Abstract
Obesity is an increasingly serious global health problem. Some studies have revealed that the gut microbiota and its metabolites make important contributions to the onset of obesity. The gut microbiota is a dynamic ecosystem composed of diverse microbial communities with key regulatory functions in host metabolism and energy balance. Disruption of the gut microbiota can result in obesity, a chronic metabolic condition characterized by the excessive accumulation of adipose tissue. Host tissues (e.g., adipose, intestinal epithelial, and muscle tissues) can modulate the gut microbiota via microenvironmental interactions that involve hormone and cytokine secretion, changes in nutrient availability, and modifications of the gut environment. The interactions between host tissues and the gut microbiota are complex and bidirectional, with important effects on host health and obesity. This review provides a comprehensive summary of gut microbiota changes associated with obesity, the functional roles of gut microbiota-derived metabolites, and the importance of the complex interactions between the gut microbiota and target tissues in the pathogenesis of obesity. It places particular emphasis on the roles of adipose tissue microenvironment interactions in the onset of obesity.
1. Introduction
Obesity, a widespread public health problem, has serious negative effects on quality of life and well-being worldwide. According to the World Health Organization, obesity rates have doubled since 1975, such that it currently affects more than 650 million adults globally [1]. This alarming trend is attributed to numerous factors, including changes in dietary habits and physical activity levels, as well as sedentary lifestyles; thus, it constitutes a primary public health concern in many countries.
The gut microbiota, a large community containing trillions of bacteria, viruses, and other microorganisms, plays a key role in the progression of obesity [2]. There is substantial evidence regarding its essential roles in energy regulation, whereby it facilitates nutrient uptake, metabolism, and storage [3]. Moreover, inherent dysbiosis within the gut microbiota has been implicated in the pathogenesis of obesity and associated metabolic morbidities [4].
In recent years, extensive research (e.g., large-scale population studies, animal models, and cellular cultures) has improved the overall understanding of the role of the gut microbiota in obesity. A pivotal study, published in 2006, demonstrated divergent gut microbiota patterns between morbidly overweight individuals and their lean counterparts, providing initial support to the notion that the gut microbiota is involved in the onset of obesity [3]. A subsequent study, published in 2013, revealed that the transfer of gut microbiota from overweight mice to lean cagemates resulted in weight gain; this finding highlighted the critical regulatory role of the gut microbiota in energy homeostasis [5]. A study published in 2014 showed that the consumption of specific probiotics could improve weight loss and enhance metabolic function among obese individuals, emphasizing the potential for gut microbiota modulation to be used in clinical treatment [6]. Since then, there have been efforts to identify the roles of gut microbiota-derived metabolites, including bile acids, short-chain fatty acids (SCFAs), and amino acids, in the development of obesity. A study published in 2016 demonstrated that the levels of specific gut microbiota-derived metabolites were increased in individuals with obesity and metabolic-related diseases, thereby strengthening the link between the gut microbiota and obesity [7]. These findings imply that the gut microbiota and its metabolites are key factors with critical effects on obesity pathogenesis.
This review provides a comprehensive synopsis of the dynamic changes and functional properties of the gut microbiota and their metabolites during the onset and progression of obesity; it also explores underlying mechanisms that govern the interactions between the microbiota and target tissues in obesity, with particular emphasis on the influential role of the microbiota in establishing the adipose tissue microenvironment. The main objective of this review is to provide a detailed framework for the current understanding of the importance of the gut microbiota and its metabolites in the context of obesity, thereby offering a valuable reference for future research endeavors focused on microbiota-related human obesity.
2. The Association between the Gut Microbiota and Obesity
There is considerable evidence of a strong association between obesity and the gut microbiota; significant changes in gut microbiota composition have been observed in obese individuals. Table 1 provides an overview of the common obesity-related changes in microbiota [8,9,10]. Several animal and human studies have demonstrated microbial changes in obesity condition. For example, Turnbaugh et al. [2] compared obese and lean individuals in mice and human volunteers and observed a reduction in Bacteroidetes and a proportional increase in Firmicutes in the obese group. They suggested that the obese microbiome has an increased capacity to harvest energy from the diet and that this trait was transmissible, indicating that the gut microbiota is an additional contributing factor to the pathophysiology of obesity. Similarly, Geurts et al. [11] found a significant higher abundance of Firmicutes, Proteobacteria, and Fibrobacteres phyla in db/db mice compared to lean mice. Furthermore, Gophna et al. [12] showed that the presence of a bacterial genus named Oscillospira, which belongs to Clostridial cluster IV, is reduced in obesity as well. They inferred that Oscillospira species were butyrate producers, and at least some of them have the ability to utilize glucuronate to help explain the observation that the presence of this genus is reduced. Treatments for obesity also lead to changes in the gut microbiota. In a dietary intervention trial for obese individuals, Remely et al. [13] observed that Lactobacillus, Clostridium cluster IV, Faecalibacterium prausnitzii, and Archaea increased during intervention, while Clostridium cluster XIVa showed a decreased abundance, indicating a marked change of gut bacterial composition through dietary intervention. Furet et al. [14] profiled gut microbita from fecal samples in control subjects and obese individuals before and after Roux-en-Y gastric bypass (RYGB) surgery, one of the most efficient procedures for the treatment of morbid obesity. They found the presence of Bacteroides/Prevotella group was lower in obese subjects, which was negatively correlated with corpulence.

Table 1.
Obesity-associated changes in the gut microbiota.
Changes in gut microbiota composition and functionality have been implicated in the onset and progression of obesity via modulation of energy metabolism, insulin sensitivity, and inflammatory signaling pathways [18]. Nevertheless, further research is needed to gain comprehensive insights into the changes in the gut microbiota and microbiota-associated metabolites in the context of obesity, with the goal of resolving the complex interactions between the gut microbiota and obesity.
3. Gut Microbiota-Derived Metabolites Associated with Obesity
The gut microbiota has a central role in the biosynthesis of various metabolites, including bile acids, amino acids, SCFAs, and neurotransmitters. These metabolites have extensive effects on energy metabolism, insulin sensitivity, and the onset of obesity and related metabolic disorders [19]. There remains a considerable lack of understanding regarding the gut microbiota’s effects on host metabolism and body weight, but the past decade has yielded important mechanistic insights into these interactions [20]. Recent research has revealed both direct and indirect effects of the gut microbiota on calorie absorption and host metabolic pathways through the production or modification of microbial or host-derived compounds [21]. This review provides a comprehensive overview of the main evidence concerning the microbiota-derived metabolites involved in obesity, as shown in Table 2.

Table 2.
Functions of microbial metabolites in obesity.
3.1. Bile Acids
Bile acids, a class of steroid compounds, are synthesized in the liver and stored in the gallbladder. Upon food intake, these molecules are released into the small intestine where they play important roles in the digestion and absorption of dietary fat, as well as fat-soluble vitamins. Bile acids also function as signaling molecules, with key contributions to systemic metabolic maintenance. These contributions are facilitated by their interactions with various nuclear receptors, including (but not limited to) the farnesoid X receptor [35], vitamin D receptor [36], pregnane X receptor [37], and androstane receptor [38], as well as membrane receptors (e.g., Takeda G protein-coupled receptor 5 [39]). These interactions regulate the secretion of key factors such as peptide YY (PYY), glucagon-like peptide-1 (GLP-1), and fibroblast growth factor 19 (FGF19); they also influence cholesterol metabolism and systemic energy expenditure [40].
Bile acids function as substrates for biotransformation by the gut microbiota, thereby influencing the composition and behavior of the microbial community within the gastrointestinal lumen. For example, the microbiota promotes biosynthesis of secondary bile acids [41,42], including deoxycholic acid and lithocholic acid. Importantly, the consumption of a high-fat diet substantially increases the levels of secondary bile acids within both fecal matter and systemic circulation [43,44,45].
