Comparative Proteomic Analysis of Exosomes Derived from Patients Infected with Non-Tuberculous Mycobacterium and Mycobacterium tuberculosis
Abstract
:1. Introduction
2. Methods
2.1. Grouping and Ethics Statement
2.2. Isolation of Exosomes
2.3. Transmission Electron Microscopy Analysis
2.4. Nanoparticle Size Analysis
2.5. Nanoflow Analysis
2.6. Extraction of Exosomal Protein
2.7. Nano-LC-MS/MS
2.8. Protein Identification and Quantification
2.9. Statistical Analysis
3. Results
3.1. Characterization of Plasma Exosomes
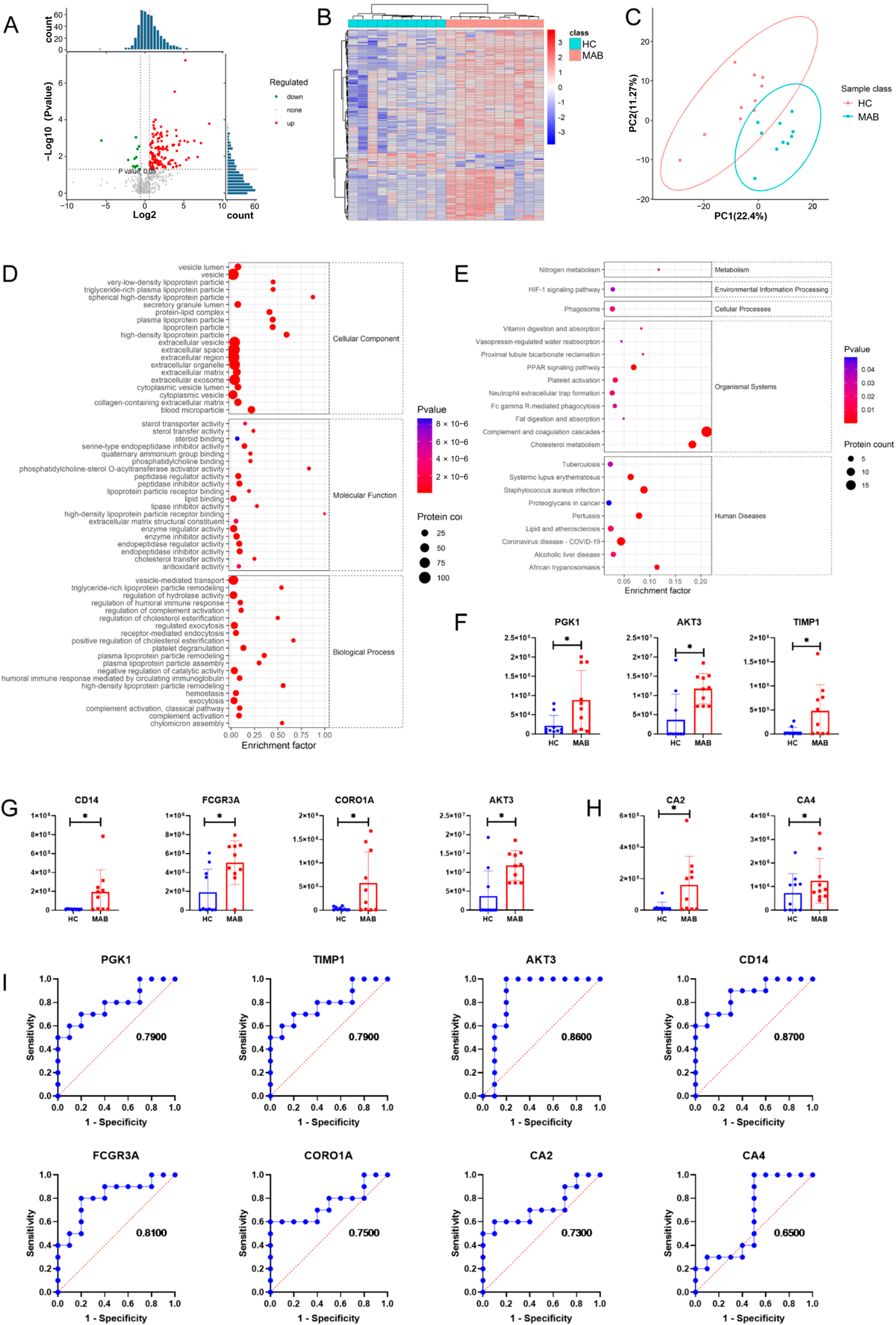
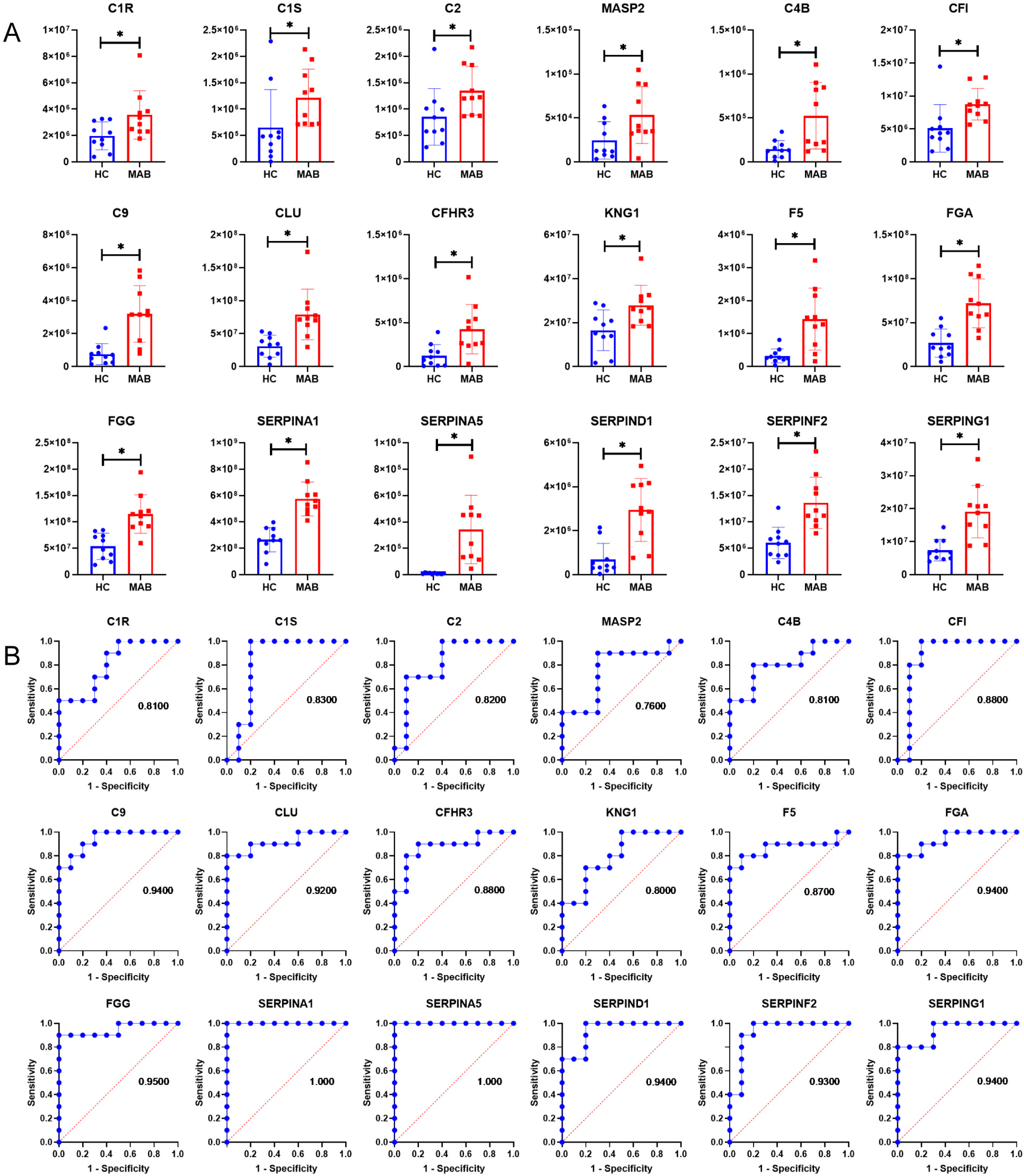
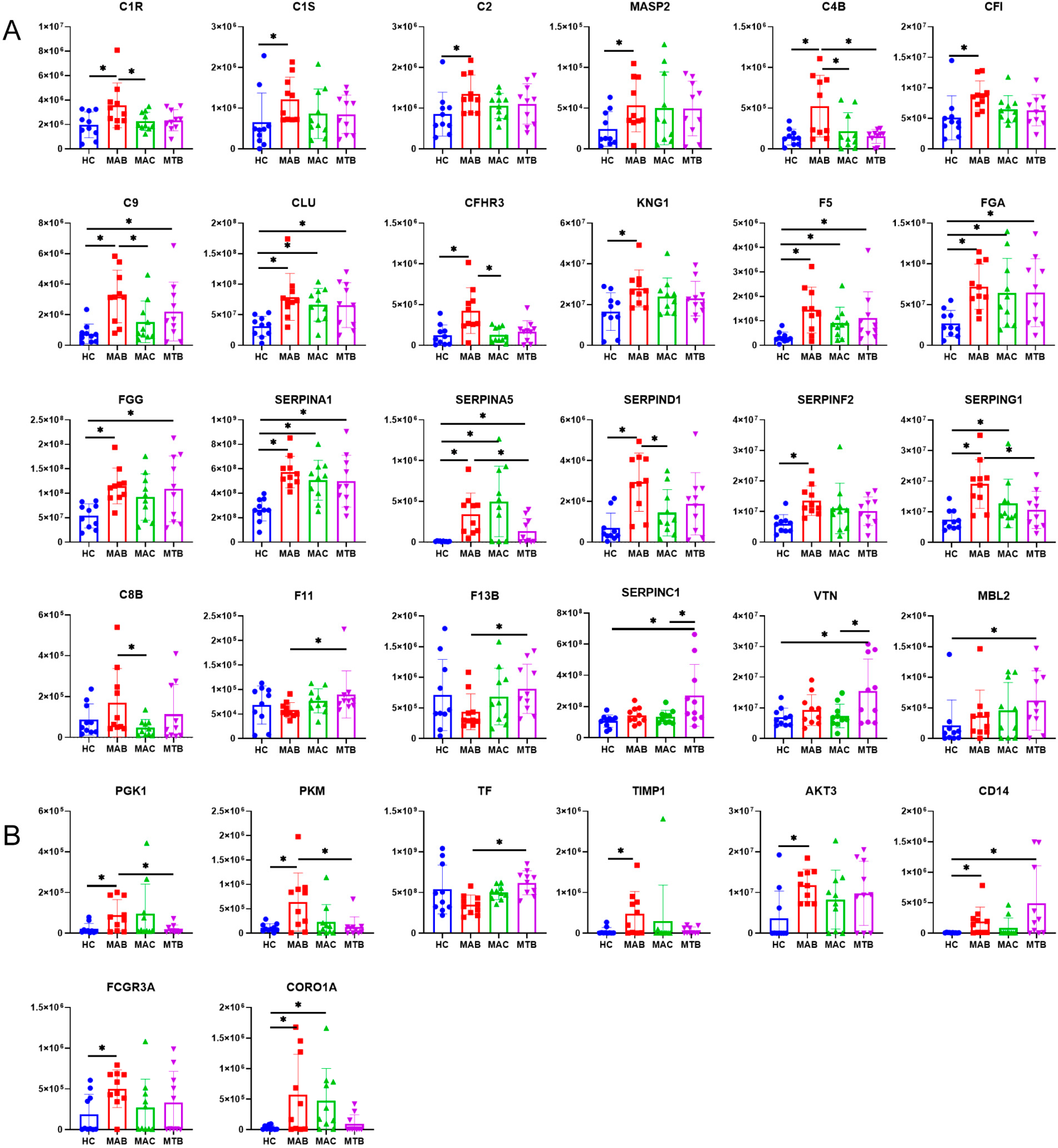
3.2. Up-Regulation of Complement Proteins in Plasma Exosomes from NTM-Infected Patients
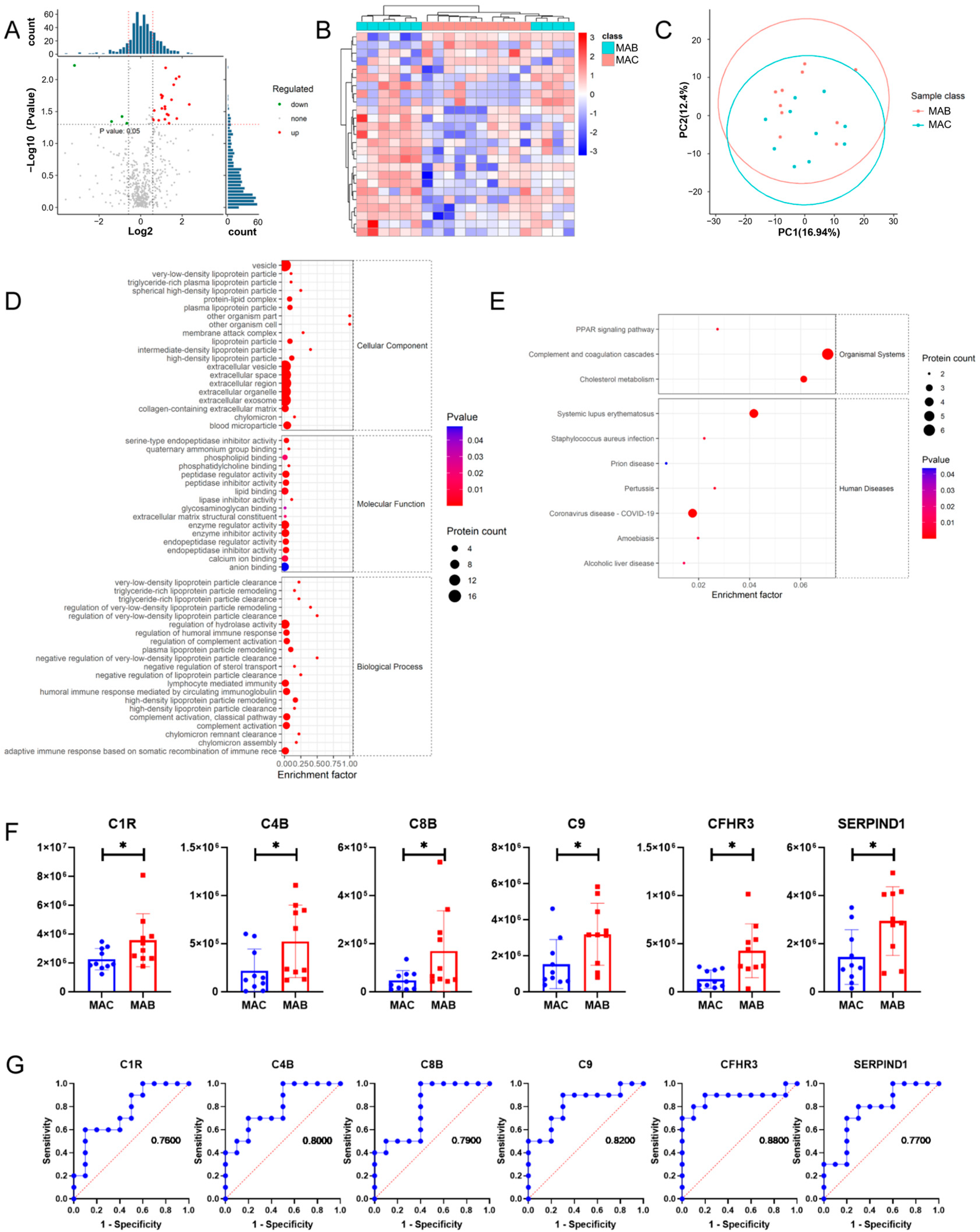
3.3. More Up-Regulated Complement Proteins in MAB-Infected Patients than MAC
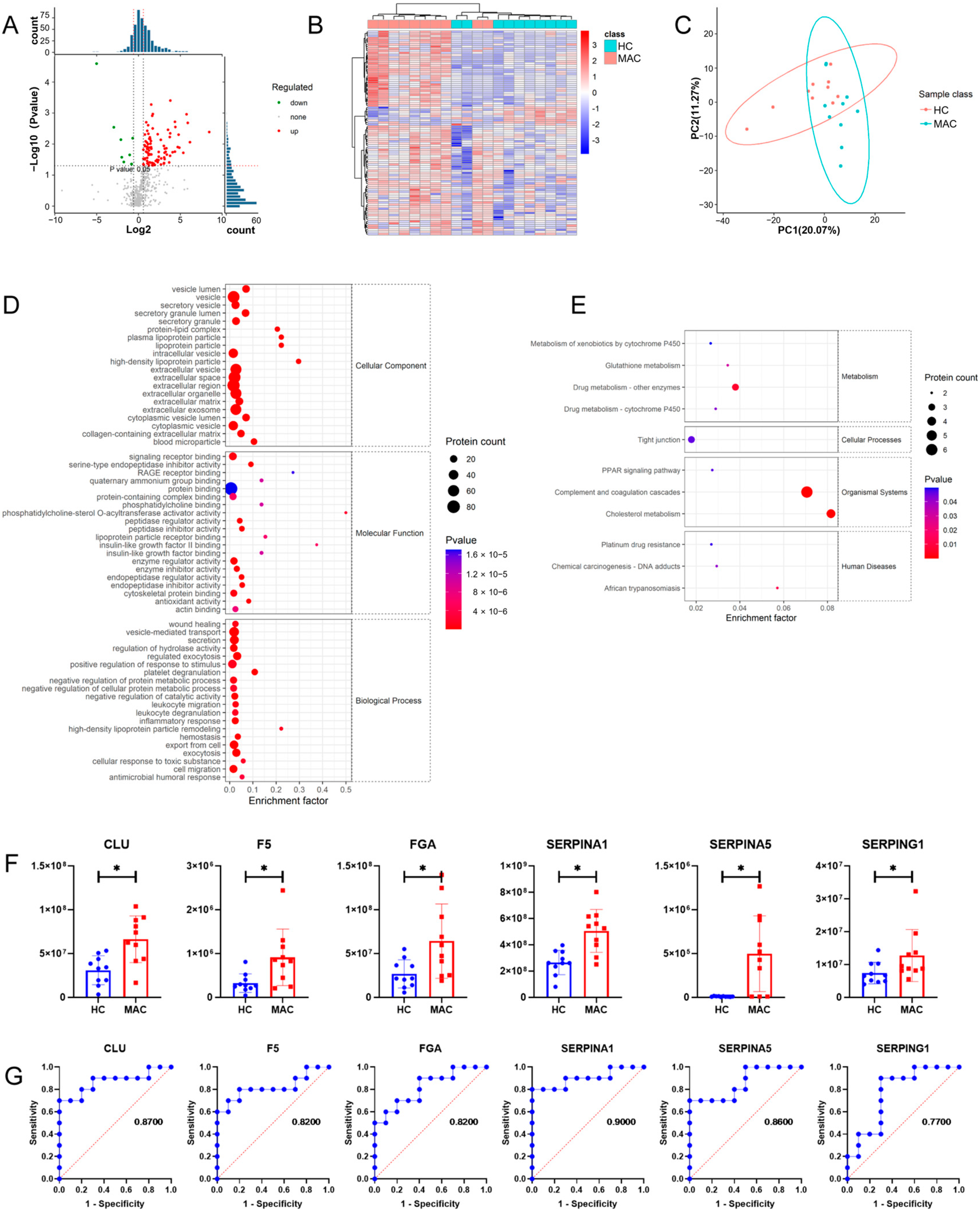
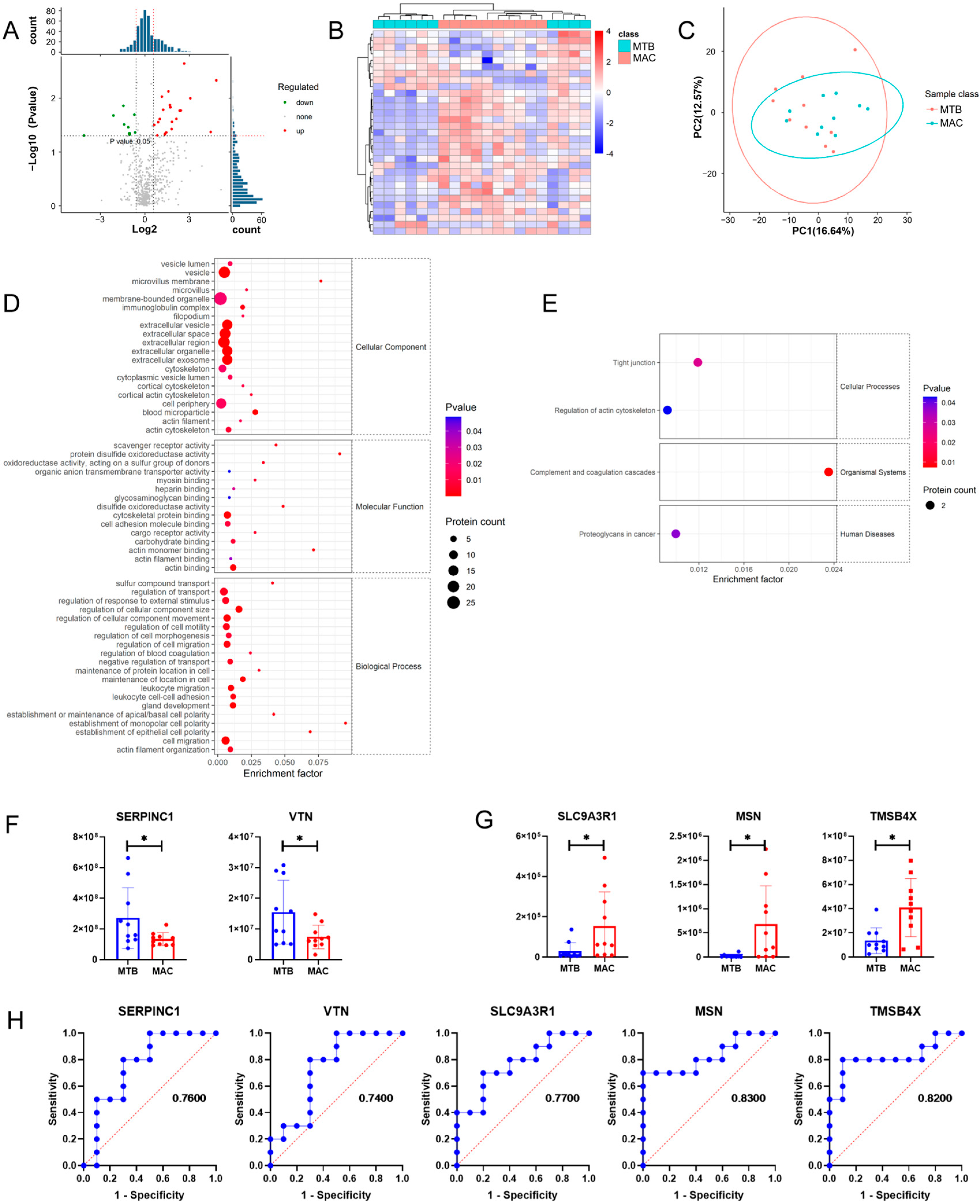
3.4. Comparison of Plasma Exosomes between NTM-Infected Patients and MTB-Infected Patients
