1. Introduction
Diesel fuel is a widely used product of oil refinery. Accidental spills occur regularly during its production, transportation, storage, and exploitation, resulting in environmental contamination [
1,
2,
3,
4,
5]. Although the dominant components of diesel fuel are medium and long chain alkanes [
6,
7,
8], which are low or non-toxic and are considered to be the most available for microbial degradation [
6,
9,
10], it remains one of the highly toxic petroleum products due to the presence of aromatic hydrocarbons and additives in its composition [
11,
12,
13,
14]. The contamination of soils with diesel fuel can lead to the inhibition of plant growth, reduction in soil respiration, increased mortality of soil invertebrates (e.g., nematodes), changes in the physical and mechanical properties of soils, and risks to human health [
12,
14,
15,
16,
17,
18].
Microorganisms play a key role in the degradation of petroleum hydrocarbons in the environment [
13,
19,
20,
21]. Actinomycetes of the genus
Rhodococcus are among the most efficient biodegraders of hydrocarbons. They can utilise various petroleum components, including linear and branched C
2–C
31 alkanes, mono and polycyclic (both light and heavy) hydrocarbons and benzothiophenes, successfully degrade complex hydrocarbon mixtures (crude oil, gasoline, and diesel fuel), are tolerant to various stresses (low and high temperature and pH, high salinity, elevated concentrations of heavy metals and solvents, drought, starvation, and oxidative stress), and are easy to maintain [
22,
23,
24,
25,
26,
27].
The biodegradation of diesel fuel by
Rhodococcus strains has been described in the literature. These bacteria have been shown to degrade 33% to 94% of diesel fuel in soil and water as monocultures or as part of consortia, to maintain high degradation activities at salinities up to 6% NaCl, and to have potential for diesel fuel desulphurisation [
28,
29,
30,
31,
32,
33]. However, the ability of
Rhodococcus bacteria to degrade diesel fuel is not fully understood. Most biodegradation experiments with
Rhodococcus cells have been carried out at low (0.1–2.0%) concentrations of diesel fuel and mesophilic (25–30 °C) temperatures [
28,
29,
30,
32,
34]. The authors of [
31] used diesel fuel concentrations of 3.0% and 4.0% and temperatures between 10 °C and 20 °C. Diesel fuel at concentrations < 3% has rather microbial promoting effects, stimulating the growth of heterotrophic prokaryotes, and leading to an increase in the metabolic activities of microorganisms. The negative effects of diesel fuel on microorganisms are more pronounced at higher concentrations [
17]. At low temperatures, the toxicity of diesel fuel increased. This is related to the reduced evaporation of toxic volatile components, increased viscosity of diesel fuel, and prevented photovolatilisation of hydrocarbons due to the high surface albedo from snow cover [
35]. As a result, hydrocarbons persist in the environment for long periods of time, particularly in cold climates such as the Arctic, Siberia, or Antarctica [
14,
17,
35,
36,
37]. Therefore, the discovery of new strains of
Rhodococcus capable of the biodegradation of diesel under such conditions may be useful for bioremediation.
The effect of nitrogen sources on this process has been carefully assessed [
31]. A less well understood effect is the influence of additional carbon sources on the biodegradation of diesel fuel. Supplementation with NH
4Cl, urea, poultry manure, or soluble organic matter facilitated 1.5–4.0 times the removal of diesel fuel [
29,
31,
35,
36,
37,
38]. It has been shown that more available carbon sources (yeast extract and glucose) accelerated the bioconversion of less available hydrophobic substrates ((–)-isopulegol, diclofenac, and drotaverine hydrochloride) by
Rhodococcus cells, although these substrates could be used as the sole carbon sources [
39,
40,
41].
The effects of externally added biosurfactants (the secondary metabolites, which are produced by bacteria in the presence of hydrophobic compounds to facilitate their utilisation) on diesel fuel biodegradation by
Rhodococcus has not been studied. However, other diesel-fuel-degrading bacteria have been exposed to biosurfactants and the influence of these biomolecules on their degradation capabilities has been demonstrated. In particular, the supplementation of
Achromobacter sp. 4(2010) and
Rahnella sp. EK12 cells with rhamnolipids and saponins resulted in an up to twofold increase in diesel fuel biodegradation [
42,
43]. Positive, neutral, and negative effects of externally added rhamnolipids have been shown on the biodegradation of diesel fuel and its blends with biodiesel by a bacterial consortium, depending on the blend composition [
44].
