Gastrointestinal Shedding of Rubulaviruses from Egyptian Rousette Bats: Temporal Dynamics and Spillover Implications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site, Ethical Clearances and Biosafety Considerations
2.2. Sample Collection and Viral Screening
2.3. Bioinformatic Analyses
2.4. Temporal Excretion Analysis
3. Results
3.1. Rubulavirus Positivity, Sequence Identity and Phylogeny
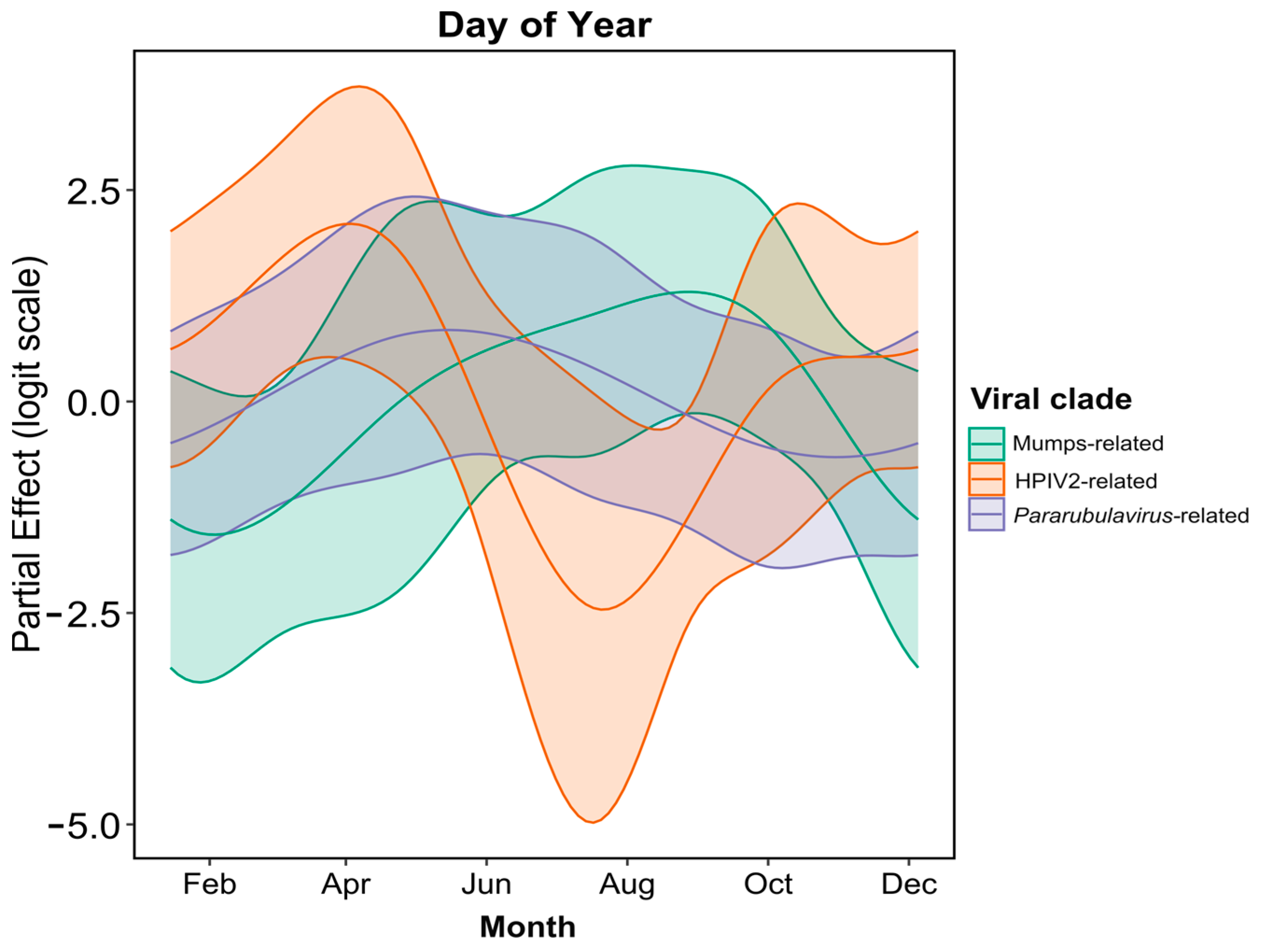
3.2. Temporal Analysis of Rubulavirus Excretion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, K.; Selleck, P.; Hooper, P.; Hyatt, A.; Gould, A.; Gleeson, L.; Westbury, H.; Hiley, L.; Selvey, L.; Rodwell, B. A morbillivirus that caused fatal disease in horses and humans. Science 1995, 268, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.B.; Goh, K.J.; Wong, K.T.; Kamarulzaman, A.; Tan, P.S.; Ksiazek, T.G.; Zaki, S.R.; Paul, G.; Lam, S.K.; Tan, C.T. Fatal encephalitis due to Nipah virus among pig-farmers in Malaysia. Lancet 1999, 354, 1257–1259. [Google Scholar] [CrossRef] [PubMed]
- Rima, B.; Balkema-Buschmann, A.; Dundon, W.G.; Duprex, P.; Easton, A.; Fouchier, R.; Kurath, G.; Lamb, R.; Lee, B.; Rota, P.; et al. ICTV Virus Taxonomy Profile: Paramyxoviridae. J. Gen. Virol. 2019, 100, 1593–1594. [Google Scholar] [CrossRef] [PubMed]
- Pavri, K.M.; Singh, K.R.; Hollinger, F.B. Isolation of a new parainfluenza virus from a frugivorous bat, Rousettus leschenaultii, collected at Poona, India. Am. J. Trop. Med. Hyg. 1971, 20, 125–130. [Google Scholar] [CrossRef]
- Drexler, J.F.; Corman, V.M.; Müller, M.A.; Maganga, G.D.; Vallo, P.; Binger, T.; Gloza-Rausch, F.; Rasche, A. Bats host major mammalian paramyxoviruses. PLoS ONE 2012, 7, e1001332. [Google Scholar] [CrossRef]
- Chua, K.B.; Wang, L.F.; Lam, S.K.; Crameri, G.; Yu, M.; Wise, T.; Boyle, D.; Hyatt, A.D.; Eaton, B.T. Tioman virus, a novel paramyxovirus isolated from fruit bats in Malaysia. Virology 2001, 283, 215–229. [Google Scholar] [CrossRef]
- Lau, S.K.; Woo, P.C.; Wong, B.H.; Wong, A.Y.; Tsoi, H.W.; Wang, M.; Lee, P.; Xu, H.; Poon, R.W.; Guo, R.; et al. Identification and complete genome analysis of three novel paramyxoviruses, Tuhoko virus 1, 2, and 3, in fruit bats from China. Virology 2010, 404, 106–116. [Google Scholar] [CrossRef]