The metabolism of the gut microbiota affects both the bioavailability and bioactivity of bile acids; these properties influence the metabolic processes in which bile acids participate, as well as the development of obesity [46]. Studies in animal models have demonstrated that gut microbiota-mediated regulation of bile acid synthesis and metabolism can influence body weight and fat distribution; the inhibition of bile acid synthesis leads to improved glucose tolerance [47] and weight loss [48]. Nierop et al. revealed that in obese and insulin-resistant individuals, the induction of weight loss by a very low-calorie diet led to increased postprandial deoxycholic acid levels and reduced resting energy expenditure [49]. Those findings highlighted the important roles of secondary bile acids in regulating energy metabolism and body weight via receptor activation, which decreases glucose and lipid synthesis, improves insulin sensitivity, and reduces energy storage [50,51,52,53].
3.2. SCFAs
SCFAs are produced in the gastrointestinal tract via microbial fermentation of carbohydrates; acetate, propionate, and butyrate are the primary SCFAs produced by this method [54]. The production of SCFAs is influenced by changes in gut microbiota structure and function; a decrease in the number of butyrate-producing bacteria is closely associated with increased energy storage and weight gain in both animal models and humans [55]. Butyrate regulates energy metabolism by interacting with various metabolic pathways; it can stimulate energy expenditure while mitigating adipocyte size via lipogenesis inhibition and the promotion of lipolysis-related genes [56]. Moreover, butyrate can enhance insulin sensitivity and glucose homeostasis by activating adenosine monophosphate-activated protein kinase (AMPK) and repressing inflammation [57].
SCFAs provide energy for the epithelial cells and influence the immune function of the mucosa, by regulating the pH and the production of mucus in the intestinal lumen. Increased mucus production is usually inferred from increased expression of the MUC2 gene encoding mucin 2, and SCFAs, particularly butyrate, stimulate MUC2 gene expression through selective acetylation/methylation of the MUC2 histone, thereby promoting mucus production [58]. Furthermore, SCFAs can enter the systemic circulation, then directly affect metabolism and function in peripheral tissues [58,59,60]. Although SCFAs serve as an immediate source of additional energy, increased levels of SCFAs are presumed to support whole-body energy regulation via reduced production of hepatic glucose and lipids [55,61].
SCFAs regulate appetite and food intake by influencing communication between the gastrointestinal tract and the central nervous system [58,59,60,62]. One of these mechanisms involves the stimulation of colonic cells that express free fatty acid receptor 2 (FFAR2) and free fatty acid receptor 3 (FFAR3) [63], which are receptors for SCFAs, especially acetate and propionate. Upon activation, these cells secrete hormones that promote satiety, such as PYY and GLP-1.
In rodents, acute or chronic administration of SCFAs can mitigate weight gain and induce weight loss [58]. For example, Hattori et al. [64] found that oral administration of acetate stimulated energy expenditure in mice compared to a water control group, and Kimura et al. [65]. reported that intraperitoneal administration of propionate increased oxygen consumption in mice compared to the phosphate group. These studies show that SCFA supplementation dramatically increases energy expenditure in rodents. Regarding the chronic perspective, Den Besten et al. [66] found that the addition of sodium acetate, sodium propionate, or sodium butyrate to a high-fat diet increased energy expenditure in mice after 10 weeks during both the 12 h day and night periods. Sahuri-Arisoylu et al. [67] also found an increase in energy expenditure on a high fat diet after 6 weeks of intraperitoneal administration of nanoparticle-derived sodium acetate during the 12 h day and night periods. Some researchers have studied the metabolism of butyrate in both acute and chronic ingestions. Interestingly, Li et al. [28] reported a reduction in energy intake following both the acute and chronic intragastric administration of butyrate, but this reduction disappeared when the mode of ingestion was changed to intravenous administration. The likely reason for this is that intragastric administration allows butyrate to reach its site of action and to interact with intestinal receptors and/or metabolism, which is not achieved with the periphery. Despite the importance of SCFAs in regulating energy metabolism and body weight, the current literature regarding SCFAs in human health is predominantly focused on exogenous fiber supplementation and endogenous SCFA production. Thus far, the findings are inconclusive because of difficulties in measuring SCFA production [58].
3.3. Amino Acids
The gut microbiota is a major source of various metabolites, including amino acids. The diverse effects of these compounds on energy balance and metabolic homeostasis are mediated by multiple mechanisms. For example, gut microbiota-derived amino acids have been consistently implicated in alterations of energy metabolism, regulation of body weight, and accumulation of adipose tissue. Notably, phenylalanine is converted to tyrosine, which serves as a precursor to catecholamines such as epinephrine and norepinephrine. By enhancing energy expenditure, promoting fat oxidation, and activating brown adipose tissue, these catecholaminergic molecules may contribute to obesity prevention [68].
Tryptophan, an essential amino acid, is utilized in protein synthesis; it also plays a key role in the gut microbiota-mediated regulation of body weight and metabolism [69]. Tryptophan and its metabolites can transmit signals locally, especially in the intestinal mucosa; they can also transmit signals to other organs, such as the brain [70]. Some tryptophan metabolites, which are derived from microbial degradation, have demonstrated robust effects on appetite regulation in various experimental models [71,72,73]. These metabolites include tryptamine, indole-3-acetic acid, and 3-indole-propionic acid, all of which serve as ligands for the aryl hydrocarbon receptor (AhR), a naturally occurring receptor for aryl hydrocarbons [74]. AhR, a sensor for environmental and physiological signals, has strong effects on intestinal barrier function, immune response, and metabolism [75,76]. Altered levels of tryptophan-derived metabolites have been detected in stool samples from individuals with metabolic syndrome; these changes were associated with reduced AhR activity [74]. Impaired microbial synthesis of AhR ligands leads to the deterioration of mucosal barrier integrity and diminished secretion of GLP-1, with subsequent contributions to the onset of metabolic syndrome, type 2 diabetes mellitus (T2DM), high body mass index, and hypertension [74].
The intestinal microbiota can synthesize branched-chain amino acids, namely leucine, isoleucine, and valine. These amino acids have been associated with the onset of insulin resistance, obesity, and subsequent metabolic disorders [7]. Some studies have shown that fasting serum BCAA levels are higher in people with type 2 diabetes than in healthy individuals [77], and that BCAA levels decrease after weight loss surgery or dietary supplementation with BCAA in obese individuals or rats [78]. These findings suggest that BCAAs or their breakdown products may play a causal role in metabolic disorders [79,80], although the exact mechanisms are not fully understood. The gut microbiota may also affect the blood levels of BCAAs by altering the expression or activity of genes involved in BCAA biosynthesis or transport. Some bacteria, such as Prevotella copri and Bacteroides vulgatus, have been found to have a high potential for BCAA biosynthesis and inward transport, and to be more abundant in people with insulin resistance than in healthy individuals [7]. These bacteria may directly affect host metabolism by increasing the production or uptake of BCAAs in the gut. This hypothesis has been tested in mice fed a high-fat diet and infected with P. copri, which resulted in increased serum BCAA levels, insulin resistance, and glucose intolerance [7].
3.4. Other Gut Microbiota-Derived Metabolites
Extensive research concerning the roles of gut microbiota-derived metabolites in obesity has been performed [81,82,83]. Some of these metabolites include phenolic acids, lipopolysaccharides, ethanol, and microbial-derived protein fermentation products. These metabolites contribute to obesity by affecting insulin sensitivity, altering energy balance, and causing low-grade inflammation. For example, phenolic acids can modulate adipocyte differentiation and lipolysis [82], lipopolysaccharides can induce inflammation and impair glucose uptake in adipose tissue, ethanol can increase hepatic lipid accumulation and insulin resistance [82], and protein fermentation products can activate pro-inflammatory pathways and reduce energy expenditure [82]. These findings highlight the need for continued research into the complex mechanisms by which gut microbiota-derived metabolites contribute to the development of obesity.