The aims of this study were as follows: to evaluate the resistance characteristics of Rhodococcus actinomycetes to diesel fuel; to study the biodegradation activities of Rhodococcus towards diesel fuel as the sole growth substrate and in the presence of additional carbon sources (specifically glucose and yeast extract); to identify new, promising biodegraders of diesel fuel, active at high (≥3%) concentrations of this pollutant and at a low (4 °C) temperature; and to assess externally added biosurfactants (glycolipids produced by Rhodococcus cells) in facilitating the diesel fuel biodegradation by these bacteria.
4. Discussion
The toxicity of diesel fuel to 16
Rhodococcus strains was determined in this study. Two strains,
R. erythropolis IEGM 275 and 587, were not included in this analysis because they were already used by our team as degraders of diesel fuel and were known to grow at 2 vol. % of this petroleum product (unpublished data). No species dependency of the resistance of the
Rhodococcus strains studied was observed, making predictions of strain resistance based on biological characteristics of species inapplicable. For example, the most sensitive (IEGM 234, MIC = 0.5 vol. % diesel fuel) and the most resistant (IEGM 231, IEGM 442 and IEGM 1263, MICs = 32.0–64.0 vol. % diesel fuel) strains were found among the representatives of the
R. ruber species with a 128-fold difference between the least and the highest toxic concentrations. Extreme levels of resistance were also found in other species (see
Table 3). However, for sensitive strains, MICs were typically bacteriostatic concentrations and the inhibitory effects of diesel fuel in 3 days should be considered as underestimated. More relevant toxic effects of diesel fuel towards
Rhodococcus cells were registered after 7 days. Comparing the resistance of strains to diesel fuel in the LB after one week, no species specificity was revealed again, but the differences between sensitive and resistant strains were less contrasting. They were no more than eight-fold and looked more like normal variations. Only
R. ruber strain IEGM 234 was 32 times more sensitive to diesel fuel than strain IEGM 231 (see
Table 3), which could be related to the biological specificity of
R. ruber IEGM 234. The theoretical bases for the differences in strain resistance could be the different permeability of the cell wall due to its hydrophobicity or thickness, different time to respond to the toxicant, and specificity of regulatory mechanisms.
It is common practice to determine the toxicity of chemical compounds in rich culture media containing sufficient amounts of nutrients and available growth substrates (e.g., LB, nutrient broth and tryptic soy broth). The inhibition of growth in these media is related to the action of a toxicant and not to a lack of nutrients/elements, a long lag phase, auxotrophic conditions, cell efforts to utilise a difficult substrate, starvation, or any other stress. Consequently, the resistance of
Rhodococcus cells to diesel fuel in the mineral RS medium, where diesel fuel was both a toxicant and a growth substrate, was predictably lower than in the LB, and all inhibitory concentrations were bactericidal. Six out of sixteen strains (
R. erythropolis IEGM 1189,
R. jostii IEGM 60,
R. opacus IEGM 717, IEGM 1157,
R. qingshegii IEGM 1359, and
R. rhodochrous IEGM 1138) almost completely lost their tolerance to diesel fuel in the RS medium, with MIC values of only 0.5–1.0 vol. % (
Table 3). Compared to other species in the mineral medium,
R. ruber seemed to be the most resistant to diesel fuel and was inhibited by no less than 8.0 vol. % diesel fuel (see
Table 3). This was in agreement with our previous works.
R. ruber was more resistant to monoaromatic hydrocarbons and survived long storage (lyophilisation and cryopreservation) better than other
Rhodococcus species [
25,
27]. This species is known to produce carotenoid pigments, and its strains have a bright orange colour. Carotenoids have been reported to protect
Rhodococcus cells from UV irradiation, cold, heat, and oxidative stress, and to be involved in biofilm development [
23,
27]. We suggest that pigments may be involved in the protection of
R. ruber cells from diesel fuel.
The resistance of most
Rhodococus strains to diesel fuel was impressively high and they survived in culture media that could be one third of the hydrophobic toxic substance. Moreover, the cells continued to grow; at least, the red-violet colour in wells with non-inhibitory concentrations of diesel fuel was as bright as that in biotic controls (
Figure S1). Increased tolerance to toxic hydrophobic compounds is typical for
Rhodococcus. Rhodococci survive in the presence of 20–80 vol. % polar (ethanol and butan-1-ol) and non-polar (toluene,
n-hexane and
n-decane) solvents. Their ability to resist the toxic effects of solvents is associated with an increase in the relative surface area, a decrease in the cell wall rigidity upon the contact with solvents, and the involvement of efflux pumps [
49].
