 2017, 243, 681–688. [Google Scholar] [CrossRef]
- Alexandre, H. Flor yeasts of Saccharomyces cerevisiae-their ecology, genetics and metabolism. Int. J. Food Microbiol. 2013, 167, 269–275. [Google Scholar] [CrossRef]
- Martínez-García, R.; Roldán-Romero, Y.; Moreno, J.; Puig-Pujol, A.; Mauricio, J.C.; García-Martínez, T. Use of a flor yeast strain for the second fermentation of sparkling wines: Effect of endogenous CO2 over-pressure on the volatilome. Food Chem. 2020, 308, 125555. [Google Scholar] [CrossRef]
- González-Jiménez, M.C.; Moreno-García, J.; García-Martínez, T.; Moreno, J.J.; Puig-Pujol, A.; Capdevilla, F.; Mauricio, J.C. Differential analysis of proteins involved in ester metabolism in two Saccharomyces cerevisiae strains during the second fermentation in sparkling wine elaboration. Microorganisms 2020, 8, 403. [Google Scholar] [CrossRef]
- Mauricio, J.C.; Moreno, J.J.; Ortega, J.M. In vitro specific activities of alcohol and aldehyde dehydrogenases from two flor yeast during controlled wine ageing. J. Agric. Food Chem. 1997, 45, 1967–1971. [Google Scholar] [CrossRef]
- Porras-Agüera, J.A.; Moreno-García, J.; Mauricio, J.C.; Moreno, J.; García-Martínez, T. First proteomic approach to identify cell death biomarkers in wine yeasts during sparkling wine production. Microorganisms 2019, 7, 542. [Google Scholar] [CrossRef]
- Ishihama, Y.; Oda, Y.; Tabata, T.; Sato, T.; Nagasu, T.; Rappsilber, J.; Mann, M. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol. Cell. Proteomics 2005, 4, 1265–1272. [Google Scholar] [CrossRef]
- Moreno-García, J.; García-Martínez, T.; Moreno, J.; Mauricio, J.C. Proteins involved in flor yeast carbon metabolism under biofilm formation conditions. Food Microbiol. 2015, 46, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Porras-Agüera, J.A.; Moreno-García, J.; González-Jiménez, M.C.; Mauricio, J.C.; Moreno, J.; García-Martínez, T. Autophagic proteome in two Saccharomyces cerevisiae strains during second fermentation for sparkling wine elaboration. Microorganisms 2020, 8, 523. [Google Scholar] [CrossRef] [PubMed]
- Seisonen, S.; Vene, K.; Koppel, K. The current practice in the application of chemometrics for correlation of sensory and gas chromatography data. Food Chem. 2016, 210, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, R.; García-Martínez, T.; Puig-Pujol, A.; Mauricio, J.C.; Moreno, J. Changes in sparkling wine aroma during the second fermentation under CO2 pressure in sealed bottle. Food Chem. 2017, 237, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Valadi, Å.; Ansell, R.; Gustafsson, L.; Adler, L.; Norbeck, J.; Blomberg, A. NADH-reductive stress in Saccharomyces cerevisiae induces the expression of the minor isoform of glyceraldehyde-3-phosphate dehydrogenase (TDH1). Curr. Genet. 2004, 45, 90–95. [Google Scholar] [PubMed]
- Esteve-Zarzoso, B.; Peris-Torán, M.J.; Garcıa-Maiquez, E.; Uruburu, F.; Querol, A. Yeast population dynamics during the fermentation and biological aging of sherry wines. Appl. Environ. Microb. 2001, 67, 2056–2061. [Google Scholar] [CrossRef]
- Carlson, M.; Botstein, D. Two differentially regulated mRNAs with different 5′ ends encode secreted and intracellular forms of yeast invertase. Cell 1982, 28, 145–154. [Google Scholar] [CrossRef]
- Byrne, S.; Howell, G. Acetaldehyde: How to Limit its Formation during Fermentation; Aust, N.Z., Ed.; Grapegrower Winemaker: Sydney, NSW, Australia, 2017; pp. 68–69. [Google Scholar]