4. Microbiota–Target Tissue Interactions in Obesity
Multiple studies have demonstrated correlations between disruptions in the taxonomic and functional properties of the gut microbiota and various histopathological phenotypes. The human gut microbial gene pool has provided insights concerning the wide range of functions exhibited by the gut microbiota, which functions as an extensive chemical factory with the ability to synthesize a diverse range of compounds that promote microbial and host survival [9,84,85,86]. Gut microbiota-derived metabolites exert extensive effects, both locally and in distant tissues; they may also facilitate microbial translocation into various tissues and organs, including human adipose tissue, by increasing intestinal permeability. Nevertheless, the host metabolism-related effects of microbes and microbial DNA in peripheral tissues remain unclear. Some members of the gut microbiota and their metabolites that are associated with host tissues (e.g., adipose tissue, intestinal epithelial cells, and muscle) are discussed below.
4.1. Adipose Tissue
Obesity is primarily characterized by irregularities in adipose tissue, which mainly comprise atypical fat distribution and adipose tissue dysfunction. Abnormal fat distribution is defined as excessive accumulation of visceral adipose tissue in the intraperitoneal and retroperitoneal regions, as well as ectopic fat deposition in non-physiological sites (e.g., liver, pancreas, heart, and skeletal muscle). Adipose tissue dysfunction involves impaired adipogenesis, adipocyte hypertrophy, and anomalous lipid metabolism [87]. The pathogenesis underlying adipose tissue dysfunction is related to the perpetuation of a vicious cycle [88] characterized by macrophage infiltration and proinflammatory polarization (M1 polarization), which triggers a cascade of inflammatory pathways that have detrimental effects on insulin signaling [89]. Moreover, the combination of aberrant adipokine production and regulation increases the likelihood of adipose tissue dysfunction.
The interaction of specific dietary factors, such as a high-fat diet and certain gut microbiota, can interfere with adipose tissue function and lead to severe adipose tissue dysfunction [90]. Tran et al. showed that Western diet-induced dysbiosis can cause adipose tissue inflammation in mice, as demonstrated by an increase in typical proinflammatory M1 macrophages and a decrease in anti-inflammatory M2 macrophages within adipose tissue [91]. They also showed that the ablation of gut microbiota could reduce this inflammatory response within adipose tissue. Additionally, experimental knockdown of the Toll-like receptor (TLR) signaling protein myeloid differentiation primary response protein 88 (MyD88) yielded a phenotype similar to the phenotype caused by microbiota ablation, supporting the notion that Western diet-induced adipose tissue inflammation does not result from lipid accumulation; instead, the microbiota and/or its metabolites activate innate immune signaling pathways, which lead to inflammation. Although gut microbiota-derived metabolites were not examined in the study by Tran et al., the results indicated that gut microbiota and their metabolites induce adipose tissue inflammation through a TLR-mediated inflammatory response. Previous research concerning LPS-mediated activation of TLRs revealed associations with the development of obesity [92], suggesting a robust relationship between gut microbiota-derived LPS and adipose tissue inflammation [93].
Additionally, the results of recent studies are consistent with the notion that gut microbiota dysbiosis represents an early indication of inflammation and obesity [94]. The gastrointestinal system interacts with the dietary components involved, which disrupts the gut microbiota’s balance and alters the secretion profiles of gut peptides [16]. These disruptions trigger an inflammatory response in the intestinal mucosa, causing damage to the epithelial barrier and enhancing LPS entry into the systemic circulation. LPS and saturated fatty acids can activate TLRs, which are receptors that recognize microbial molecules, on macrophages or intestinal epithelial cells [95]. This leads to low-grade systemic inflammation. TLRs are also expressed in adipose tissue, where they can be activated by LPS and induce the secretion of inflammatory cytokines, such as TNF-α, IL-6, IL-8, and MCP-1, by macrophages and adipocytes [96,97,98,99]. These cytokines can attract more inflammatory cells to adipose tissue and worsen the inflammation. Chronic inflammation can also affect the gut microbiota and cause dysbiosis [100]. For example, obesity decreases the abundance of Akkermansia muciniphila in mice, with a concomitant increase in circulating C-reactive protein [101]. IL-36, which stimulates Akkermansia proliferation, plays a key role in protecting against obesity and metabolic disorders [102]. Overall, the interactions between the gut microbiota and inflammation are highly complex; they often involve gut bacteria-driven immune activation, systemic low-grade inflammation leading to adipose inflammation, and the resultant adipose tissue dysfunction that perpetuates further systemic inflammation, insulin resistance, and (ultimately) obesity (Figure 1).
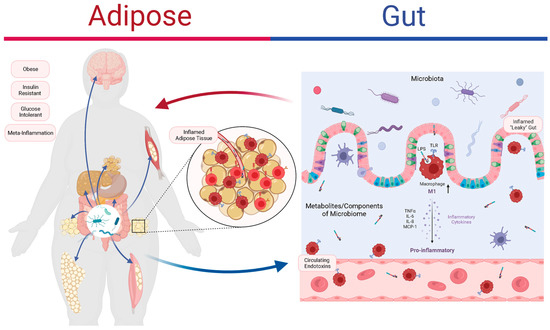
Figure 1.
The role of the gut microbiota and inflammation in adipose tissue dysfunction and obesity. Obesity is associated with abnormal fat distribution and adipocyte dysfunction, which result in chronic inflammation and insulin resistance. The gut microbiota can affect the intestinal barrier function, the immune system, and the adipose tissue metabolism. The gut microbiota produces various metabolites, such as lipopolysaccharides (LPS), that can activate Toll-like receptors (TLRs) on macrophages and intestinal epithelial cells (IECs), triggering inflammatory pathways that impair insulin signaling and promote adipose tissue inflammation. The adipose tissue secretes hormones and cytokines, such as tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6), that can modulate the gut microbiota’s composition and function. The interactions between the gut microbiota and the adipose tissue are complex and bidirectional, forming a vicious cycle that leads to adipose tissue dysfunction and obesity. Figure created using BioRender.com.
4.2. Intestinal Epithelium
Gut barrier dysfunction and gut microbiota dysbiosis are linked to a diverse array of pathological conditions, including obesity, T2DM, and inflammatory bowel disease. Intestinal epithelial cells play a key role in maintaining the health of the gut microbiota. These cells form a physical barrier between the gut microbiota and the underlying tissues, preventing the migration of harmful bacteria and small molecules into the systemic circulation. Tight junctions between the epithelial cells function as gatekeepers that regulate the entry of nutrients and other substances into the intestinal epithelium. Dysregulation involving these junctions can lead to gut dysbiosis and intestinal permeability, triggering various pathological conditions. Thus, the preservation of epithelial cell integrity is essential to maintain gastrointestinal health and prevent gut microbiota-related diseases.
The Firmicutes phylum, including the Lactobacillus genus, has been linked to obesity and T2DM [103]. However, some genera in this phylum (e.g., Lactobacillus paracasei and Lactobacillus plantarum) can help to prevent weight gain [104,105]. Decreased relative abundances of Bacteroides and Bifidobacterium, as well as the butyrate-producing Faecalibacterium prausnitzii and Roseburia intestinalis, have been associated with obesity [106,107,108]. In T2DM patients, bariatric surgery increases the relative abundance of F. prausnitzii while improving glucose homeostasis, low-grade inflammation, and intestinal epithelial permeability [14,109]. F. prausnitzii is a key producer of butyrate, which serves as an important energy source for intestinal epithelial cells [110]. Multiple butyrate-mediated mechanisms (e.g., mucin synthesis [111], reorganization of tight junctions, and upregulation of occludin and Zonula occludens protein 1 [112,113]) can reduce local inflammation and improve intestinal barrier permeability. The species Bacteroides vulgatus and Bacteroides dorei may provide benefits for T2DM patients by increasing Zonula occludens protein 1 (ZO-1) expression and enhancing epithelial barrier function [114]. These bacteria produce bacteriocins, a class of proteins that inhibit the growth of specific microbes and could reduce the abundance of harmful species [115]. Additionally, a mucin-degrading species in the Verrucomicrobia phylum, Akkermansia muciniphila, colonizes the intestinal mucus layer and enhances intestinal barrier integrity; these effects are mediated by promotion of mucin production (direct mechanism) [116] and by interactions with other bacteria (indirect mechanism) [117,118]. A. muciniphila has been linked to reductions in wasting, insulin sensitivity, and low-grade inflammation [117,119]. Furthermore, the relative abundances of Gram-negative bacteria (primary producers of LPS) are altered in obesity; the dysregulated gut microbiota tends to shift towards the more proinflammatory LPS from Proteus spp., rather than the less potent LPS from Bacteroides spp. [120,121]. Gut microbiota dysregulation can lead to increased levels of proinflammatory endotoxins, thereby exacerbating low-grade inflammation by enhancing transepithelial permeability; these changes promote the circulation of proinflammatory LPS [118,122].