Three strains were selected for biodegradation experiments:
R. ruber IEGM 231 and IEGM 442, which showed a high (inhibition concentrations were 8.0–64.0 vol. %) resistance to diesel fuel in both the LB and RS medium, and
Rhodococcus sp. IEGM 1276, which was not very resistant to diesel fuel in the RS medium but showed the highest (MIC = 64.0 vol. %) resistance in the LB (see
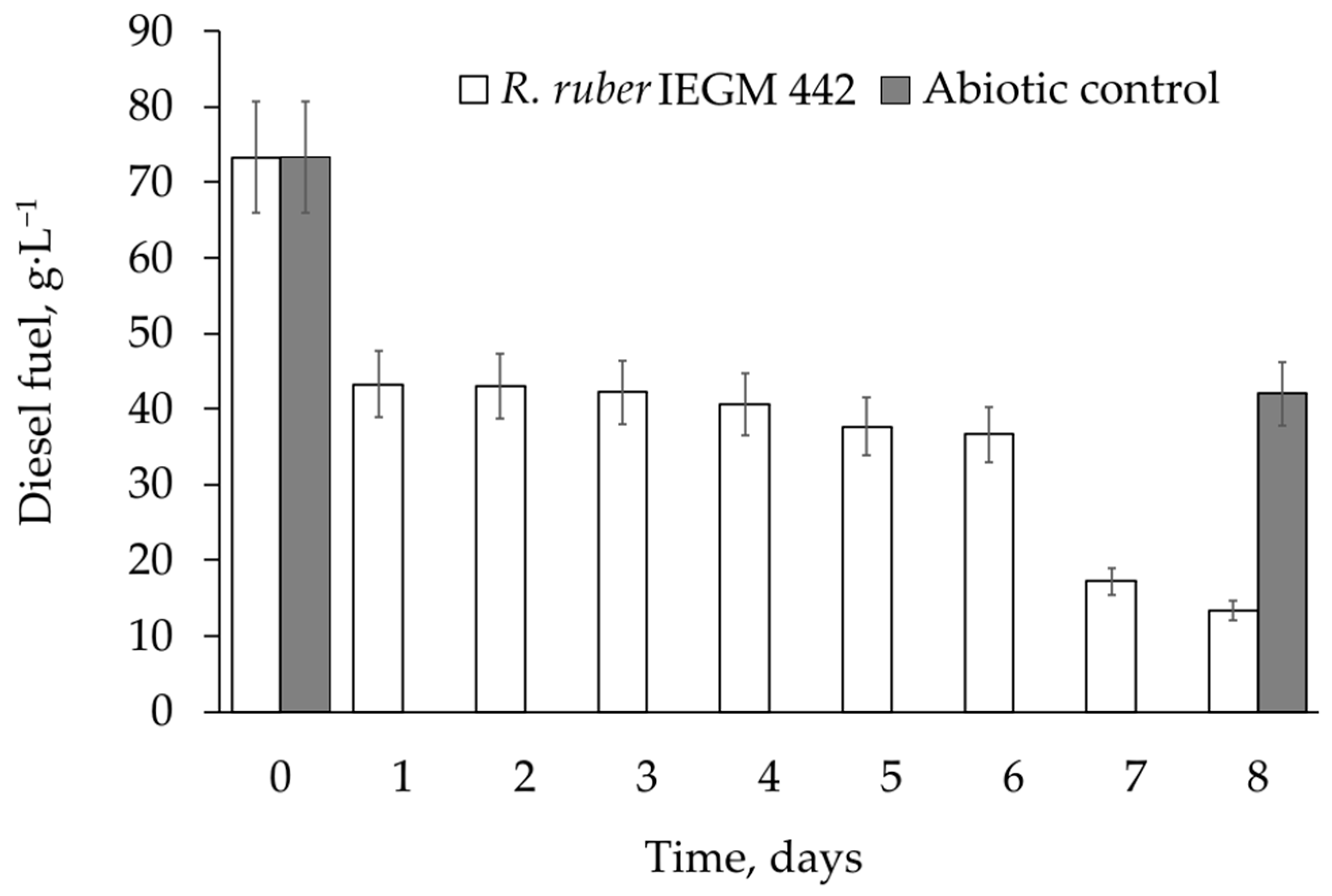
Table 3). These strains degraded from 33% to 44% of 2.0 vol. % diesel fuel at 28°C in 8 days (
Table 4). Combined with the removal of diesel fuel due to evaporation (abiotic losses), the total removal of this petroleum toxicant was between 75% and 86% (see
Table 4), which was similar to published efficiencies [
6,
29,
30,
31,
32,
33]. Particularly promising results for ecobiotechnology were obtained with
R. ruber IEGM 442, which was able to degrade 8.0 vol. % diesel fuel with the same efficiency as at 2.0 vol. %, and
Rhodococcus sp. IEGM 1276, which was able to degrade 59% of 2.0 vol. % diesel fuel at 4 °C. Notably, the last strain was psychrophilic and was two times less active at 28 °C (
