Assessment of ISO Method 15216 to Quantify Hepatitis E Virus in Bottled Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus Strains
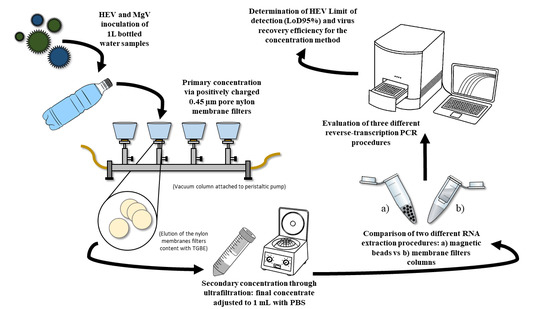
2.2. Detection Limit and Efficiency of the Procedure to Concentrate HEV in Bottled Water according to ISO 15216-1:2017
2.3. RNA Extraction and RT-qPCR Assays
2.4. Statistical Analysis
3. Results and Discussion
Limit of Detection and Efficiency of HEV Concentration Procedure in Bottled Water Based on ISO 15216-1:2017
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Zhao, C.; Qi, Y.; Geng, Y. Hepatitis E virus. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2016; Volume 948, pp. 1–16. [Google Scholar]
- World Health Orgnization. Waterborne Outbreaks of Hepatitis E: Recognition, Investigation and Control; World Health Orgnization: Geneva, Switzerland, 2014; ISBN 9789-2415-07608. [Google Scholar]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernandez Escamez, P.S.; Herman, L.; Koutsoumanis, K.; Lindqvist, R.; Nørrung, B.; et al. Public health risks associated with hepatitis E virus (HEV) as a food-borne pathogen. EFSA J. 2017, 15, e04886. [Google Scholar]
- Fenaux, H.; Chassaing, M.; Berger, S.; Gantzer, C.; Bertrand, I.; Schvoerer, E. Transmission of hepatitis E virus by water: An issue still pending in industrialized countries. Water Res. 2019, 151, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Li, C.; Hagedorn, C.H. Phylogenetic analysis of global hepatitis E virus sequences: Genetic diversity, subtypes and zoonosis. Rev. Med. Virol. 2006, 16, 5–36. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, F.M.; Di Bartolo, I.; Ostanello, F.; Trevisani, M. Hepatitis E Virus: An Emerging Zoonotic and Foodborne Pathogen; Springer: Amsterdam, The Netherlands, 2013; pp. 1–88. [Google Scholar]
- Van der Poel, W. Food and environmental routes of Hepatitis E virus transmission. Curr. Opin. Virol. 2014, 4, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Van der Poel, W.; Rzezutka, A.; Hepatitis, E. Global Water Pathogen Project; Meschke, J.S., Girones, R., Eds.; Michigan State University: East Lansing, Michigan, 2019. [Google Scholar]
- Boccia, D.; Guthmann, J.-P.; Klovstad, H.; Hamid, N.; Tatay, M.; Ciglenecki, I.; Nizou, J.-Y.; Nicand, E.; Guerin, P.J. High Mortality Associated with an Outbreak of Hepatitis E among Displaced Persons in Darfur, Sudan. Clin. Infect. Dis. 2006, 42, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Latorre, L.; Carratala, A.; Rodriguez-Manzano, J.; Calgua, B.; Hundesa, A.; Girones, R. Occurrence of water-borne enteric viruses in two settlements based in Eastern Chad: Analysis of hepatitis E virus, hepatitis A virus and human adenovirus in water sources. J. Water Health 2011, 9, 515–524. [Google Scholar] [CrossRef] [PubMed]
- UNHCR UNHCR steps up measures to rein in Hepatitis E among South Sudanese refugees in Ethiopia. Available online: https://www.unhcr.org/news/ (accessed on 12 May 2020).