The induction of low-grade inflammation in the presence of a damaged intestinal barrier has been attributed to the proinflammatory LPS produced by Gram-negative bacteria [93]. Under normal circumstances, the intestinal epithelium provides protection against LPS translocation; however, models of diet-induced obesity and T2DM (e.g., db/db mice) display increased levels of circulating LPS and subsequent inflammation [93]. Moreover, mice with diet-induced obesity and db/db mice exhibit increased transepithelial permeability and disrupted ZO-1 distribution in the intestinal mucosa, compared with lean control mice [123]. This significant alteration of intestinal barrier permeability has been linked to decreased levels of the porosity-regulating tight junction proteins ZO-1 and occludin [90,124]. Furthermore, studies in humans have revealed higher gastrointestinal epithelial permeability among individuals with obesity [125]. However, despite the potential role of obesity in intestinal barrier dysfunction, hyperglycemia is considered the predominant factor that promotes intestinal barrier disruption in obese and T2DM mice [126]. This disruption has been directly linked to hyperglycemia and can be remedied by glycemic control [126]. Similarly, poor glycemic control in humans has been linked to increased entry of microbial products into the bloodstream [126]. The severity of hepatic steatosis in obese individuals has also been linked to an increase in intestinal permeability [123,127]. These observations suggest a strong linkage between changes in intestinal barrier function and the onset of obesity-related complications.
4.3. Muscle
Skeletal muscle, which comprises approximately 40% of the human body mass, is responsible for numerous critical functions including thermoregulation and the modulation of glucose/amino acid metabolism. Although it is physically distinct from the gut, skeletal muscle is influenced by gut-derived signals that arise from interactions between gut microbiota and host tissue; these interactions involve microbes, metabolites, gut peptides, LPS, and ILs. These signals form a link between gut microbiota activity and skeletal muscle function; modulation of these signals influences systemic or tissue inflammation and insulin sensitivity, helping to regulate muscle function. Disruptions in gut microbiota composition can lead to muscle atrophy, weakness, and poor exercise performance. Additionally, some microbial metabolites, such as SCFAs, have direct effects on muscle health and function, highlighting the complex interactions between gut microbiota and muscle physiology. Thus, the gut microbiota represents a promising new target for the prevention and treatment of muscle-related diseases.
Considering the diverse array of gut microbiota-derived metabolites, research has focused on the potential for SCFAs to mediate interactions among the gut microbiota, gastrointestinal physiology, and muscle insulin sensitivity. Comparative analyses have revealed that exercise interventions are associated with greater abundances of SCFA-producing microbial taxa, compared with the abundances in individuals with sarcopenia. SCFAs are primarily generated by microbial anaerobic fermentation of nondigestible dietary fibers, mainly within the distal ileum and colon. Acetate, propionate, and butyrate comprise the predominant SCFA profile within the colon, totaling more than 95% of the total SCFA content. Upon entry into enterocytes, butyrate drives the citric acid cycle through acetyl-CoA, satisfying up to 60–70% of colonocyte metabolic needs [55]. The remaining SCFAs are transported through the portal vein to the liver, which absorbs up to 80% of the available propionate and 40% of the available acetate for subsequent utilization in gluconeogenesis [55,128]. Finally, a small subset of SCFAs, predominantly acetate, is transported to skeletal muscle.
SCFAs are important for maintaining glucose and lipid homeostasis, regulating inflammation, and establishing connections between the gut and distant tissues [55,129]. There is empirical evidence that SCFA supplementation can enhance muscle mass and strength, particularly in germ-free and antibiotic-treated rodents [130,131,132,133]. Notably, acetate supplementation (via dietary intake or subcutaneous injection) enhances glucose uptake and glycogen content while reducing lipid accumulation in rat skeletal muscles [134]. In mice, oral supplementation of butyrate protects against oxidative stress and loss of muscle mass; it also increases mitochondrial function and increases the number of type I fibers in skeletal muscles [133,135]. Although SCFAs may provide metabolic benefits for skeletal muscles (e.g., acetate is present in peripheral blood), butyrate and propionate may only be present in small amounts in peripheral blood. However, the effects of SCFAs may be indirectly achieved via the secretion of GLP-1, a gut hormone that stimulates insulin secretion, glucose storage in the liver, and glucose uptake in skeletal muscles [135]. Moreover, SCFAs can increase blood flow to muscles and exert anti-inflammatory effects through epigenetic mechanisms [136]. Similar to propionate, succinate serves as a substrate for gluconeogenesis. The detection of enterocyte-derived glucose—produced from succinate—in the portal vein can increase satiety, energy expenditure, glucose tolerance, and insulin sensitivity [137,138].
4.4. Other Target Organs or Tissues
In addition to its effects on adipose tissue, intestinal epithelium, and muscle, the gut microbiota interacts with other physiological targets and organs during the development of obesity. The gut microbiota is reportedly involved in the regulation of the hypothalamic–pituitary–adrenal axis, a key factor in energy balance and metabolism [139]. This axis is regulated by a complex network of signaling pathways involving the central nervous system, gut microbiota, and other peripheral tissues, including the liver and adipose tissue.
Recent evidence suggests that the gut microbiota helps to modulate the gut–liver axis, a key regulatory component in metabolic homeostasis. Specifically, the liver can be influenced by gut microbiota-derived metabolites, which exert downstream effects on hepatic metabolism, gene expression, and insulin sensitivity [140,141].
Moreover, the gut microbiota affects the cardiovascular system by influencing blood pressure, lipoprotein metabolism, and systemic inflammation [142]. Additionally, the gut microbiota has regulatory effects on the immune system; gut microbiota dysregulation has been associated with the onset of obesity-related inflammatory and metabolic disorders [143].
5. Study Strengths and Limitations
This review provides an updated overview of the current literature on the gut microbiota and obesity, covering various aspects of the gut microbiota–host tissue interactions and the gut microbiota-derived metabolites, and emphasizing the roles of adipose tissue microenvironment interactions in the onset of obesity. However, this review has some limitations that need to be acknowledged. One limitation is that most of the studies included are based on rodent models, which may not fully reflect the human situation. Another limitation is that it does not adequately address the effects of various dietary factors on the gut microbiota and obesity. Future research should investigate how different components of the Western diet, such as trans-fatty acids, easily digestible carbohydrates, antibiotic residues, hormones, preservatives, or pesticides, influence the gut microbiota and obesity.
6. Conclusions
The cumulative findings of numerous studies highlight the complex and multifaceted link between intestinal microflora and obesity. These studies have demonstrated that gut microbiota dysregulation influences energy equilibrium and can contribute to the onset of obesity. Furthermore, these studies have identified potential mechanisms by which the gut microbiota and its metabolites affect the onset of obesity, including the production of endogenous metabolites, regulation of systemic inflammation, and modulation of the adipose tissue microenvironment.
Future research in this area is needed to elucidate the mechanisms that underlie the relationships of the gut microbiota and its metabolites with obesity. Such studies will contribute key insights concerning the viability of microbiota-centered interventions for human health. Accordingly, continued investigation of the interactions between the gut microbiota and its metabolites in obesity has important implications for efforts to manage the growing obesity pandemic.
Author Contributions
Conceptualization, C.W., F.Y. and S.Z.; writing—original draft preparation, C.W., Z.Y. and Y.J.; writing—review and editing, C.W., Z.Y., Z.S., F.Y. and S.Z.; visualization, C.W.; supervision, F.Y. and S.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China [grant numbers 31770979]; the Pre-research Foundation of Zhejiang University (2021) to Zhong Shen [grant numbers 519600-I52104]; Cao Guangbiao High Sci-Tech Development Fund of Zhejiang University [2020QN026].