Table 4). Thus, both strains were promising for bioremediation:
R. ruber IEGM 442—at a mesophilic temperature (28 °C) but with high diesel fuel contamination (up to 8 vol. %), and
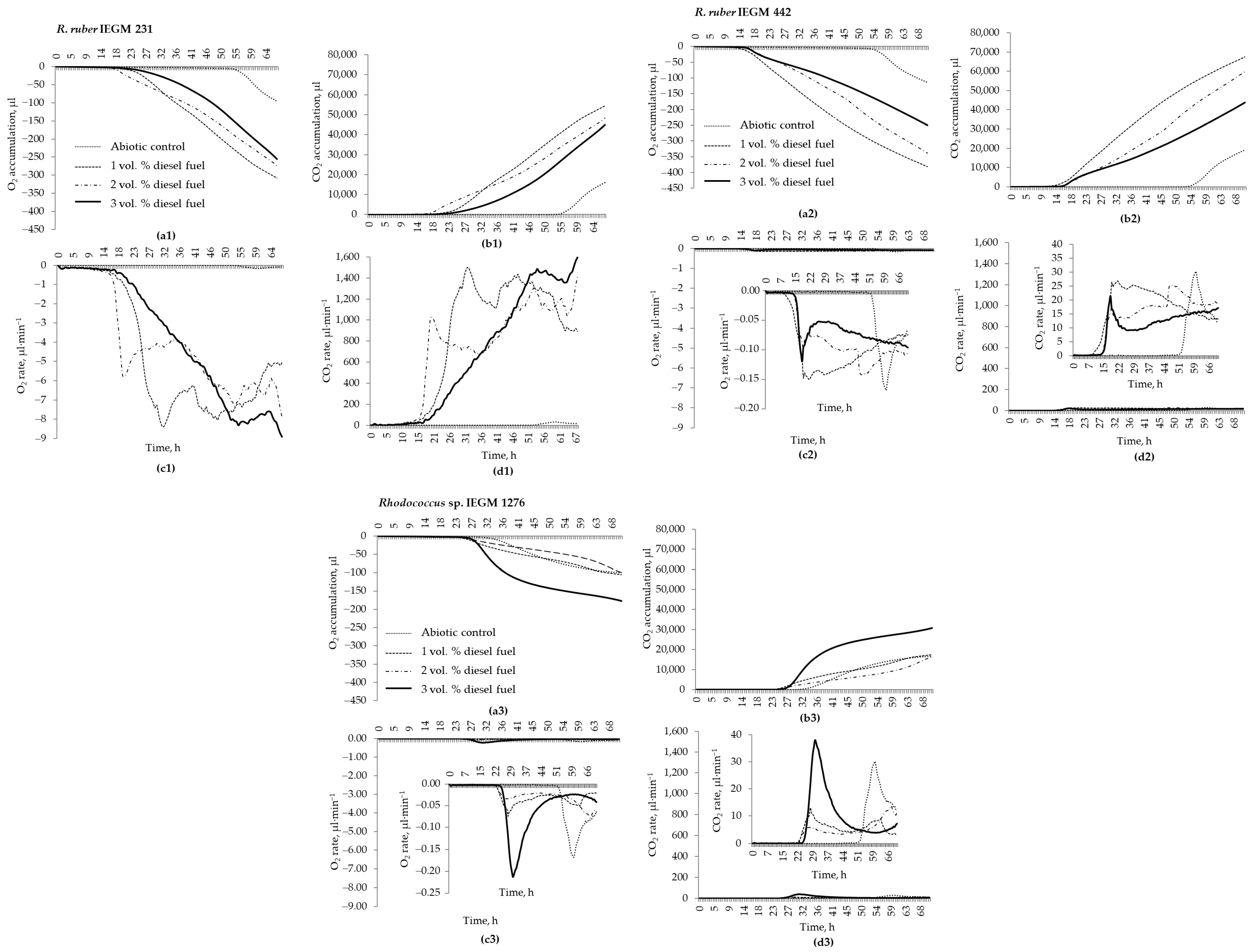
Rhodococcus sp. IEGM 1276—with lower contamination levels (up to 2–3 vol. %) but under cold (4 °C) conditions. Although the respiratory activity of
Rhodococcus sp. IEGM 1276 increased with increasing diesel fuel concentrations from 1.0 vol. % to 3.0 vol. % (
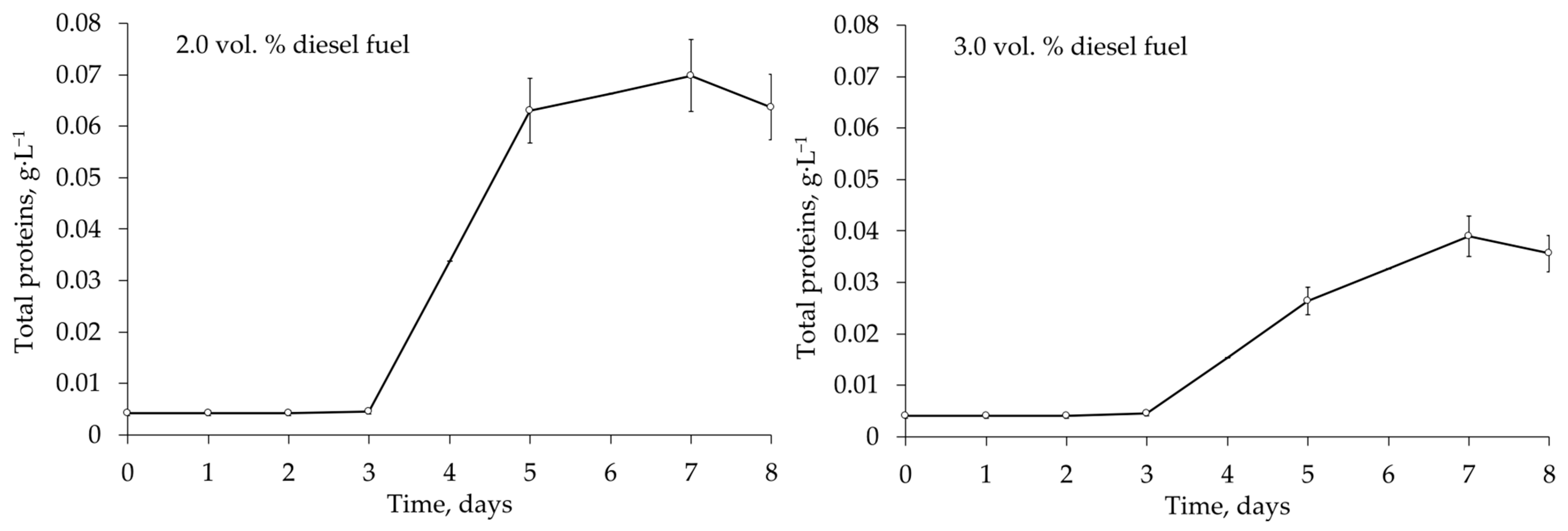
Figure 5), this activity was the lowest among the strains tested (
Figure 5). However, respiration experiments were carried out at of 28 °C, a non-optimal growth temperature for this strain, which may not be relevant for assessing the biodegradation ability of psychrophilic bacteria.
Unfortunately, the complete removal of diesel fuel by selected
Rhodococcus strains was not achieved in this work. This could be due to a short period of biodegradation (8 days) and the gradual accumulation of recalcitrant components of diesel fuel, such as isoprenoids, dominated by phytane and pristane, polyaromatic hydrocarbons, and organosulphur compounds in the growth medium [
6,
11,
33]. From the respirometry and growth kinetics data, we assumed that a stage of active metabolism of diesel fuel was relatively rapid, but biodegradation did not stop after 8 days, when the process was no longer monitored. This active stage, similar in duration, was observed in all experimental variants and resulted, for example, in the same biodegradation percentages for the
R. ruber IEGM 442 cells at 2.0 and 8.0 vol. % diesel fuel (see
Table 4). As shown in
Figure 2, the active biodegradation of 8 vol. % diesel fuel began only after 6 days, while at lower diesel fuel concentrations, the lag phase ranged from 13–24 h to 3 days, after which the cells started to metabolise diesel fuel (
Figure 5 and
Figure 6). It seems that rhodococci would not have enough time to degrade as much diesel fuel at 8.0 vol. % as at 2 vol. %. Thus, assuming that active metabolism occurs within 2–5 days, all available components of diesel fuel could be significantly degraded in 8 days, and similar biodegradation percentages could be achieved at all studied diesel fuel concentrations.