3.5. Comprehensive Analysis of Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stout, J.E.; Koh, W.J.; Yew, W.W. Update on pulmonary disease due to non-tuberculous mycobacteria. Int. J. Infect. Dis. 2016, 45, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Prevots, D.R.; Marras, T.K. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: A review. Clin. Chest Med. 2015, 36, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J., Jr.; Andrejak, C.; Bottger, E.C.; Brozek, J.; Griffith, D.E.; Guglielmetti, L.; et al. Treatment of nontuberculous mycobacterial pulmonary disease: An official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur. Respir. J. 2020, 71, e1–e36. [Google Scholar] [CrossRef]
- Rajendran, P.; Padmapriyadarsini, C.; Mondal, R. Nontuberculous mycobacterium: An emerging pathogen: Indian perspective. Int. J. Mycobacteriol. 2021, 10, 217–227. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Li, C.; Yu, Y.; Yi, Y.; Wang, J.; Chen, D. Exosome-Induced Regulation in Inflammatory Bowel Disease. Front. Immunol. 2019, 10, 1464. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yao, Y.; Chen, X.; Wu, J.; Gu, T.; Tang, X. Host derived exosomes-pathogens interactions: Potential functions of exosomes in pathogen infection. Biomed. Pharmacother. 2018, 108, 1451–1459. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Zhang, M.; Xie, Y.; Li, S.; Ye, X.; Jiang, Y.; Tang, L.; Wang, J. Proteomics Analysis of Exosomes From Patients With Active Tuberculosis Reveals Infection Profiles and Potential Biomarkers. Front. Microbiol. 2021, 12, 800807. [Google Scholar] [CrossRef]
- Sun, Y.F.; Pi, J.; Xu, J.F. Emerging Role of Exosomes in Tuberculosis: From Immunity Regulations to Vaccine and Immunotherapy. Front. Immunol. 2021, 12, 628973. [Google Scholar] [CrossRef]
- Kim, C.J.; Kim, N.H.; Song, K.H.; Choe, P.G.; Kim, E.S.; Park, S.W.; Kim, H.B.; Kim, N.J.; Kim, E.C.; Park, W.B.; et al. Differentiating rapid- and slow-growing mycobacteria by difference in time to growth detection in liquid media. Diagn. Microbiol. Infect. Dis. 2013, 75, 73–76. [Google Scholar] [CrossRef]
- World Health, O.; International Union Against, T.; Lung, D.; Royal Netherlands Tuberculosis, A. Revised international definitions in tuberculosis control. Int. J. Tuberc. Lung Dis. 2001, 5, 213–215. [Google Scholar]