Acknowledgments
We would like to express our sincere gratitude to Ningxin Chen from Chronic Disease Research Institute for her valuable assistance in the revision process of this review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 18 October 2022).
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Delzenne, N.M. The gut microbiome as therapeutic target. Pharmacol. Ther. 2011, 130, 202–212. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef]
- Sanchez, M.; Darimont, C.; Drapeau, V.; Emady-Azar, S.; Lepage, M.; Rezzonico, E.; Ngom-Bru, C.; Berger, B.; Philippe, L.; Ammon-Zuffrey, C.; et al. Effect of Lactobacillus rhamnosusCGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. Br. J. Nutr. 2014, 111, 1507–1519. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X.; et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Jensen, B.A.H.; Varin, T.V.; Servant, F.; Van Blerk, S.; Richard, D.; Marceau, S.; Surette, M.; Biertho, L.; Lelouvier, B.; et al. Type 2 diabetes influences bacterial tissue compartmentalisation in human obesity. Nat. Metab. 2020, 2, 233–242. [Google Scholar] [CrossRef]
- Thingholm, L.B.; Rühlemann, M.C.; Koch, M.; Fuqua, B.; Laucke, G.; Boehm, R.; Bang, C.; Franzosa, E.A.; Hübenthal, M.; Rahnavard, G.; et al. Obese Individuals with and without Type 2 Diabetes Show Different Gut Microbial Functional Capacity and Composition. Cell Host Microbe 2019, 26, 252–264.e10. [Google Scholar] [CrossRef]
- Geurts, L.; Lazarevic, V.; Derrien, M.; Everard, A.; Van Roye, M.; Knauf, C.; Valet, P.; Girard, M.; Muccioli, G.G.; François, P.; et al. Altered gut microbiota and endocannabinoid system tone in obese and diabetic leptin-resistant mice: Impact on apelin regulation in adipose tissue. Front. Microbiol. 2011, 2, 149. [Google Scholar] [CrossRef]
- Gophna, U.; Konikoff, T.; Nielsen, H.B. Oscillospira and related bacteria-From metagenomic species to metabolic features. Environ. Microbiol. 2017, 19, 835–841. [Google Scholar] [CrossRef]
- Remely, M.; Tesar, I.; Hippe, B.; Gnauer, S.; Rust, P.; Haslberger, A. Gut microbiota composition correlates with changes in body fat content due to weight loss. Benef. Microbes 2015, 6, 431–439. [Google Scholar] [CrossRef]
- Furet, J.-P.; Kong, L.-C.; Tap, J.; Poitou, C.; Basdevant, A.; Bouillot, J.-L.; Mariat, D.; Corthier, G.; Doré, J.; Henegar, C.; et al. Differential adaptation of human gut microbiota to bariatric surgery–induced weight loss. Diabetes 2010, 59, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, M.A.; Hoffmann, C.; Sherrill–Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.-Y.; Knight, R.; Ahima, R.S.; Bushman, F.; Wu, G.D. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009, 137, 1716–1724.E2. [Google Scholar] [CrossRef]
- Ellis, C.L.; Lee, J.; Hartman, A.L.; Rutledge, J.C.; Raybould, H.E.; Klingbeil, E.; Little, T.J.; Cvijanovic, N.; DiPatrizio, N.V.; Argueta, D.A.; et al. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am. J. Physiol. Liver Physiol. 2010, 299, G440–G448. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, M.; Wang, S.; Han, R.; Cao, Y.; Hua, W.; Mao, Y.; Zhang, X.; Pang, X.; Wei, C.; et al. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010, 4, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Cavallari, J.F.; Schertzer, J.D. Intestinal Microbiota Contributes to Energy Balance, Metabolic Inflammation, and Insulin Resistance in Obesity. J. Obes. Metab. Syndr. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Heiss, C.N.; Olofsson, L.E. Gut Microbiota-Dependent Modulation of Energy Metabolism. J. Innate Immun. 2018, 10, 163–171. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Visconti, A.; Le Roy, C.I.; Rosa, F.; Rossi, N.; Martin, T.C.; Mohney, R.P.; Li, W.; de Rinaldis, E.; Bell, J.T.; Venter, J.C.; et al. Interplay between the human gut microbiome and host metabolism. Nat. Commun. 2019, 10, 4505. [Google Scholar] [CrossRef]
- Mollard, R.C.; Sénéchal, M.; MacIntosh, A.C.; Hay, J.; Wicklow, B.A.; Wittmeier, K.D.; Sellers, E.A.; Dean, H.J.; Ryner, L.; Berard, L.; et al. Dietary determinants of hepatic steatosis and visceral adiposity in overweight and obese youth at risk of type 2 diabetes. Am. J. Clin. Nutr. 2014, 99, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Petrus, P.; Lecoutre, S.; Dollet, L.; Wiel, C.; Sulen, A.; Gao, H.; Tavira, B.; Laurencikiene, J.; Rooyackers, O.; Checa, A.; et al. Glutamine Links Obesity to Inflammation in Human White Adipose Tissue. Cell Metab. 2020, 31, 375–390.e11. [Google Scholar] [CrossRef]
- López-Gonzales, E.; Lehmann, L.; Ruiz-Ojeda, F.J.; Hernández-Bautista, R.; Altun, I.; Onogi, Y.; Khalil, A.E.; Liu, X.; Israel, A.; Ussar, S. L-Serine Supplementation Blunts Fasting-Induced Weight Regain by Increasing Brown Fat Thermogenesis. Nutrients 2022, 14, 1922. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Bäckhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016, 22, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Pathak, P.; Xie, C.; Nichols, R.G.; Ferrell, J.M.; Boehme, S.; Krausz, K.W.; Patterson, A.D.; Gonzalez, F.J.; Chiang, J.Y.L. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology 2018, 68, 1574–1588. [Google Scholar] [CrossRef]
- Sun, L.; Xie, C.; Wang, G.; Wu, Y.; Wu, Q.; Wang, X.; Liu, J.; Deng, Y.; Xia, J.; Chen, B.; et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat. Med. 2018, 24, 1919–1929. [Google Scholar] [CrossRef]
- Li, Z.; Yi, C.-X.; Katiraei, S.; Kooijman, S.; Zhou, E.; Chung, C.K.; Gao, Y.; van den Heuvel, J.K.; Meijer, O.C.; Berbée, J.F.P.; et al. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut 2018, 67, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Van Deuren, T.; Blaak, E.E.; Canfora, E.E. Butyrate to combat obesity and obesity-associated metabolic disorders: Current status and future implications for therapeutic use. Obes. Rev. 2022, 23, e13498. [Google Scholar] [CrossRef]
- Hernández, M.A.G.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef]
- Chambers, E.S.; Byrne, C.S.; Morrison, D.J.; Murphy, K.G.; Preston, T.; Tedford, C.; Garcia-Perez, I.; Fountana, S.; Serrano-Contreras, J.I.; Holmes, E.; et al. Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: A randomised cross-over trial. Gut 2019, 68, 1430–1438. [Google Scholar] [CrossRef]
- Virtue, A.T.; McCright, S.J.; Wright, J.M.; Jimenez, M.T.; Mowel, W.K.; Kotzin, J.J.; Joannas, L.; Basavappa, M.G.; Spencer, S.P.; Clark, M.L.; et al. The gut microbiota regulates white adipose tissue inflammation and obesity via a family of microRNAs. Sci. Transl. Med. 2019, 11, eaav1892. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Furuzono, T.; Yamakuni, K.; Li, Y.; Kim, Y.; Takahashi, H.; Ohue-Kitano, R.; Jheng, H.; Takahashi, N.; Kano, Y.; et al. 10-oxo-12(Z)-octadecenoic acid, a linoleic acid metabolite produced by gut lactic acid bacteria, enhances energy metabolism by activation of TRPV1. FASEB J. 2017, 31, 5036–5048. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Pierce, K.A.; Jedrychowski, M.P.; Garrity, R.; Winther, S.; Vidoni, S.; Yoneshiro, T.; Spinelli, J.B.; Lu, G.Z.; Kazak, L.; et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 2018, 560, 102–106. [Google Scholar] [CrossRef]