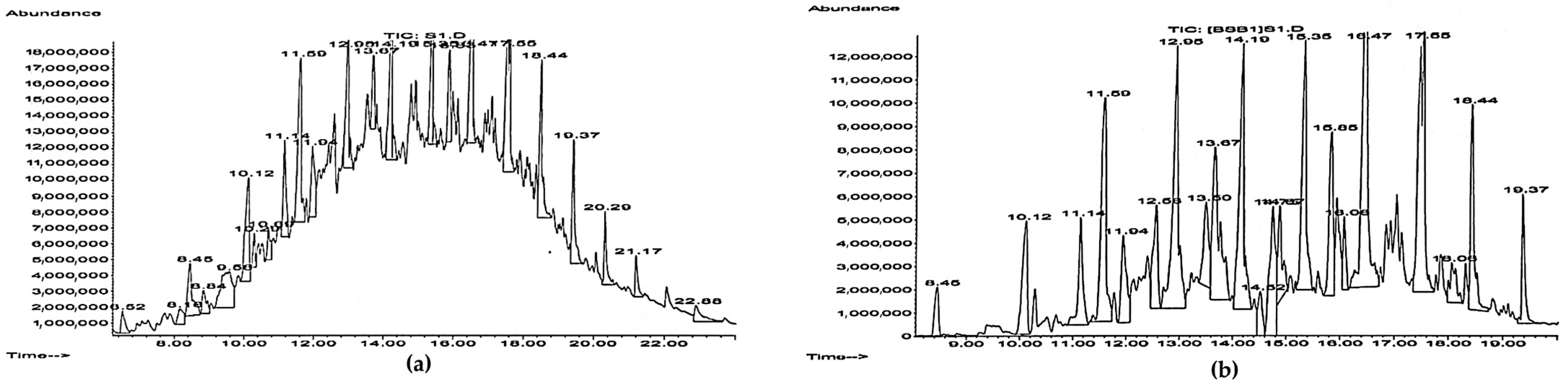
The GC-MS analysis (
Figure 3 and
Figure 4) showed a significant amount of undegraded
n-alkanes and no pronounced changes in representative
n-alkanes (C
15–C
17) during the biodegradation of 8.0 vol. % diesel fuel by
R. ruber IEGM 442. This could be related to the preferential consumption of lower molecular weight alkanes, e.g.,
n-decane or
n-tetradecane, which were not detected in all samples of residual diesel fuel. This finding was partially supported by respiration curves having a zigzag character (
Figure 5), which suggested a sequential biodegradation of individual diesel fuel components by individual
Rhodococus strains. The bacterial preference of certain compounds would depend on their abundance and availability. The latter depends on the mobility of hydrocarbons in NAPLs (non-aqueous phase liquids, e.g., diesel fuel in our study) and their relative solubility in water [
22,
24]. This is partially demonstrated in the experiments with
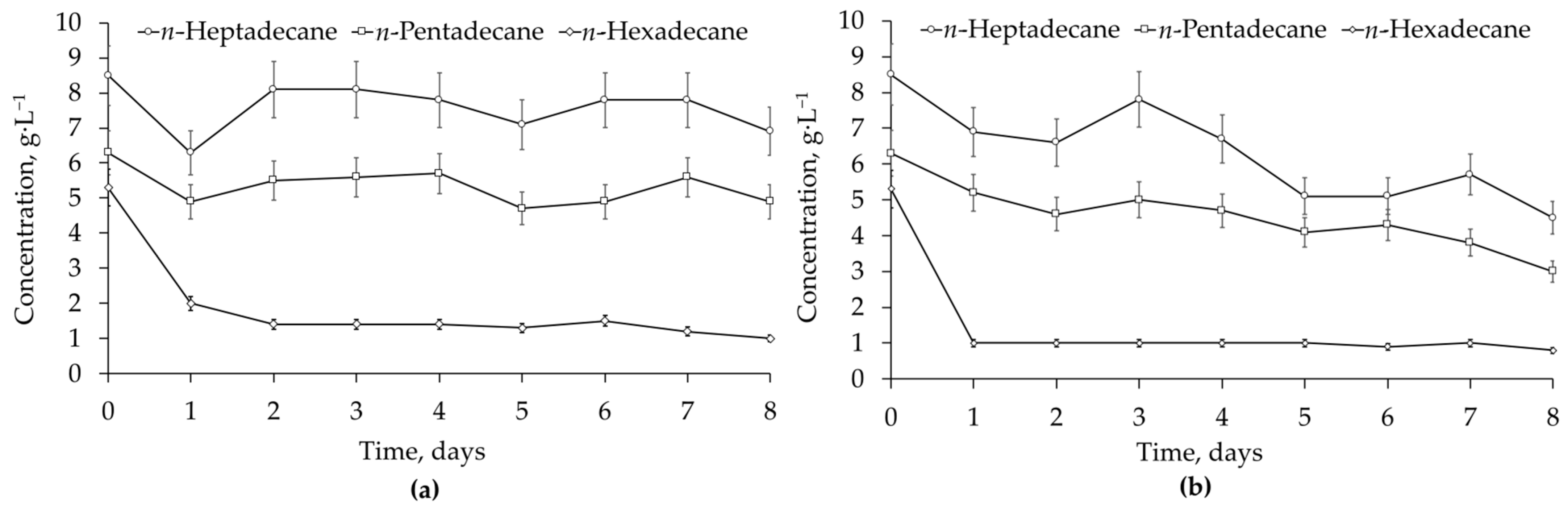
Rhodococcus-biosurfactants. Their presence enhanced the biodegradation of representative alkanes, namely, they accelerated the removal of
n-hexadecane and stimulated the degradation of
n-pentadecane and
n-heptadecane (
Figure 4). Biosurfactants, added at double the CMC value, emulsified and dispersed NAPLs, thereby facilitating their contact with cells and the transportation of emulsified hydrocarbons through the cell wall to the membrane for further oxidation [
22].