- Subedi, P.; Schneider, M.; Philipp, J.; Azimzadeh, O.; Metzger, F.; Moertl, S.; Atkinson, M.J.; Tapio, S. Comparison of methods to isolate proteins from extracellular vesicles for mass spectrometry-based proteomic analyses. Anal. Biochem. 2019, 584, 113390. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Mei, Z.; Zeng, C.; Liu, S. metaX: A flexible and comprehensive software for processing metabolomics data. BMC Bioinform. 2017, 18, 183. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, H.; An, J.; Zhang, X.; Wang, W.; Wang, Y.; Xue, Z.; Li, S.; Pang, Y. Transition between Mycobacterium tuberculosis and nontuberculous mycobacteria in recurrent “tuberculosis” patients. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 1127–1132. [Google Scholar] [CrossRef]
- Saptawati, L.; Primaningtyas, W.; Dirgahayu, P.; Sutanto, Y.S.; Wasita, B.; Suryawati, B.; Nuryastuti, T.; Probandari, A. Characteristics of clinical isolates of nontuberculous mycobacteria in Java-Indonesia: A multicenter study. PLoS Negl. Trop. Dis. 2022, 16, e0011007. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Tang, L.; Garcia, R.C. Extracellular Vesicles in Mycobacterial Infections: Their Potential as Molecule Transfer Vectors. Front. Immunol. 2019, 10, 1929. [Google Scholar] [CrossRef] [PubMed]