- Makishima, M.; Okamoto, A.Y.; Repa, J.J.; Tu, H.; Learned, R.M.; Luk, A.; Hull, M.V.; Lustig, K.D.; Mangelsdorf, D.J.; Shan, B. Identification of a Nuclear Receptor for Bile Acids. Science 1999, 284, 1362–1365. [Google Scholar] [CrossRef] [PubMed]
- Makishima, M.; Lu, T.T.; Xie, W.; Whitfield, G.K.; Domoto, H.; Evans, R.M.; Haussler, M.R.; Mangelsdorf, D.J. Vitamin D Receptor As an Intestinal Bile Acid Sensor. Science 2002, 296, 1313–1316. [Google Scholar] [CrossRef] [PubMed]
- Ihunnah, C.A.; Jiang, M.; Xie, W. Nuclear receptor PXR, transcriptional circuits and metabolic relevance. Biochim. et Biophys. Acta (BBA) Mol. Basis Dis. 2011, 1812, 956–963. [Google Scholar] [CrossRef]
- Wagner, M.; Halilbasic, E.; Marschall, H.-U.; Zollner, G.; Fickert, P.; Langner, C.; Zatloukal, K.; Denk, H.; Trauner, M. CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology 2005, 42, 420–430. [Google Scholar] [CrossRef]
- Kawamata, Y.; Fujii, R.; Hosoya, M.; Harada, M.; Yoshida, H.; Miwa, M.; Fukusumi, S.; Habata, Y.; Itoh, T.; Shintani, Y.; et al. A G Protein-coupled Receptor Responsive to Bile Acids. J. Biol. Chem. 2003, 278, 9435–9440. [Google Scholar] [CrossRef]
- McGlone, E.R.; Bloom, S.R. Bile acids and the metabolic syndrome. Ann. Clin. Biochem. Int. J. Biochem. Lab. Med. 2018, 56, 326–337. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Harris, S.C.; Bhowmik, S.; Kang, D.-J.; Hylemon, P.B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 2016, 7, 22–39. [Google Scholar] [CrossRef] [PubMed]
- Duparc, T.; Plovier, H.; Marrachelli, V.G.; Van Hul, M.; Essaghir, A.; Ståhlman, M.; Matamoros, S.; Geurts, L.; Pardo-Tendero, M.M.; Druart, C.; et al. Hepatocyte MyD88 affects bile acids, gut microbiota and metabolome contributing to regulate glucose and lipid metabolism. Gut 2016, 66, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; An, Y.; Tang, H.; Wang, Y. Alterations of Bile Acids and Gut Microbiota in Obesity Induced by High Fat Diet in Rat Model. J. Agric. Food Chem. 2019, 67, 3624–3632. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-Y.; Iii, M.G.; Lee, A.A.; Wu, X.; Zhou, S.-Y.; Owyang, C. Secondary bile acids mediate high-fat diet–induced upregulation of R-spondin 3 and intestinal epithelial proliferation. J. Clin. Investig. 2022, 7, e148309. [Google Scholar] [CrossRef] [PubMed]
- Vallim, T.Q.d.A.; Tarling, E.J.; Edwards, P.A. Pleiotropic Roles of Bile Acids in Metabolism. Cell Metab. 2013, 17, 657–669. [Google Scholar] [CrossRef]
- Watanabe, M.; Houten, S.M.; Mataki, C.; Christoffolete, M.A.; Kim, B.W.; Sato, H.; Messaddeq, N.; Harney, J.W.; Ezaki, O.; Kodama, T.; et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006, 439, 484–489. [Google Scholar] [CrossRef]
- Li, F.; Jiang, C.; Krausz, K.W.; Li, Y.; Albert, I.; Hao, H.; Fabre, K.M.; Mitchell, J.B.; Patterson, A.D.; Gonzalez, F.J. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat. Commun. 2013, 4, 2348. [Google Scholar] [CrossRef]
- Van Nierop, F.S.; Kulik, W.; Endert, E.; Schaap, F.G.; Damink, S.W.O.; Romijn, J.A.; Soeters, M.R. Effects of acute dietary weight loss on postprandial plasma bile acid responses in obese insulin resistant subjects. Clin. Nutr. 2016, 36, 1615–1620. [Google Scholar] [CrossRef]
- Sayin, S.I.; Wahlström, A.; Felin, J.; Jäntti, S.; Marschall, H.-U.; Bamberg, K.; Angelin, B.; Hyötyläinen, T.; Orešič, M.; Bäckhed, F. Gut Microbiota Regulates Bile Acid Metabolism by Reducing the Levels of Tauro-beta-muricholic Acid, a Naturally Occurring FXR Antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef]
- Parséus, A.; Sommer, N.; Sommer, F.; Caesar, R.; Molinaro, A.; Ståhlman, M.; Greiner, T.U.; Perkins, R.; Bäckhed, F. Microbiota-induced obesity requires farnesoid X receptor. Gut 2017, 66, 429–437. [Google Scholar] [CrossRef]
- Swann, J.R.; Want, E.J.; Geier, F.M.; Spagou, K.; Wilson, I.D.; Sidaway, J.E.; Nicholson, J.K.; Holmes, E. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4523–4530. [Google Scholar] [CrossRef] [PubMed]
- Pols, T.W.; Noriega, L.G.; Nomura, M.; Auwerx, J.; Schoonjans, K. The Bile Acid Membrane Receptor TGR5: A Valuable Metabolic Target. Dig. Dis. 2011, 29, 37–44. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, H.; Tu, X.; Gao, Z. The Role of Short-Chain Fatty Acids of Gut Microbiota Origin in Hypertension. Front. Microbiol. 2021, 12, 730809. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef] [PubMed]
- Canani, R.B.; Di Costanzo, M.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; Van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Rastelli, M.; Cani, P.D.; Knauf, C. The Gut Microbiome Influences Host Endocrine Functions. Endocr. Rev. 2019, 40, 1271–1284. [Google Scholar] [CrossRef]
- Régnier, M.; Van Hul, M.; Knauf, C.; Cani, P.D. Gut microbiome, endocrine control of gut barrier function and metabolic diseases. J. Endocrinol. 2021, 248, R67–R82. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- De Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Hattori, M.; Kondo, T.; Kishi, M.; Yamagami, K. A Single Oral Administration of Acetic Acid Increased Energy Expenditure in C57BL/6J Mice. Biosci. Biotechnol. Biochem. 2010, 74, 2158–2159. [Google Scholar] [CrossRef]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035. [Google Scholar] [CrossRef]
- den Besten, G.; Bleeker, A.; Gerding, A.; Van Eunen, K.; Havinga, R.; Van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.-J.; et al. Short-Chain Fatty Acids Protect Against High-Fat Diet–Induced Obesity via a PPARgamma-Dependent Switch From Lipogenesis to Fat Oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef]
- Sahuri-Arisoylu, M.; Brody, L.P.; Parkinson, J.R.; Parkes, H.; Navaratnam, N.; Miller, A.D.; Thomas, E.L.; Frost, G.; Bell, J.D. Reprogramming of hepatic fat accumulation and ’browning’ of adipose tissue by the short-chain fatty acid acetate. Int. J. Obes. 2016, 40, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Liu, W.; Piao, M.; Zhu, H. A review of the relationship between the gut microbiota and amino acid metabolism. Amino Acids 2017, 49, 2083–2090. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Mu, C.-L.; Farzi, A.; Zhu, W.-Y. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. Int. Rev. J. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Ye, L.; Bae, M.; Cassilly, C.D.; Jabba, S.V.; Thorpe, D.W.; Martin, A.M.; Lu, H.-Y.; Wang, J.; Thompson, J.D.; Lickwar, C.R.; et al. Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host Microbe 2021, 29, 179–196.e9. [Google Scholar] [CrossRef]
- Bhattarai, Y.; Williams, B.B.; Battaglioli, E.J.; Whitaker, W.R.; Till, L.; Grover, M.; Linden, D.R.; Akiba, Y.; Kandimalla, K.K.; Zachos, N.C.; et al. Gut Microbiota-Produced Tryptamine Activates an Epithelial G-Protein-Coupled Receptor to Increase Colonic Secretion. Cell Host Microbe 2018, 23, 775–785.e5. [Google Scholar] [CrossRef]