- Baker, K.S.; Todd, S.; Marsh, G.; Fernandez-Loras, A.; Suu-Ire, R.; Wood, J.L.N.; Wang, L.F.; Murcia, P.R.; Cunningham, A.A. Co-circulation of diverse paramyxoviruses in an urban African fruit bat population. J. Gen. Virol. 2012, 93, 850–856. [Google Scholar] [CrossRef]
- Baker, K.S.; Todd, S.; Marsh, G.A.; Crameri, G.; Barr, J.; Kamins, A.O.; Peel, A.J.; Yu, M.; Hayman, D.T.; Nadjm, B.; et al. Novel, potentially zoonotic paramyxoviruses from the African straw-colored fruit bat Eidolon helvum. J. Virol. 2013, 87, 1348–1358. [Google Scholar] [CrossRef]
- Baker, K.S.; Tachedjian, M.; Barr, J.; Marsh, G.A.; Todd, S.; Crameri, G.; Crameri, S.; Smith, I.; Holmes, C.E.G.; Suu-Ire, R.; et al. Achimota Pararubulavirus 3: A New Bat-Derived Paramyxovirus of the Genus Pararubulavirus. Viruses 2020, 12, 1236. [Google Scholar] [CrossRef]
- Mortlock, M.; Dietrich, M.; Weyer, J.; Paweska, J.T.; Markotter, W. Co-Circulation and Excretion Dynamics of Diverse Rubula- and Related Viruses in Egyptian Rousette Bats from South Africa. Viruses 2019, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Conrardy, C.; Tao, Y.; Kuzmin, I.V.; Niezgoda, M.; Agwanda, B.; Breiman, R.F.; Anderson, L.J.; Rupprecht, C.E.; Tong, S. Molecular detection of adenoviruses, rhabdoviruses, and paramyxoviruses in bats from Kenya. Am. J. Trop. Med. Hyg. 2014, 91, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Paskey, A.C.; Lim, X.F.; Ng, J.H.J.; Rice, G.K.; Chia, W.N.; Philipson, C.W.; Foo, R.; Cer, R.Z.; Long, K.A.; Lueder, M.R.; et al. Genomic Characterization of a Relative of Mumps Virus in Lesser Dawn Bats of Southeast Asia. Viruses 2023, 15, 659. [Google Scholar] [CrossRef]
- Pawełczyk, M.; Kowalski, M.L. The Role of Human Parainfluenza Virus Infections in the Immunopathology of the Respiratory Tract. Curr. Allergy Asthma Rep. 2017, 17, 16. [Google Scholar] [CrossRef]
- Philbey, A.W.; Kirkland, P.D.; Ross, A.D.; Davis, R.J.; Gleeson, A.B.; Love, R.J.; Daniels, P.W.; Gould, A.R.; Hyatt, A.D. An Apparently New Virus (Family Paramyxoviridae) Infectious for Pigs, Humans, and Fruit Bats. Emerg. Infect. Dis. 1998, 4, 269–271. [Google Scholar] [CrossRef]
