Temperature Shift Experiments Suggest That Metabolic Impairment and Enhanced Rates of Photorespiration Decrease Organic Acid Levels in Soybean Leaflets Exposed to Supra-Optimal Growth Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Quantification of Leaf Components
2.3. Statistical Comparisons
3. Results
3.1. Growth Temperature Effects on Foliar Organic Acids in Ambient CO2 Treatment
| Compound | 36 °C | 36 to 28 °C | 28 °C | 28 to 36 °C | p |
|---|---|---|---|---|---|
| mg g−1 DW | |||||
| 39 Pa CO2 | |||||
| malonate | 4.69 ± 0.57 | 4.54 ± 0.39 | 6.80 ± 0.57 | 6.12 ± 0.51 | * |
| glycerate | 1.15 ± 0.12 | 1.25 ± 0.11 | 1.33 ± 0.16 | 1.13 ± 0.15 | ns |
| fumarate | 0.26 ± 0.07 | 0.32 ± 0.08 | 0.36 ± 0.06 | 0.29 ± 0.05 | * |
| succinate | 0.15 ± 0.03 | 0.16 ± 0.02 | 0.27 ± 0.04 | 0.21 ± 0.03 | * |
| malate | 5.30 ± 0.43 | 6.31 ± 0.76 | 10.66 + 0.85 | 9.15 ± 0.75 | * |
| citrate | 2.17 ± 0.42 | 4.79 ± 0.53 | 13.02 ± 1.08 | 7.92 ± 0.85 | ** |
| 70 Pa CO2 | |||||
| malonate | 7.12 ± 0.86 | 4.86 ± 0.33 | 6.98 ± 0.66 | 6.91 ± 0.74 | ns |
| glycerate | 0.67 ± 0.05 | 0.66 ± 0.06 | 0.76 ±0 .06 | 0.92 ± 0.80 | * |
| fumarate | 0.65 ± 0.10 | 0.56 ± 0.10 | 0.66 ± 0.10 | 0.80 ± 0.09 | ns |
| succinate | 0.13 ± 0.01 | 0.14 ± 0.01 | 0.21 ± 0.20 | 0.18 ± 0.02 | * |
| malate | 7.88 ± 0.72 | 7.33 ± 0.50 | 12.12 ± 1.30 | 10.79 ± 1.10 | ** |
| citrate | 4.99 ± 0.88 | 5.12 ± 0.69 | 11.52 ± 1.79 | 7.97 ± 1.23 | ** |
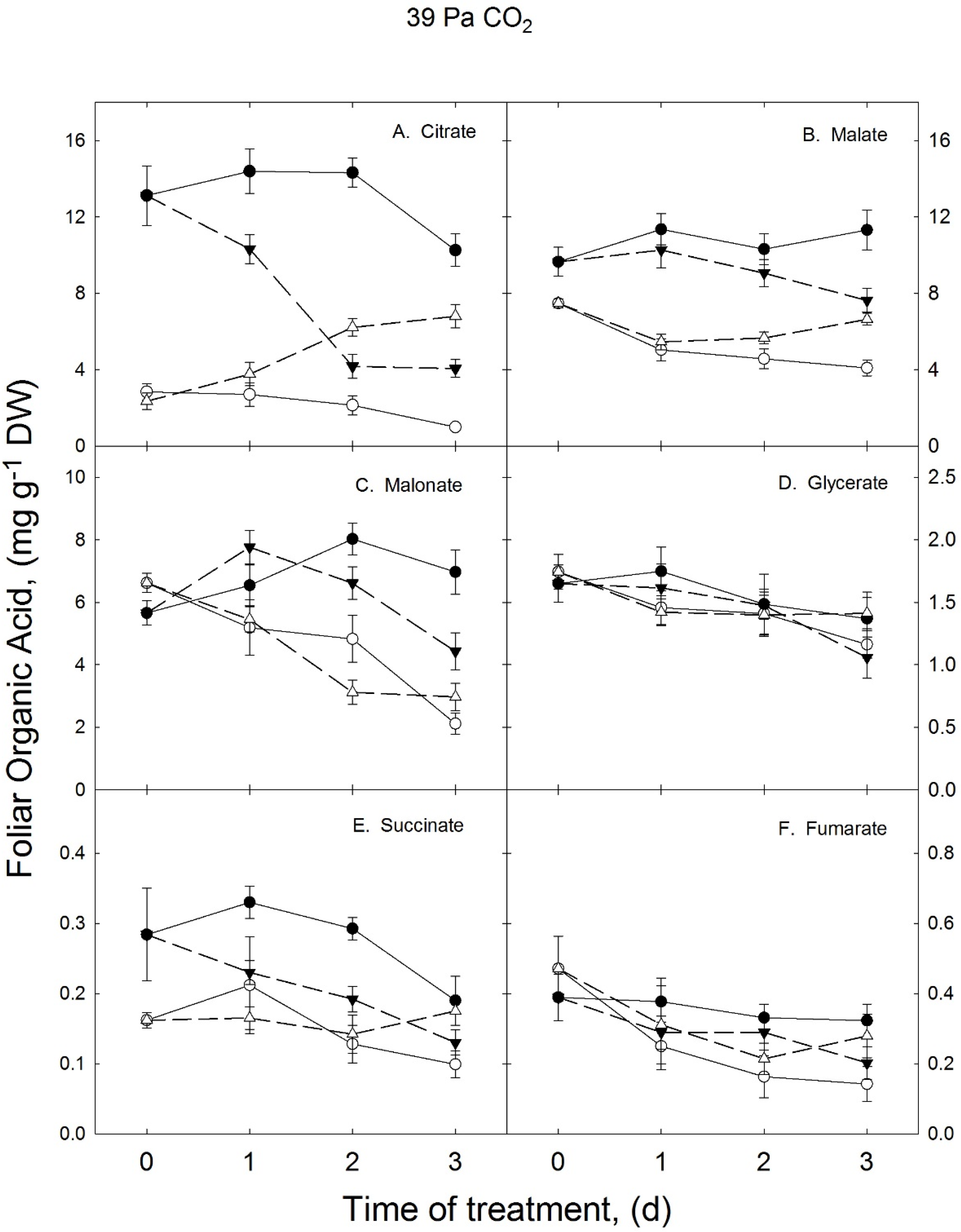
3.2. Growth Temperature Effects on Foliar Organic Acids in the Elevated CO2 Treatment
| Temperature | Malonate | Glycerate | Fumarate | Succinate | Malate | Citrate |
|---|---|---|---|---|---|---|
| p | ||||||
| 28/20 °C | ns | ** | ** | ns | ** | * |
| 36/30 °C | ns | ** | ** | ns | ns | ** |
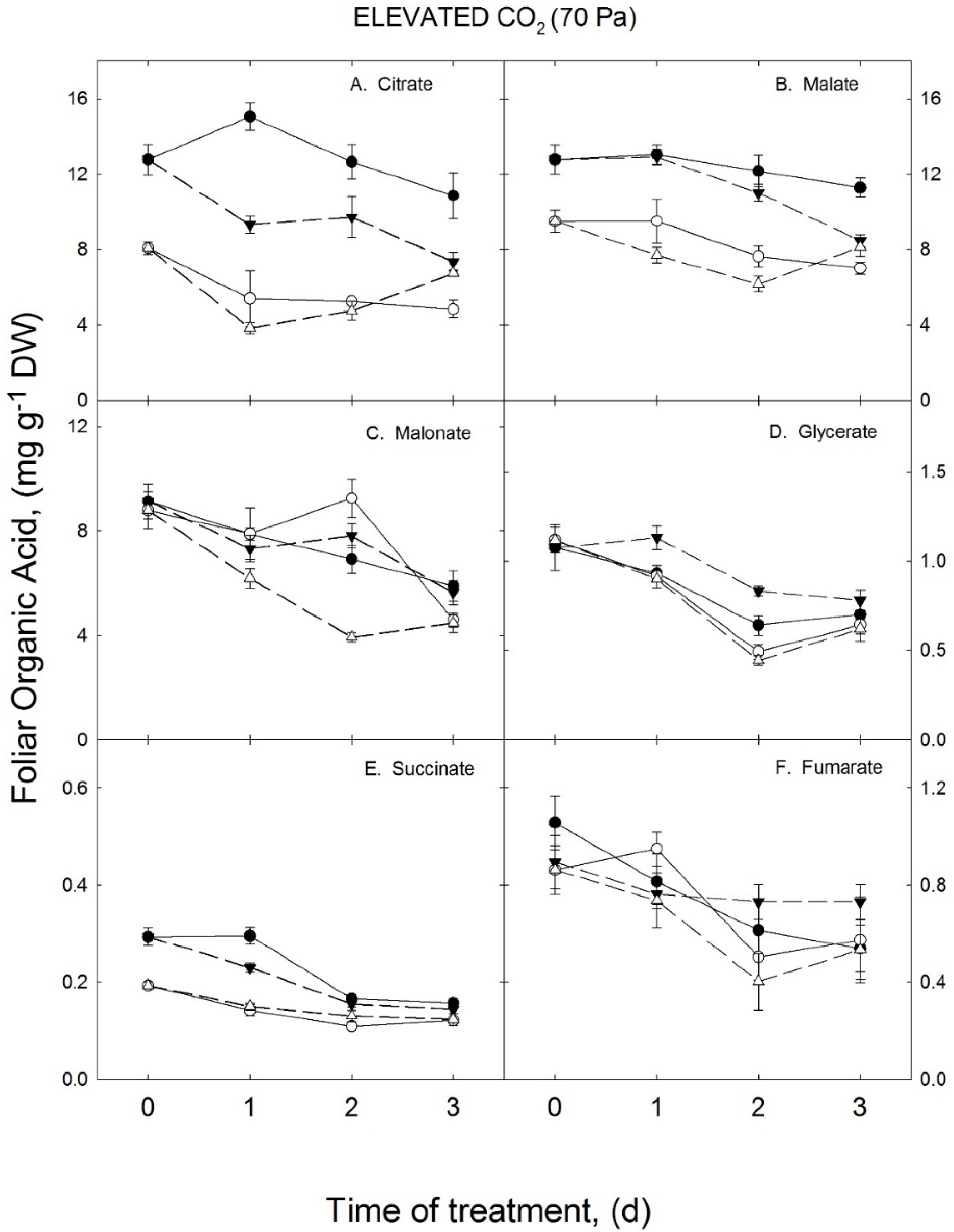
3.3. Species Differences in the Effects of Enhanced Growth Temperatures on Foliar Organic Acid Levels
| Compound | Zea Mays | Capsicum Annum | Phaseolus Vulgaris | |||
|---|---|---|---|---|---|---|
| 28/20 °C | 36/28 °C | 28/20 °C | 36/28 °C | 28/20 °C | 36/28 °C | |
| μg g−1 DW | ||||||
| Maleic | 141 | 179 | 5184 | 3277 | 297 | 89 |
| Malic | 9886 | 13612 | 7292 | 11090 | 37570 | 9850 |