- Chimerel, C.; Emery, E.; Summers, D.K.; Keyser, U.; Gribble, F.M.; Reimann, F. Bacterial Metabolite Indole Modulates Incretin Secretion from Intestinal Enteroendocrine L Cells. Cell Rep. 2014, 9, 1202–1208. [Google Scholar] [CrossRef]
- Natividad, J.M.; Agus, A.; Planchais, J.; Lamas, B.; Jarry, A.C.; Martin, R.; Michel, M.-L.; Chong-Nguyen, C.; Roussel, R.; Straube, M.; et al. Impaired Aryl Hydrocarbon Receptor Ligand Production by the Gut Microbiota Is a Key Factor in Metabolic Syndrome. Cell Metab. 2018, 28, 737–749.e4. [Google Scholar] [CrossRef] [PubMed]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef]
- Postal, B.G.; Ghezzal, S.; Aguanno, D.; André, S.; Garbin, K.; Genser, L.; Brot-Laroche, E.; Poitou, C.; Soula, H.; Leturque, A.; et al. AhR activation defends gut barrier integrity against damage occurring in obesity. Mol. Metab. 2020, 39, 101007. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A Branched-Chain Amino Acid-Related Metabolic Signature that Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef]
- Jang, C.; Oh, S.F.; Wada, S.; Rowe, G.C.; Liu, L.; Chan, M.C.; Rhee, J.; Hoshino, A.; Kim, B.; Ibrahim, A.; et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat. Med. 2016, 22, 421–426. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Cani, P.D.; Van Hul, M.; Lefort, C.; Depommier, C.; Rastelli, M.; Everard, A. Microbial regulation of organismal energy homeostasis. Nat. Metab. 2019, 1, 34–46. [Google Scholar] [CrossRef]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef]
- Massier, L.; Chakaroun, R.; Tabei, S.; Crane, A.; Didt, K.D.; Fallmann, J.; Von Bergen, M.; Haange, S.-B.; Heyne, H.; Stumvoll, M.; et al. Adipose tissue derived bacteria are associated with inflammation in obesity and type 2 diabetes. Gut 2020, 69, 1796–1806. [Google Scholar] [CrossRef]
- Velmurugan, G.; Dinakaran, V.; Rajendhran, J.; Swaminathan, K. Blood Microbiota and Circulating Microbial Metabolites in Diabetes and Cardiovascular Disease. Trends Endocrinol. Metab. 2020, 31, 835–847. [Google Scholar] [CrossRef]
- Cani, P.D.; Van Hul, M. Microbial signatures in metabolic tissues: A novel paradigm for obesity and diabetes? Nat. Metab. 2020, 2, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Aru, J.; Freeman, J.; Hudson, R.; Janssen, I.; Van Pelt, D.W.; Guth, L.M.; Wang, A.Y.; Horowitz, J.F.; Veiga-Lopez, A.; et al. Abdominal adiposity and insulin resistance in obese men. Am. J. Physiol. Metab. 2002, 282, E657–E663. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet-Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.Q.; Bretin, A.; Adeshirlarijaney, A.; Yeoh, B.S.; Vijay-Kumar, M.; Zou, J.; Denning, T.L.; Chassaing, B.; Gewirtz, A.T. “Western Diet”-Induced Adipose Inflammation Requires a Complex Gut Microbiota. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 313–333. [Google Scholar] [CrossRef]
- Erridge, C. Endogenous ligands of TLR2 and TLR4: Agonists or assistants? J. Leukoc. Biol. 2010, 87, 989–999. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Bleau, C.; Karelis, A.D.; St-Pierre, D.H.; Lamontagne, L. Crosstalk between intestinal microbiota, adipose tissue and skeletal muscle as an early event in systemic low-grade inflammation and the development of obesity and diabetes. Diabetes/Metab. Res. Rev. 2014, 31, 545–561. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.E.; Gabler, N.K.; Walker-Daniels, J.; Spurlock, M.E. Tlr-4 Deficiency Selectively Protects Against Obesity Induced by Diets High in Saturated Fat. Obesity 2008, 16, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Al-Mass, A.; Atizado, V.; Al-Hubail, A.; Al-Ghimlas, F.; Al-Arouj, M.; Bennakhi, A.; Dermime, S.; Behbehani, K. Elevated expression of the toll like receptors 2 and 4 in obese individuals: Its significance for obesity-induced inflammation. J. Inflamm. 2012, 9, 48. [Google Scholar] [CrossRef]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef]
- Talukdar, S.; Oh, D.Y.; Bandyopadhyay, G.; Li, D.; Xu, J.; McNelis, J.; Lu, M.; Li, P.; Yan, Q.; Zhu, Y.; et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 2012, 18, 1407–1412. [Google Scholar] [CrossRef]
- Creely, S.J.; McTernan, P.G.; Kusminski, C.M.; Fisher, F.M.; Da Silva, N.F.; Khanolkar, M.; Evans, M.; Harte, A.L.; Kumar, S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E740–E747. [Google Scholar] [CrossRef]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef]
- Giannoudaki, E.; Hernandez-Santana, Y.E.; Mulfaul, K.; Doyle, S.L.; Hams, E.; Fallon, P.G.; Mat, A.; O’shea, D.; Kopf, M.; Hogan, A.E.; et al. Interleukin-36 cytokines alter the intestinal microbiome and can protect against obesity and metabolic dysfunction. Nat. Commun. 2019, 10, 4003. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Anishetty, S. A meta-metabolome network of carbohydrate metabolism: Interactions between gut microbiota and host. Biochem. Biophys. Res. Commun. 2012, 428, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.-J. Gut bacteria alteration in obese people and its relationship with gene polymorphism. World J. Gastroenterol. 2011, 17, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, T.F.S.; Grześkowiak, Ł.M.; Salminen, S.; Laitinen, K.; Bressan, J.; Peluzio, M.D.C.G. Faecal levels of Bifidobacterium and Clostridium coccoides but not plasma lipopolysaccharide are inversely related to insulin and HOMA index in women. Clin. Nutr. 2013, 32, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Andoh, A.; Nishida, A.; Takahashi, K.; Inatomi, O.; Imaeda, H.; Bamba, S.; Kito, K.; Sugimoto, M.; Kobayashi, T. Comparison of the gut microbial community between obese and lean peoples using 16S gene sequencing in a Japanese population. J. Clin. Biochem. Nutr. 2016, 59, 65–70. [Google Scholar] [CrossRef]
- Hippe, B.; Remely, M.; Aumueller, E.; Pointner, A.; Magnet, U.; Haslberger, A. Faecalibacterium prausnitzii phylotypes in type two diabetic, obese, and lean control subjects. Benef. Microbes 2016, 7, 511–517. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Carlsson, A.H.; Yakymenko, O.; Olivier, I.; Håkansson, F.; Postma, E.; Keita, A.V.; Söderholm, J.D. Faecalibacterium prausnitziisupernatant improves intestinal barrier function in mice DSS colitis. Scand. J. Gastroenterol. 2013, 48, 1136–1144. [Google Scholar] [CrossRef]
- Duncan, S.H.; Hold, G.; Harmsen, H.J.M.; Stewart, C.S.; Flint, H.J. Growth requirements and fermentation products of Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 2141–2146. [Google Scholar] [CrossRef]
- Willemsen, L.E.M.; Koetsier, M.A.; Van Deventer, S.J.H.; Van Tol, E.A.F. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E1 and E2 production by intestinal myofibroblasts. Gut 2003, 52, 1442–1447. [Google Scholar] [CrossRef]