- Albariño, C.G.; Foltzer, M.; Towner, J.S.; Rowe, L.A.; Campbell, S.; Jaramillo, C.M.; Bird, B.H.; Reeder, D.M.; Vodzak, M.E.; Rota, P. Novel Paramyxovirus Associated with Severe Acute Febrile Disease, South Sudan and Uganda, 2012. Emerg. Infect. Dis. 2014, 20, 211–216. [Google Scholar] [CrossRef]
- Amman, B.R.; Albariño, C.G.; Bird, B.H.; Nyakarahuka, L.; Sealy, T.K.; Balinandi, S.; Schuh, A.J.; Campbell, S.M.; Ströher, U.; Jones, M.E.; et al. A Recently Discovered Pathogenic Paramyxovirus, Sosuga Virus, is Present in Rousettus aegyptiacus Fruit Bats at Multiple Locations in Uganda. J. Wildl. Dis. 2015, 51, 774–779. [Google Scholar] [CrossRef]
- Amman, B.R.; Schuh, A.J.; Sealy, T.K.; Spengler, J.R.; Welch, S.R.; Kirejczyk, S.G.M.; Albariño, C.G.; Nichol, S.T.; Towner, J.S. Experimental Infection of Egyptian Rousette Bats (Rousettus aegyptiacus) with Sosuga Virus Demonstrates Potential Transmission Routes for a Bat-Borne Human Pathogenic Paramyxovirus. PLoS Negl. Trop. Dis. 2020, 14, e0008092. [Google Scholar] [CrossRef]
- Amman, B.R.; Koroma, A.H.; Schuh, A.J.; Conteh, I.; Sealy, T.K.; Foday, I.; Johnny, J.; Bakarr, I.A.; Whitmer, S.L.M.; Wright, E.A. Sosuga Virus Detected in Egyptian Rousette Bats (Rousettus aegyptiacus) in Sierra Leone. Viruses 2024, 16, 648. [Google Scholar] [CrossRef]
- Geldenhuys, M.; Ross, N.; Dietrich, M.; de Vries, J.L.; Mortlock, M.; Epstein, J.H.; Weyer, J.; Pawęska, J.T.; Markotter, W. Viral Maintenance and Excretion Dynamics of Coronaviruses within an Egyptian Rousette Fruit Bat Maternal Colony: Considerations for Spillover. Sci. Rep. 2023, 13, 15829. [Google Scholar] [CrossRef]
- Bouckaert, R.; Heled, J.; Kühnert, D.; Vaughan, T.; Wu, C.-H.; Xie, D.; Suchard, M.A.; Rambaut, A.; Drummond, A.J. BEAST 2: A Software Platform for Bayesian Evolutionary Analysis. PLoS Comput. Biol. 2014, 10, e1003537. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- R Core Team 2023. R: A Language and Environment for Statistical Computing. Available online: https://cran.rstudio.com/ (accessed on 11 November 2024).