- Rahman, J.; Singh, P.; Merle, N.S.; Niyonzima, N.; Kemper, C. Complement’s favourite organelle-Mitochondria? Br. J. Pharmacol. 2021, 178, 2771–2785. [Google Scholar] [CrossRef] [PubMed]
- Ling, M.; Murali, M. Analysis of the Complement System in the Clinical Immunology Laboratory. Clin. Lab. Med. 2019, 39, 579–590. [Google Scholar] [CrossRef]
- Conigliaro, P.; Triggianese, P.; Ballanti, E.; Perricone, C.; Perricone, R.; Chimenti, M.S. Complement, infection, and autoimmunity. Curr. Opin. Rheumatol. 2019, 31, 532–541. [Google Scholar] [CrossRef]
- Jagatia, H.; Tsolaki, A.G. The Role of Complement System and the Immune Response to Tuberculosis Infection. Medicina 2021, 57, 84. [Google Scholar] [CrossRef]
- Debreczeni, M.L.; Nemeth, Z.; Kajdacsi, E.; Schwaner, E.; Mako, V.; Masszi, A.; Doleschall, Z.; Rigo, J.; Walter, F.R.; Deli, M.A.; et al. MASP-1 Increases Endothelial Permeability. Front. Immunol. 2019, 10, 991. [Google Scholar] [CrossRef] [PubMed]
- Lenhart-Pendergrass, P.M.; Malcolm, K.C.; Wheeler, E.; Rysavy, N.M.; Poch, K.; Caceres, S.; Calhoun, K.M.; Martiniano, S.L.; Nick, J.A. Deficient Complement Opsonization Impairs Mycobacterium avium Killing by Neutrophils in Cystic Fibrosis. Microbiol. Spectr. 