- Miao, W.; Wu, X.; Wang, K.; Wang, W.; Wang, Y.; Li, Z.; Liu, J.; Li, L.; Peng, L. Sodium Butyrate Promotes Reassembly of Tight Junctions in Caco-2 Monolayers Involving Inhibition of MLCK/MLC2 Pathway and Phosphorylation of PKCβ2. Int. J. Mol. Sci. 2016, 17, 1696. [Google Scholar] [CrossRef]
- Ma, X.; Fan, P.X.; Li, L.S.; Qiao, S.Y.; Zhang, G.; Li, D.F. Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions. J. Anim. Sci. 2012, 90, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Emoto, T.; Yamashita, T.; Watanabe, H.; Hayashi, T.; Tabata, T.; Hoshi, N.; Hatano, N.; Ozawa, G.; Sasaki, N.; et al. Bacteroides vulgatus and Bacteroides dorei Reduce Gut Microbial Lipopolysaccharide Production and Inhibit Atherosclerosis. Circulation 2018, 138, 2486–2498. [Google Scholar] [CrossRef] [PubMed]
- Drissi, F.; Merhej, V.; Angelakis, E.; El Kaoutari, A.; Carrière, F.; Henrissat, B.; Raoult, D. Comparative genomics analysis of Lactobacillus species associated with weight gain or weight protection. Nutr. Diabetes 2014, 4, e109. [Google Scholar] [CrossRef]
- Ottman, N.; Reunanen, J.; Meijerink, M.; Pietilä, T.E.; Kainulainen, V.; Klievink, J.; Huuskonen, L.; Aalvink, S.; Skurnik, M.; Boeren, S.; et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS ONE 2017, 12, e0173004. [Google Scholar] [CrossRef] [PubMed]
- Macchione, I.G.; Lopetuso, L.R.; Ianiro, G.; Napoli, M.; Gibiino, G.; Rizzatti, G.; Petito, V.; Gasbarrini, A.; Scaldaferri, F. Akkermansia muciniphila: Key player in metabolic and gastrointestinal disorders. Eur. Rev. Med. Pharm. Sci. 2019, 23, 8075–8083. [Google Scholar]
- Chelakkot, C.; Choi, Y.; Kim, D.-K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.-S.; Jee, Y.-K.; Gho, Y.S.; et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef] [PubMed]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef]
- Salguero, M.V.; Al-Obaide, M.A.I.; Singh, R.; Siepmann, T.; Vasylyeva, T.L. Dysbiosis of Gram-negative gut microbiota and the associated serum lipopolysaccharide exacerbates inflammation in type 2 diabetic patients with chronic kidney disease. Exp. Ther. Med. 2019, 18, 3461–3469. [Google Scholar] [CrossRef]
- D’hennezel, E.; Abubucker, S.; Murphy, L.O.; Cullen, T.W. Total Lipopolysaccharide from the Human Gut Microbiome Silences Toll-Like Receptor Signaling. Msystems 2017, 2, e00046-17. [Google Scholar] [CrossRef]
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.-E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Miele, L.; Valenza, V.; La Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Mascianà, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G.; et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009, 49, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Poritz, L.S.; Garver, K.I.; Green, C.; Fitzpatrick, L.; Ruggiero, F.; Koltun, W.A. Loss of the Tight Junction Protein ZO-1 in Dextran Sulfate Sodium Induced Colitis. J. Surg. Res. 2007, 140, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Wilbrink, J.; Bernards, N.; Mujagic, Z.; Van Avesaat, M.; Pijls, K.; Klaassen, T.; Van Eijk, H.; Nienhuijs, S.; Stronkhorst, A.; Wilms, E.; et al. Intestinal barrier function in morbid obesity: Results of a prospective study on the effect of sleeve gastrectomy. Int. J. Obes. 2019, 44, 368–376. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Grosheva, I.; Zheng, D.; Soffer, E.; Blacher, E.; Braverman, S.; Tengeler, A.C.; Barak, O.; Elazar, M.; et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 2018, 359, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Damms-Machado, A.; Louis, S.; Schnitzer, A.; Volynets, V.; Rings, A.; Basrai, M.; Bischoff, S.C. Gut permeability is related to body weight, fatty liver disease, and insulin resistance in obese individuals undergoing weight reduction. Am. J. Clin. Nutr. 2017, 105, 127–135. [Google Scholar] [CrossRef]
- Boets, E.; Gomand, S.V.; Deroover, L.; Preston, T.; Vermeulen, K.; De Preter, V.; Hamer, H.M.; Van den Mooter, G.; De Vuyst, L.; Courtin, C.M.; et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: A stable isotope study. J. Physiol. 2017, 595, 541–555. [Google Scholar] [CrossRef]
- Saint-Criq, V.; Lugo-Villarino, G.; Thomas, M. Dysbiosis, malnutrition and enhanced gut-lung axis contribute to age-related respiratory diseases. Ageing Res. Rev. 2020, 66, 101235. [Google Scholar] [CrossRef]
- Lahiri, S.; Kim, H.; Garcia-Perez, I.; Reza, M.M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H.; et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 2019, 11, eaan5662. [Google Scholar] [CrossRef]
- Nay, K.; Jollet, M.; Goustard, B.; Baati, N.; Vernus, B.; Pontones, M.; Lefeuvre-Orfila, L.; Bendavid, C.; Rué, O.; Mariadassou, M.; et al. Gut bacteria are critical for optimal muscle function: A potential link with glucose homeostasis. Am. J. Physiol. Metab. 2019, 317, E158–E171. [Google Scholar] [CrossRef]
- Okamoto, T.; Morino, K.; Ugi, S.; Nakagawa, F.; Lemecha, M.; Ida, S.; Ohashi, N.; Sato, D.; Fujita, Y.; Maegawa, H. Microbiome potentiates endurance exercise through intestinal acetate production. Am. J. Physiol. Metab. 2019, 316, E956–E966. [Google Scholar] [CrossRef]
- Walsh, M.E.; Bhattacharya, A.; Sataranatarajan, K.; Qaisar, R.; Sloane, L.; Rahman, M.; Kinter, M.; Van Remmen, H. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell 2015, 14, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fu, C.; Li, F. Acetate Affects the Process of Lipid Metabolism in Rabbit Liver, Skeletal Muscle and Adipose Tissue. Animals 2019, 9, 799. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.; Murphy, K.G.; Frost, G.; Chambers, E.S. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat. Metab. 2020, 2, 840–848. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Produced Succinate Improves Glucose Homeostasis via Intestinal Gluconeogenesis. Cell Metab. 2016, 24, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Dardevet, D.; Moore, M.C.; Remond, D.; Everett-Grueter, C.A.; Cherrington, A.D. Regulation of hepatic metabolism by enteral delivery of nutrients. Nutr. Res. Rev. 2006, 19, 161–173. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’mahony, S.M. The microbiome-gut-brain axis: From bowel to behavior. Neurogastroenterol. Motil. 2011, 23, 187–192. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Bhoori, S.; Castelli, C.; Putignani, L.; Rivoltini, L.; Del Chierico, F.; Sanguinetti, M.; Morelli, D.; Sterbini, F.P.; Petito, V.; et al. Hepatocellular Carcinoma Is Associated With Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease. Hepatology 2019, 69, 107–120. [Google Scholar] [CrossRef]
- Wong, V.W.-S.; Tse, C.-H.; Lam, T.-T.Y.; Wong, G.L.-H.; Chim, A.M.-L.; Chu, W.C.-W.; Yeung, D.K.-W.; Law, P.T.-W.; Kwan, H.S.; Yu, J.; et al. Molecular Characterization of the Fecal Microbiota in Patients with Nonalcoholic Steatohepatitis–A Longitudinal Study. PLoS ONE 2013, 8, e62885. [Google Scholar] [CrossRef]
- Ahmadmehrabi, S.; Tang, W.W. Gut microbiome and its role in cardiovascular diseases. Curr. Opin. Cardiol. 2017, 32, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).