- Givens, C.E.; Kolpin, D.W.; Borchardt, M.A.; Duris, J.W.; Moorman, T.B.; Spencer, S.K. Detection of hepatitis E virus and other livestock-related pathogens in Iowa streams. Sci. Total Environ. 2016, 566–567, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Girones, R.; Carratalà, A.; Calgua, B.; Calvo, M.; Rodriguez-Manzano, J.; Emerson, S. Chlorine inactivation of hepatitis e virus and human adenovirus 2 in water. J. Water Health 2014, 12, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Latorre, L.; Gonzales-Gustavson, E.; Hundesa, A.; Sommer, R.; Rosina, G. UV disinfection and flocculation-chlorination sachets to reduce hepatitis E virus in drinking water. Int. J. Hyg. Environ. Health 2016, 219, 405–411. [Google Scholar] [CrossRef] [PubMed]
- ISO 15216-1. ISO 15216-1:2017—Microbiology of the Food Chain—Horizontal Method for Determination of Hepatitis A Virus and Norovirus Using Real-Time RT-PCR—Part 1: Method for Quantification; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- Lowther, J.A.; Bosch, A.; Butot, S.; Ollivier, J.; Mäde, D.; Rutjes, S.A.; Hardouin, G.; Lombard, B.; In’t Veld, P.; Leclercq, A. Validation of EN ISO method 15216—Part 1—Quantification of hepatitis A virus and norovirus in food matrices. Int. J. Food Microbiol. 2019, 288, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Bronzwaer, S.; Kass, G.; Robinson, T.; Tarazona, J.; Verhagen, H.; Verloo, D.; Vrbos, D.; Hugas, M. Food Safety Regulatory Research Needs 2030. EFSA J. 2019, 17, e170622. [Google Scholar]
- Baylis, S.A.; Sakata, H.; Okada, Y.; Mizusawa, S.; Hanschmann, K.-M.O.; Nübling, C.M.; Matsubayashi, K.; Blümel, J.; Mizusawa, S.; Matsubayashi, K.; et al. World Health Organization International Standard to Harmonize Assays for Detection of Hepatitis E Virus RNA. Emerg. Infect. Dis. 2013, 19, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Gerba, C.P.; Betancourt, W.Q.; Kitajima, M.; Rock, C.M. Reducing uncertainty in estimating virus reduction by advanced water treatment processes. Water Res. 2018, 133, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, J.; Eiden, M.; Vina-Rodriguez, A.; Fast, C.; Dremsek, P.; Lange, E.; Ulrich, R.G.; Groschup, M.H. Natural and experimental hepatitis E virus genotype 3-infection in European wild boar is transmissible to domestic pigs. Vet. Res. 2014, 45, 121. [Google Scholar] [CrossRef]
- Jothikumar, N.; Cromeans, T.L.; Robertson, B.H.; Meng, X.J.; Hill, V.R. A broadly reactive one-step real-time RT-PCR assay for rapid and sensitive detection of hepatitis E virus. J. Virol. Methods 2006, 131, 65–71. [Google Scholar] [CrossRef]
- Girón-Callejas, A.; Clark, G.; Irving, W.L.; McClure, C.P. In silico and in vitro interrogation of a widely used HEV RT-qPCR assay for detection of the species Orthohepevirus A. J. Virol. Methods 2015, 214, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, W.; Vásquez-García, A.; Bracho, M.A.; Alcaraz, M.J.; Aznar, R.; Sánchez, G. Hepatitis E virus in lettuce and water samples: A method-comparison study. Int. J. Food Microbiol. 2018, 277, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Wilrich, C.; Wilrich, P.