- Mojzita, D.; Hohmann, S. Pdc2 coordinates expression of the THI regulon in the yeast Saccharomyces cerevisiae. Mol. Genet. Genom. 2006, 276, 147–161. [Google Scholar] [CrossRef]
- Moreno-García, J.; García-Martínez, T.; Millán, M.C.; Mauricio, J.C.; Moreno, J. Proteins involved in wine aroma compounds metabolism by a Saccharomyces cerevisiae flor-velum yeast strain grown in two conditions. Food Microbiol. 2015, 51, 1–9. [Google Scholar] [CrossRef]
- Ugliano, M.; Henschke, P.A. Yeasts and Wine Flavour. In Wine Chemistry and Biochemistry; Springer: Berlin/Heidelberg, Germany, 2009; pp. 313–392. [Google Scholar]
- Remize, F.; Andrieu, E.; Dequin, S. Engineering of the pyruvate dehydrogenase bypass in Saccharomyces cerevisiae: Role of the cytosolic Mg2+ and mitochondrial K+ acetaldehyde dehydrogenases Ald6p and Ald4p in acetate formation during alcoholic fermentation. Appl. Environ. Microbiol. 2000, 66, 3151–3159. [Google Scholar] [CrossRef]
- Pigeau, G.M.; Inglis, D.L. Response of wine yeast (Saccharomyces cerevisiae) aldehyde dehydrogenases to acetaldehyde stress during Icewine fermentation. J. Appl. Microbiol. 2007, 103, 1576–1586. [Google Scholar] [CrossRef] [PubMed]
- González, V.F. Aroma, aromas y olores del vino. In Análisis Sensorial y Cata de los Vinos de España; Dialnet: Logroño, Spain, 2001; pp. 207–233. [Google Scholar]
- Vilela-Moura, A.; Schuller, D.; Mendes-Faia, A.; Silva, R.D.; Chaves, S.R.; Sousa, M.J.; Corte-Real, M. The impact of acetate metabolism on yeast fermentative performance and wine quality: Reduction of volatile acidity of grape musts and wines. Appl. Microbiol. Biotechnol. 2011, 89, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Boubekeur, S.; Camougrand, N.; Bunoust, O.; Rigoulet, M.; Guérin, B. Participation of acetaldehyde dehydrogenases in ethanol and pyruvate metabolism of the yeast Saccharomyces cerevisiae. Eur. J. Biochem. 2001, 268, 5057–5065. [Google Scholar] [CrossRef] [PubMed]
- Verduyn, C.; Postma, E.; Scheffers, W.A.; Van Dijken, J.P. Physiology of Saccharomyces cerevisiae in anaerobic glucose-limited chemostat cultures. J. Gen. Microbiol. 1990, 136, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Romano, P.; Suzzi, G. Origin and Production of Acetoin during Wine Yeast Fermentation. Appl. Environ. Microbiol. 1996, 62, 309–315. [Google Scholar] [CrossRef]
- Michnick, S.; Roustan, J.L.; Remize, F.; Barre, P.; Dequin, S. Modulation of glycerol and ethanol yields during alcoholic fermentation in Saccharomyces cerevisiae strains overexpressed or disrupted for GPD1 encoding glycerol 3-phosphate dehydrogenase. Yeast 1997, 13, 783–793. [Google Scholar] [CrossRef]
- Romano, P.; Brandolini, V.; Ansaloni, C.; Menziani, E. The production of 2,3-butanediol as a differentiating character in wine yeasts. World J. Microbiol. Biotechnol. 1998, 14, 649–653. [Google Scholar] [CrossRef]
- Borrull, A.; Poblet, M.; Rozès, N. New insights into the capacity of commercial wine yeasts to grow on sparkling wine media. Factor screening for improving wine yeast selection. Food Microbiol. 2015, 48, 41–48. [Google Scholar] [CrossRef]
- Kemp, B.; Alexandre, H.; Robillard, B.; Marchal, R. Effect of production phase on bottle-fermented sparkling wine quality. J. Agric. Food Chem. 2015, 63, 19–38. [Google Scholar] [CrossRef]