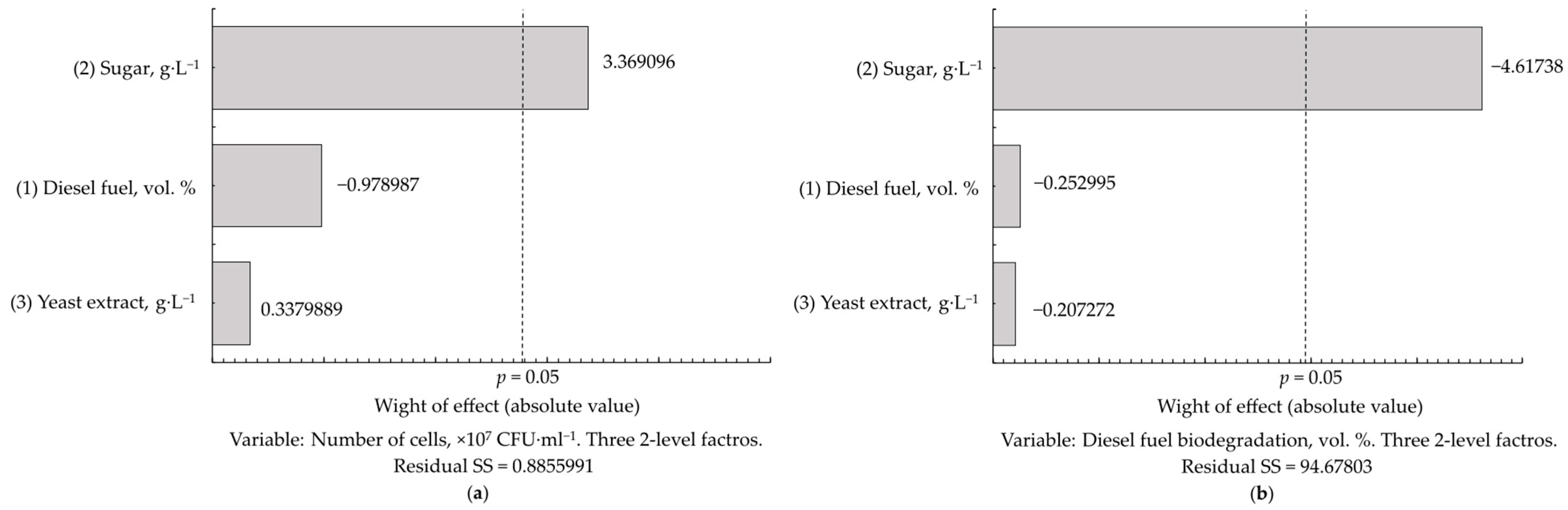
Another important finding of this study is the absence of catabolite repression in the assimilation of diesel fuel, and apparently all other hydrocarbons, by
Rhodococcus. As revealed in co-substrate experiments, diesel fuel can be metabolised simultaneously with carbohydrates (
Table 8). The best growth of
Rhodococcus cells (2.40×10
7 CFU·mL
−1) was obtained with the simultaneous presence of diesel fuel and sugar in the culture medium (
Table 7). The lack of catabolite repression is a useful adaptation of
Rhodococcus, allowing the cell population to be maintained in competition with rapidly growing microbial species and to metabolise a great variety of organic compounds (as growth or co-metabolic substrates, or for the neutralization of their toxic effects). However, an increased (>2.5 g·L
−1) sugar concentration inhibited the biodegradation of diesel fuel by two to three times (
Figure 7a and
Table 8), probably due to the interfering mutual influence of parallel metabolic pathways; the dominance of one of them could probably depend on the ratio between substrate concentrations. This should be taken into account when designing bioremediation processes, and the monitoring of carbohydrate concentrations would be recommended for organic-rich and hydrocarbon-contaminated sites.