2023, 11, e0327922. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, R.; Sutherland, J.S.; Goletti, D.; de Paus, R.A.; Dijkstra, D.J.; van Moorsel, C.H.M.; Veltkamp, M.; Vestjens, S.M.T.; Bos, W.J.W.; Petrone, L.; et al. Expression and production of the SERPING1-encoded endogenous complement regulator C1-inhibitor in multiple cohorts of tuberculosis patients. Mol. Immunol. 2020, 120, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, R.; Sutherland, J.S.; Goletti, D.; de Paus, R.A.; van Moorsel, C.H.M.; Veltkamp, M.; Vestjens, S.M.T.; Bos, W.J.W.; Petrone, L.; Del Nonno, F.; et al. Complement Component C1q as Serum Biomarker to Detect Active Tuberculosis. Front. Immunol. 2018, 9, 2427. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. HIF-1 and mechanisms of hypoxia sensing. Curr. Opin. Cell Biol. 2001, 13, 167–171. [Google Scholar] [CrossRef]
- Jiang, Q.; Qiu, Y.; Kurland, I.J.; Drlica, K.; Subbian, S.; Tyagi, S.; Shi, L. Glutamine Is Required for M1-like Polarization of Macrophages in Response to Mycobacterium tuberculosis Infection. mBio 2022, 13, e0127422. [Google Scholar] [CrossRef]