- Goold, H.D.; Kroukamp, H.; Williams, T.C.; Paulsen, I.T.; Varela, C.; Pretorius, I.S. Yeast′s balancing act between ethanol and glycerol production in low-alcohol wines. Microb. Biotechnol. 2017, 10, 264–278. [Google Scholar] [CrossRef]
- Hohmann, S. An integrated view on a eukaryotic osmoregulation system. Curr. Genet. 2015, 61, 373–382. [Google Scholar] [CrossRef] [PubMed]
- García-Mauricio, J.C.; García-Martínez, T. Role of Yeasts in Sweet Wines. In Book Sweet, Reinforced and Fortified Wines: Grape Biochemistry, Technology and Vinification; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Remize, F.; Cambon, B.; Barnavon, L.; Dequin, S. Glycerol formation during wine fermentation is mainly linked to Gpd1p and is only partially controlled by the HOG pathway. Yeast 2003, 20, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Nissen, T.L.; Kielland Brandt, M.C.; Nielsen, J.; Villadsen, J. Optimization of ethanol production in Saccharomyces cerevisiae by metabolic engineering of the ammonium assimilation. Metab. Eng. 2000, 2, 69–77. [Google Scholar] [CrossRef] [PubMed]





| Flor Yeast | Conventional Yeast | |||||
|---|---|---|---|---|---|---|
| T0 | MF | EF | T0 | MF | EF | |
| Ethanol (% v/v) | 10.23 ± 0.02 | 10.76 ± 0.04 | 11.4 ± 0.1 | 10.23 ± 0.02 | 10.85 ± 0.04 | 11.60 ± 0.03 |
| Acetaldehyde (mg/L) | 87 ± 1 | 132 ± 1 | 87 ± 16 | 87.2 ± 1.1 | 133.9 ± 7.3 | 85.2 ± 0.2 |
| Acetoin (mg/L) | 19 ± 1 | 61 ± 1 | 31 ± 2 | 19.3 ± 1.3 | 127.5 ± 11.8 | 24.5 ± 6.3 |
| 2,3-butanediol (mg/L) | 171 ± 7 | 221 ± 4 | 200 ± 33 | 171 ± 6 | 166 ± 4 | 192 ± 12 |
| Acetic acid (g/L) | 0.23 ± 0.02 | 0.20 ± 0.00 | 0.28 ± 0.02 | 0.23 ± 0.02 | 0.20 ± 0.00 | 0.22 ± 0.00 |
| Glycerol (mg/L) | 4020 ± 656 | 4493 ± 164 | 4227 ± 297 | 4019 ± 655 | 4557 ± 212 | 3513 ± 163 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Jiménez, M.d.C.; García-Martínez, T.; Mauricio, J.C.; Sánchez-León, I.; Puig-Pujol, A.; Moreno, J.; Moreno-García, J. Comparative Study of the Proteins Involved in the Fermentation-Derived Compounds in Two Strains of Saccharomyces cerevisiae during Sparkling Wine Second Fermentation. Microorganisms 2020, 8, 1209. https://doi.org/10.3390/microorganisms8081209
González-Jiménez MdC, García-Martínez T, Mauricio JC, Sánchez-León I, Puig-Pujol A, Moreno J, Moreno-García J. Comparative Study of the Proteins Involved in the Fermentation-Derived Compounds in Two Strains of Saccharomyces cerevisiae during Sparkling Wine Second Fermentation. Microorganisms. 2020; 8(8):1209. https://doi.org/10.3390/microorganisms8081209
Chicago/Turabian StyleGonzález-Jiménez, María del Carmen, Teresa García-Martínez, Juan Carlos Mauricio, Irene Sánchez-León, Anna Puig-Pujol, Juan Moreno, and Jaime Moreno-García. 2020. "Comparative Study of the Proteins Involved in the Fermentation-Derived Compounds in Two Strains of Saccharomyces cerevisiae during Sparkling Wine Second Fermentation" Microorganisms 8, no. 8: 1209. https://doi.org/10.3390/microorganisms8081209
APA StyleGonzález-Jiménez, M. d. C., García-Martínez, T., Mauricio, J. C., Sánchez-León, I., Puig-Pujol, A., Moreno, J., & Moreno-García, J. (2020). Comparative Study of the Proteins Involved in the Fermentation-Derived Compounds in Two Strains of Saccharomyces cerevisiae during Sparkling Wine Second Fermentation. Microorganisms, 8(8), 1209. https://doi.org/10.3390/microorganisms8081209