5. Conclusions
This study provides new data on the biology of Rhodococcus actinomycetes, biodegraders of diesel fuel, and specific details on the microbial degradation of diesel fuel. A high resistance of Rhodococcus cells to diesel fuel was demonstrated. The growth of Rhodococcus cells can be significantly inhibited on first contact with this petroleum product, but after a period of time (e.g., 6–7 days in this study), they adapt to its toxic effect and grow in the presence of up to 32.0 vol. % diesel fuel. Although resistance is a rather specific feature of a particular strain, R. ruber strains are the most promising in terms of their resistance and degradation activity. According to our results, the inhibitory concentrations of diesel fuel in the RS medium against representatives of R. ruber are 8.0–16.0 vol. %, and some R. ruber strains (e.g., IEGM 442) are able to degrade diesel fuel at these high concentrations. Another important point for bioremediation is the dependence of strain degradation activities on temperature. The strain Rhodococcus sp. IEGM 1276 was most active in degrading diesel fuel at 4 °C. There was no evidence of catabolite repression in the biodegradation of diesel fuel by Rhodococcus, which occurred in the presence of other carbon sources, such as granular sugar and yeast extract. This feature gives Rhodococcus a competitive advantage and they grow better in media containing both diesel fuel and sugar. However, the high concentration of additional carbon sources has a negative effect on the efficiency of diesel fuel biodegradation. In particular, sugar at concentrations > 2.5 g·L−1 inhibits the biodegradation process. The recommended growth conditions are 1.3 g·L−1 of granular sugar and 0.25 g·L−1 of yeast extract, which allowed for the biodegradation of 71–88% diesel fuel.
The strains selected in this work on the basis of their resistance and degradation activities are R. ruber IEGM 231, IEGM 442, and Rhodococccus sp. IEGM 1276. They can be recommended for the bioremediation of diesel fuel-contaminated sites. R. ruber IEGM 442 was best suited for heavy contamination (up to 8.0 vol. % diesel fuel) at a standard temperature (28 °C) and Rhodococcus sp. IEGM 1276 for light contamination (not more than 2.0 vol. % diesel fuel) at a low temperature (4 °C). R. ruber IEGM 231 is suitable for diesel fuel contamination up to 3.0 vol. % at 28 °C. However, it has the highest respiratory rates under these conditions, which is its advantage over R. ruber IEGM 442. Further identification of Rhodococcus sp. IEGM 1276 is required for bioremediation applications. Its draft genome has been sequenced and can be provisionally assigned to Gordonia amicalis (DDBJ/ENA/GenBank acc. no JAPWIL010000001–JAPWIL010000081, accessed 15 November 2024). We follow the polyphasic taxonomy approach and intend to harmonise phenotypic and genotypic data before the final strain identification, which is the subject of future research.
The use of externally added
Rhodococcus-biosurfactants at a concentration of 1.4 g·L
−1 has proven to be efficient for hydrocarbon biodegradation. Although no statistically significant effects of the biosurfactants on the total removal of diesel fuel were determined, the biodegradation of individual
n-alkanes (
n-pentadecane,
n-hexadecane, and
n-heptadecane) was stimulated, which may accelerate the bioremediation process. No additional contamination with
n-hexadecane was detected when biosurfactants were added, although they were produced by the
R. ruber IEGM 231 cells grown with 3 vol. %
n-hexadecane (see
Section 2.1). The total removal of more than 71% of diesel fuel in 8 days can be expected when using selected strains and biosurfactants under the optimised growth conditions.
This work requires further investigation, focusing on field trials, the development of consortia, and the construction of stable biocatalysts. Selected
Rhodococcus strains appear to be environmentally compatible, with no possible adverse effects on indigenous microorganisms.
Rhodococcus actinomycetes are ubiquitous in biotopes throughout the world, are dominant in hydrocarbon-contaminated ecosystems, are tolerant to fluctuations in abiotic factors, and their abundance returns to background levels after the end of the intensive phase of bioremediation, without harmful effects on microbial communities [
23,
27,
50].