- Cohen, L.E.; Fagre, A.C.; Chen, B.; Carlson, C.J.; Becker, D.J. Coronavirus Sampling and Surveillance in Bats from 1996–2019: A Systematic Review and Meta-Analysis. Nat. Microbiol. 2023, 8, 1176–1186. [Google Scholar] [CrossRef]
- Schatz, J.; Ohlendorf, B.; Busse, P.; Pelz, G.; Dolch, D.; Teubner, J.; Encarnação, J.A.; Mühle, R.U.; Fischer, M.; Hoffmann, B.; et al. Twenty Years of Active Bat Rabies Surveillance in Germany: A Detailed Analysis and Future Perspectives. Epidemiol. Infect. 2014, 142, 1155–1166. [Google Scholar] [CrossRef]
- Schatz, J.; Freuling, C.M.; Auer, E.; Goharriz, H.; Harbusch, C.; Johnson, N.; Kaipf, I.; Mettenleiter, T.C.; Mühldorfer, K.; Mühle, R.-U.; et al. Enhanced Passive Bat Rabies Surveillance in Indigenous Bat Species from Germany—A Retrospective Study. PLoS Negl. Trop. Dis. 2014, 8, e2835. [Google Scholar] [CrossRef]
- Begeman, L.; Kooi, E.A.; van Weezep, E.; Van De Bildt, M.W.; Reusken, C.B.; Lina, P.H.; Koopmans, M.P.; van den Brand, J.M.; Kuiken, T. Faeces as a Novel Material to Estimate Lyssavirus Prevalence in Bat Populations. Zoonoses Public Health 2020, 67, 198–202. [Google Scholar] [CrossRef]
- Jacobsen, N.H.G.; du Plessis, E. Observations on the Ecology and Biology of the Cape Fruit Bat Rousettus aegyptiacus leachi in the Eastern Transvaal. S. Afr. J. Sci. 1976, 72, 270–273. [Google Scholar]
- Mortlock, M.; Geldenhuys, M.; Dietrich, M.; Epstein, J.H.; Weyer, J.; Pawęska, J.T.; Markotter, W. Seasonal Shedding Patterns of Diverse Henipavirus-Related Paramyxoviruses in Egyptian Rousette Bats. Sci. Rep. 2021, 11, 24262. [Google Scholar] [CrossRef]
- Pawęska, J.T.; Jansen van Vuren, P.; Kemp, A.; Storm, N.; Grobbelaar, A.A.; Wiley, M.R.; Palacios, G.; Markotter, W. Marburg Virus Infection in Egyptian Rousette Bats, South Africa, 2013–2014. Emerg. Infect. Dis. 2018, 24, 1134–1137. [Google Scholar] [CrossRef]
- Wood, M.R.; de Vries, J.L.; Epstein, J.H. Variations in Small-Scale Movements of Rousettus aegyptiacus, a Marburg Virus Reservoir across a Seasonal Gradient. Front. Zool. 2023, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Penzhorn, B.; Rautenbach, I. Reproduction of the Egyptian Fruit Bat (Rousettus aegyptiacus) in the Southern Tropics. S. Afr. J. Wildl. Res. 1988, 18, 88–92. [Google Scholar]