-T. Estimation of the POD function and the LOD of a qualitative microbiological measurement method. J. AOAC Int. 2009, 92, 1763–1772. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Ferrando, E.; Randazzo, W.; Pérez-Cataluña, A.; Sánchez, G. HEV Occurrence in Waste and Drinking Water Treatment Plants. Front. Microbiol. 2020, 10, 2937. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.B.; Simmonds, P.; Izopet, J.; Oliveira-Filho, E.F.; Ulrich, R.G.; Johne, R.; Koenig, M.; Jameel, S.; Harrison, T.J.; Meng, X.-J.; et al. Proposed reference sequences for hepatitis E virus subtypes. J. Gen. Virol. 2016, 97, 537–542. [Google Scholar] [CrossRef] [PubMed]

| Assay | Amplification Region | Primers and Probe | Sequence 5’-3’ | RT-qPCR Conditions | Location * | Reference |
|---|---|---|---|---|---|---|
| RT-qPCR1 | ORF3 | HEV.Fa | GTGCCGGCGGTGGTTTC | RT 50 °C for 30’ | 5296–5377 (81 nt) | [20] |
| Hev.Fb | GTGCCGGCGGTGGTTTCTG | 95 °C for 15’ | ||||
| HEV.R | GCGAAGGGGTTGGTTGGATG | PCR (45x) | ||||
| HEV.P | FAM-TGACMGGGT/ZEN/TGATTCTCAGCC/3IABkFQ | 95 °C for 10’’ | ||||
| 55 °C for 20’’ | ||||||
| 72 °C for 15’’ | ||||||
| RT-qPCR2 | ORF3 | N/A | N/A | RT 45 °C for 10’ | N/A | Ceeram (hepatitis@ceeramTools) |
| 95 °C for 10’ | ||||||
| PCR (40x) | ||||||
| 95 °C for 15’’ | ||||||
| 60 °C for 45’’ | ||||||
| RT-qPCR3 | ORF3 | JVHEVF | GGTGGTTTCTGGGGTGAC | RT 50 °C for 30’ | 5304–5373 (69 nt) | [21,22] |
| JVHEVRmod | AGGGGTTGGTTGGRTGRA | 95 °C for 2’ | ||||
| JVHEVPmod | TGATTCTCAGCCCTTCGC | PCR (45x) | ||||
| 95 °C for 15’’ | ||||||
| 60 °C for 40’’ |
| Extraction Method | RT-qPCR | Levels of Inoculated HEV (IU/L) | LoD95%a (IU/L) | ||
|---|---|---|---|---|---|
| 1 × 105 | 1 × 104 | 1 × 103 | |||
| MN | RT-qPCR1 | 4/4 b | 4/4 | 0/4 | 1.25 × 104 |
| RT-qPCR2 | 4/4 | 4/4 | 0/4 | 1.25 × 104 | |
| RT-qPCR3 | 4/4 | 4/4 | 0/4 | 1.25 × 104 | |
| NS | RT-qPCR1 | 4/4 | 4/4 | 2/4 | 4.26 × 103 |
| RT-qPCR2 | 4/4 | 4/4 | 0/4 | 1.25 × 104 | |
| RT-qPCR3 | 4/4 | 4/4 | 0/4 | 1.25 × 104 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuevas-Ferrando, E.; Martínez-Murcia, A.; Pérez-Cataluña, A.; Sánchez, G.; Randazzo, W. Assessment of ISO Method 15216 to Quantify Hepatitis E Virus in Bottled Water. Microorganisms 2020, 8, 730. https://doi.org/10.3390/microorganisms8050730
Cuevas-Ferrando E, Martínez-Murcia A, Pérez-Cataluña A, Sánchez G, Randazzo W. Assessment of ISO Method 15216 to Quantify Hepatitis E Virus in Bottled Water. Microorganisms. 2020; 8(5):730. https://doi.org/10.3390/microorganisms8050730
Chicago/Turabian StyleCuevas-Ferrando, Enric, Antonio Martínez-Murcia, Alba Pérez-Cataluña, Gloria Sánchez, and Walter Randazzo. 2020. "Assessment of ISO Method 15216 to Quantify Hepatitis E Virus in Bottled Water" Microorganisms 8, no. 5: 730. https://doi.org/10.3390/microorganisms8050730
APA StyleCuevas-Ferrando, E., Martínez-Murcia, A., Pérez-Cataluña, A., Sánchez, G., & Randazzo, W. (2020). Assessment of ISO Method 15216 to Quantify Hepatitis E Virus in Bottled Water. Microorganisms, 8(5), 730. https://doi.org/10.3390/microorganisms8050730