| Adipic | 179 | 133 | 5040 | 4323 | 205 | 172 |
| Quinic | 104 | 217 | 3323 | 3805 | 40 | 19 |
| 2-Oxoglutaric | ND | ND | ND | ND | 56 | 37 |
| Aconitic | 39075 | 36984 | 63 | 63 | 54 | 23 |
| Shikimic | 1008 | 827 | 202 | 195 | 86 | 102 |
| Citric | 1189 | 904 | 7539 | 12133 | 12043 | 11682 |
| Malonic | 55 | 56 | 944 | 583 | 6991 | 329 |
| Glyceric | 2898 | 2981 | 2833 | 2211 | 2667 | 1912 |
| Fumaric | 32 | 56 | 28 | 33 | 288 | 49 |
| Succinic | 83 | 95 | 69 | 100 | 403 | 144 |
4. Discussion
Supplementary Files
Supplementary File 1Acknowledgments
Conflicts of Interest
References
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Mittler, R.; Finka, A.; Goloubinoff, P. How do plants feel the heat? Trends Biochem. Sci. 2011, 37, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Schöffl, F.; Prandl, R.; Reindl, A. Molecular responses to heat stress. In Molecular Responses to Cold, Drought, Heat and Salt Stress in Higher Plants; Shinozaki, K., Yamaguchi-Shinozaki, K., Eds.; R.G. Landes Co.: Austin, TX, USA, 1999; pp. 81–98. [Google Scholar]
- Vierling, E. The roles of heat shock proteins in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 579–620. [Google Scholar] [CrossRef]
- Wang, W.; Vincour, B.; Shoseyov, O.; Altman, A. Role of heat shock proteins and molecular chaperones in abiotic stress response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Sharp, R.E.; Ober, E.S.; Wu, Y. Regulation of root growth at low water potentials. In Interacting Stresses on Plants in a Changing Climate; Jackson, M.B., Black, C.R., Eds.; Springer-Verlag: Berlin, Germany, 2013; pp. 557–572. [Google Scholar]
- Shen, B.; Jensen, R.G.; Bohnert, H.J. Increased resistance to oxidative stress in transgenic plants by targeting mannitol biosynthesis to chloroplasts. Plant Physiol. 1997, 113, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.; Kopka, J.; Haskell, D.W.; Zhao, W.; Schiller, K.C.; Gatzke, N.; Sung, D.Y.; Guy, C.L. Exploring the temperature stress metabolome of Arabidopsis. Plant Physiol. 2004, 136, 4159–4168. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M. Rising CO2 levels and their potential significance for carbon flow in photosynthetic cells. Plant Cell Environ. 1991, 14, 741–762. [Google Scholar] [CrossRef]