- Peel, A.J.; Wells, K.; Giles, J.; Boyd, V.; Burroughs, A.; Edson, D.; Crameri, G.; Baker, M.L.; Field, H.; Wang, L.F.; et al. Synchronous Shedding of Multiple Bat Paramyxoviruses Coincides with Peak Periods of Hendra Virus Spillover. Emerg. Microbes Infect. 2019, 8, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Pantin-Jackwood, M.J.; Costa-Hurtado, M.; Miller, P.J.; Afonso, C.L.; Spackman, E.; Kapczynski, D.R.; Shepherd, E.; Smith, D.; Swayne, D.E. Experimental Co-Infections of Domestic Ducks with a Virulent Newcastle Disease Virus and Low or Highly Pathogenic Avian Influenza Viruses. Vet. Microbiol. 2015, 177, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Amman, B.R.; Carroll, S.A.; Reed, Z.D.; Sealy, T.K.; Balinandi, S.; Swanepoel, R.; Kemp, A.; Erickson, B.R.; Comer, J.A.; Campbell, S.; et al. Seasonal Pulses of Marburg Virus Circulation in Juvenile Rousettus aegyptiacus Bats Coincide with Periods of Increased Risk of Human Infection. PLoS Pathog. 2012, 8, e1002877. [Google Scholar] [CrossRef]
- Krüger, N.; Sauder, C.; Hüttl, S.; Papies, J.; Voigt, K.; Herrler, G.; Hardes, K.; Steinmetzer, T.; Örvell, C.; Drexler, J.F.; et al. Entry, Replication, Immune Evasion, and Neurotoxicity of Synthetically Engineered Bat-Borne Mumps Virus. Cell Rep. 2018, 25, 312–320.e7. [Google Scholar] [CrossRef]
- Dalton, M.; Sanderson, B.; Robinson, L.J.; Homer, C.S.; Pomat, W.; Danchin, M.; Vaccher, S. Impact of COVID-19 on Routine Childhood Immunisations in Low-and Middle-Income Countries: A Scoping Review. PLoS Glob. Public Health 2023, 3, e0002268. [Google Scholar] [CrossRef]
- Wang, D.; Yang, X.; Ren, Z.; Hu, B.; Zhao, H.; Yang, K.; Shi, P.; Zhang, Z.; Feng, Q.; Nawenja, C.V.; et al. Substantial Viral Diversity in Bats and Rodents from East Africa: Insights into Evolution, Recombination, and Cocirculation. Microbiome 2024, 12, 72. [Google Scholar] [CrossRef]
- O’Shea, T.J.; Cryan, P.M.; Cunningham, A.A.; Fooks, A.R.; Hayman, D.T.; Luis, A.D.; Peel, A.J.; Plowright, R.K.; Wood, J.L. Bat Flight and Zoonotic Viruses. Emerg. Infect. Dis. 2014, 20, 741–745. [Google Scholar] [CrossRef]
- Mutere, F.A. Breeding Cycles in Tropical Bats in Uganda. J. Appl. Ecol. 1968, 5, 8P–9P. [Google Scholar]



| Representative Virus Sequence (Number of Detections) | Additional Positive Samples * | Highest Similarity (%) to Classified Rubulavirus Species # | |||
|---|---|---|---|---|---|
| Virus | Nucleotide | Virus | Amino Acid | ||
| UPE64_R_aeg_fecal_pooled | - | ThkPV1 | 73% | AchPV2 | 85% |
| UPE68_R_aeg_fecal_pooled (3) | UPE071; UPE074 | ThkPV1/AchPV2 | 73% | AchPV2 | 82% |
| UPE151_R_aeg_fecal_pooled (4) | UPE212; UPE224; UPE236 | AchPV2 | 80% | ThkPV2/AchPV1 | 82% |
| UPE154_R_aeg_fecal_pooled (4) | UPE214; UPE2112; UPE2340 | ThkPV1/AchPV2 | 70% | ThkPV2 | 82% |
| UPE155_R_aeg_fecal_pooled (2) | UPE110 | AchPV2/HPIV4a | 73% | AchPV1 | 76% |
| UPE207_R_aeg_fecal_pooled (8) | UPE210; UPE294; UPE1483; UPE1551; UPE1556; UPE1558; UPE1667 | HPIV2 | 80% | HPIV2 | 90% |
| UPE229_R_aeg_fecal_pooled | - | AchPV2 | 75% | ThkPV1/2 | 81% |