- Shi, L.; Eugenin, E.A.; Subbian, S. Immunometabolism in Tuberculosis. Front. Immunol. 2016, 7, 150. [Google Scholar] [CrossRef]
- Li, Q.; Xie, Y.; Cui, Z.; Huang, H.; Yang, C.; Yuan, B.; Shen, P.; Shi, C. Activation of hypoxia-inducible factor 1 (Hif-1) enhanced bactericidal effects of macrophages to Mycobacterium tuberculosis. Tuberculosis 2021, 126, 102044. [Google Scholar] [CrossRef] [PubMed]
- Teran, G.; Li, H.; Catrina, S.B.; Liu, R.; Brighenti, S.; Zheng, X.; Grunler, J.; Nylen, S.; Carow, B.; Rottenberg, M.E. High Glucose and Carbonyl Stress Impair HIF-1-Regulated Responses and the Control of Mycobacterium tuberculosis in Macrophages. mBio 2022, 13, e0108622. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Z.; Ren, S.; Zhao, H. Exosomes derived from mycobacterium tuberculosis-infected MSCs induce a pro-inflammatory response of macrophages. Aging 2021, 13, 11595–11609. [Google Scholar] [CrossRef]
- Kaushik, A.C.; Wu, Q.; Lin, L.; Li, H.; Zhao, L.; Wen, Z.; Song, Y.; Wu, Q.; Wang, J.; Guo, X.; et al. Exosomal ncRNAs profiling of mycobacterial infection identified miRNA-185-5p as a novel biomarker for tuberculosis. Brief. Bioinform. 