- Gibson, L.R.; Mullen, R.E. Influence of day and night temperature on soybean seed yield. Crop Sci. 1996, 36, 98–104. [Google Scholar] [CrossRef]
- Sicher, R.C. Combined effects of CO2 enrichment and elevated growth temperatures on metabolites in soybean leaflets. Planta 2013, 238, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.V.V.; Boote, K.J.; Vu, J.C.V.; Allen, L.H., Jr. The carbohydrate metabolism enzymes sucrose-P synthase and ADG-pyrophosphorylase in phaseolus bean leaves are up-regulated at elevated growth carbon dioxide and temperature. Plant Sci. 2004, 166, 1565–1573. [Google Scholar] [CrossRef]
- Guo, C.; Oosterhuis, D.M. Pinitol occurrence in soybean plants as affected by temperature and plant growth regulators. J. Exp. Bot. 1995, 46, 249–253. [Google Scholar] [CrossRef]
- Yu, J.; Du, H.; Xu, M.; Huang, B. Metabolic responses of heat stress under elevated atmospheric CO2 concentration in a cool-season grass species. J. Am. Soc. Hortic. Sci. 2012, 137, 221–228. [Google Scholar]
- Sicher, R.C. Effects of CO2 enrichment on soluble amino acids and organic acids in barley primary leaves as a function of age, photoperiod and chlorosis. Plant Sci. 2008, 174, 576–582. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A.; Leakey, A.D.B.; Heady, L.E.; Gibon, Y.; Stitt, M.; Schurr, U. Does elevated [CO2] alter diurnal C uptake and the balance of C and N metabolites in growing fully expanded soybean leaves. J. Exp. Bot. 2007, 58, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Roessner, T.U.; Luedemann, A.; Brust, D.; Fiehn, O.; Linke, T.; Willmitzer, L.; Fernie, A.R. Metabolic profiling allows comprehensive phenotyping of genetically or environmentally modified plant systems. Plant Cell 2001, 13, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Sweetlove, L.J.; Beard, K.F.M.; Nunes-Nesi, A.; Fernie, A.R.; Ratcliffe, R.G. Not just a circle: Flux modes in the plant TCA cycle. Trends Plant Sci. 2010, 15, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Pick, T.R.; Bräutigam, A.; Schulz, M.A.; Obata, T.; Fernie, A.R.; Weber, A.P.M. PLGG1, a plastidic glycolate glycerate transporter, is required for photorespiration and defines a unique class of metabolite transporters. Proc. Natl. Acad. Sci. USA 2013, 110, 3185–3190. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D. Effects of moderate heat stress on photosynthesis: Importance of thylakoid reactions, rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant Cell Environ. 2005, 28, 269–277. [Google Scholar] [CrossRef]
- Douce, R.; Bourguignon, J.; Neuburger, M.; Rébeillé, F. The glycine decarboxylase system: A fascinating complex. Trends Plant Sci. 2001, 6, 167–176. [Google Scholar] [CrossRef]
- Thelen, J.J.; Miernyk, J.A.; Randall, D.D. Partial purification and characterization of the maize mitochondrial pyruvate dehydrogenase complex. Plant Physiol. 1998, 11, 1443–1450. [Google Scholar] [CrossRef]
- Fernie, A.R.; Carrari, F.; Sweetlove, L.J. Respiratory metabolism: Glycolysis, the TCA cycle and mitochondrial electron transport. Curr. Opin. Plant Biol. 2004, 7, 254–261. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sicher, R.C. Temperature Shift Experiments Suggest That Metabolic Impairment and Enhanced Rates of Photorespiration Decrease Organic Acid Levels in Soybean Leaflets Exposed to Supra-Optimal Growth Temperatures. Metabolites 2015, 5, 443-454. https://doi.org/10.3390/metabo5030443
Sicher RC. Temperature Shift Experiments Suggest That Metabolic Impairment and Enhanced Rates of Photorespiration Decrease Organic Acid Levels in Soybean Leaflets Exposed to Supra-Optimal Growth Temperatures. Metabolites. 2015; 5(3):443-454. https://doi.org/10.3390/metabo5030443
Chicago/Turabian StyleSicher, Richard C. 2015. "Temperature Shift Experiments Suggest That Metabolic Impairment and Enhanced Rates of Photorespiration Decrease Organic Acid Levels in Soybean Leaflets Exposed to Supra-Optimal Growth Temperatures" Metabolites 5, no. 3: 443-454. https://doi.org/10.3390/metabo5030443
APA StyleSicher, R. C. (2015). Temperature Shift Experiments Suggest That Metabolic Impairment and Enhanced Rates of Photorespiration Decrease Organic Acid Levels in Soybean Leaflets Exposed to Supra-Optimal Growth Temperatures. Metabolites, 5(3), 443-454. https://doi.org/10.3390/metabo5030443