| UPE243_R_aeg_fecal_pooled | - | ThkPV2/SOSV | 73% | SOSV | 79% |
| UPE310_R_aeg_fecal_pooled (10) | UPE386; UPE776; UPE780; UPE781; UPE1568; UPE1570; UPE1582; UPE1586; UPE1745 | HPIV2 | 75% | HPIV2 | 95% |
| UPE696_R_aeg_fecal_pooled (11) | UPE541; UPE697; UPE698; UPE750; UPE751; UPE1749; UPE1875; UPE2116; UPE2446; UPE2585 | HPIV2/SV41 | 78% | HPIV2 | 94% |
| UPE1001_R_aeg_fecal_pooled | - | HPIV2 | 78% | HPIV2 | 92% |
| UPE1007_R_aeg_fecal_pooled | - | HPIV4a | 77% | SOSV | 82% |
| UPE1016_R_aeg_fecal_pooled | - | AchPV1 | 76% | ThkPV2 | 82% |
| UPE1024_R_aeg_fecal_pooled | - | ThkPV2 | 76% | AchPV1 | 82% |
| UPE1669_R_aeg_fecal_pooled | - | PIV5 | 76% | HPIV2 | 87% |
| UPE1764_R_aeg_fecal_pooled | - | HPIV2 | 74% | HPIV2 | 90% |
| UPE1839_R_aeg_fecal_pooled (4) | UPE563; UPE1845; UPE1872 | MuV | 76% | MuV | 97% |
| UPE1889_R_aeg_fecal_pooled | - | HPIV2 | 76% | HPIV2 | 87% |
| UPE2137_R_aeg_fecal_pooled | - | MuV | 75% | MuV | 95% |
| UPE2151_R_aeg_fecal_pooled | - | HPIV2/SV41 | 79% | HPIV2 | 92% |
| UPE2428_R_aeg_fecal_pooled | - | HPIV2 | 77% | HPIV2 | 90% |
| Viral Cluster Analyzed | Chi-Square | p-Value # |
|---|---|---|
| Within-year variation | ||
| Mumps-related | 5.243 | 0.026279 * |
| Human parainfluenza 2-related | 29.108 | 3.26 × 10−7 *** |
| Pararubulavirus-related | 14.894 | 3.72 × 10−4 *** |
| Variation across the study period | ||
| Mumps-related | 0.616 | 0.432806 |
| Human parainfluenza 2-related | 0.002 | 0.969013 |
| Pararubulavirus-related | 21.155 | 3.01 × 10−5 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muvengi, T.S.; Mortlock, M.; Kain, M.P.; Markotter, W. Gastrointestinal Shedding of Rubulaviruses from Egyptian Rousette Bats: Temporal Dynamics and Spillover Implications. Microorganisms 2024, 12, 2505. https://doi.org/10.3390/microorganisms12122505
Muvengi TS, Mortlock M, Kain MP, Markotter W. Gastrointestinal Shedding of Rubulaviruses from Egyptian Rousette Bats: Temporal Dynamics and Spillover Implications. Microorganisms. 2024; 12(12):2505. https://doi.org/10.3390/microorganisms12122505
Chicago/Turabian StyleMuvengi, Tauya S., Marinda Mortlock, Morgan P. Kain, and Wanda Markotter. 2024. "Gastrointestinal Shedding of Rubulaviruses from Egyptian Rousette Bats: Temporal Dynamics and Spillover Implications" Microorganisms 12, no. 12: 2505. https://doi.org/10.3390/microorganisms12122505
APA StyleMuvengi, T. S., Mortlock, M., Kain, M. P., & Markotter, W. (2024). Gastrointestinal Shedding of Rubulaviruses from Egyptian Rousette Bats: Temporal Dynamics and Spillover Implications. Microorganisms, 12(12), 2505. https://doi.org/10.3390/microorganisms12122505