2021, 22, bbab210. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zheng, X.; Ma, J.; Gu, J.; Sha, W. Comparative Proteomic Analysis of Exosomes Derived from Patients Infected with Non-Tuberculous Mycobacterium and Mycobacterium tuberculosis. Microorganisms 2023, 11, 2334. https://doi.org/10.3390/microorganisms11092334
Wang L, Zheng X, Ma J, Gu J, Sha W. Comparative Proteomic Analysis of Exosomes Derived from Patients Infected with Non-Tuberculous Mycobacterium and Mycobacterium tuberculosis. Microorganisms. 2023; 11(9):2334. https://doi.org/10.3390/microorganisms11092334
Chicago/Turabian StyleWang, Li, Xubin Zheng, Jun Ma, Jin Gu, and Wei Sha. 2023. "Comparative Proteomic Analysis of Exosomes Derived from Patients Infected with Non-Tuberculous Mycobacterium and Mycobacterium tuberculosis" Microorganisms 11, no. 9: 2334. https://doi.org/10.3390/microorganisms11092334
APA StyleWang, L., Zheng, X., Ma, J., Gu, J., & Sha, W. (2023). Comparative Proteomic Analysis of Exosomes Derived from Patients Infected with Non-Tuberculous Mycobacterium and Mycobacterium tuberculosis. Microorganisms, 11(9), 2334. https://doi.org/10.3390/microorganisms11092334





