Changing Patterns of Antihyperglycaemic Treatment among Patients with Type 2 Diabetes in Hungary between 2015 and 2020—Nationwide Data from a Register-Based Analysis
Abstract
:1. Introduction
2. Material and Methods
- antihyperglycaemic agents (in monotherapy or in combination) in 2015–2020 (assessing prevalence of patients with different treatment regimes)
- antihyperglycaemic agents as initial therapy in 2015–2020 (assessing incidence of patients with initial therapy)
- novel drugs with cardiovascular/renal benefit (either in monotherapy or in combination) after initial therapy with MET or SU (assessing incidence of patients with such treatment after initial MET or SU therapy; for comparison, DPP-4-inhibitors and INS were also analysed).
3. Results
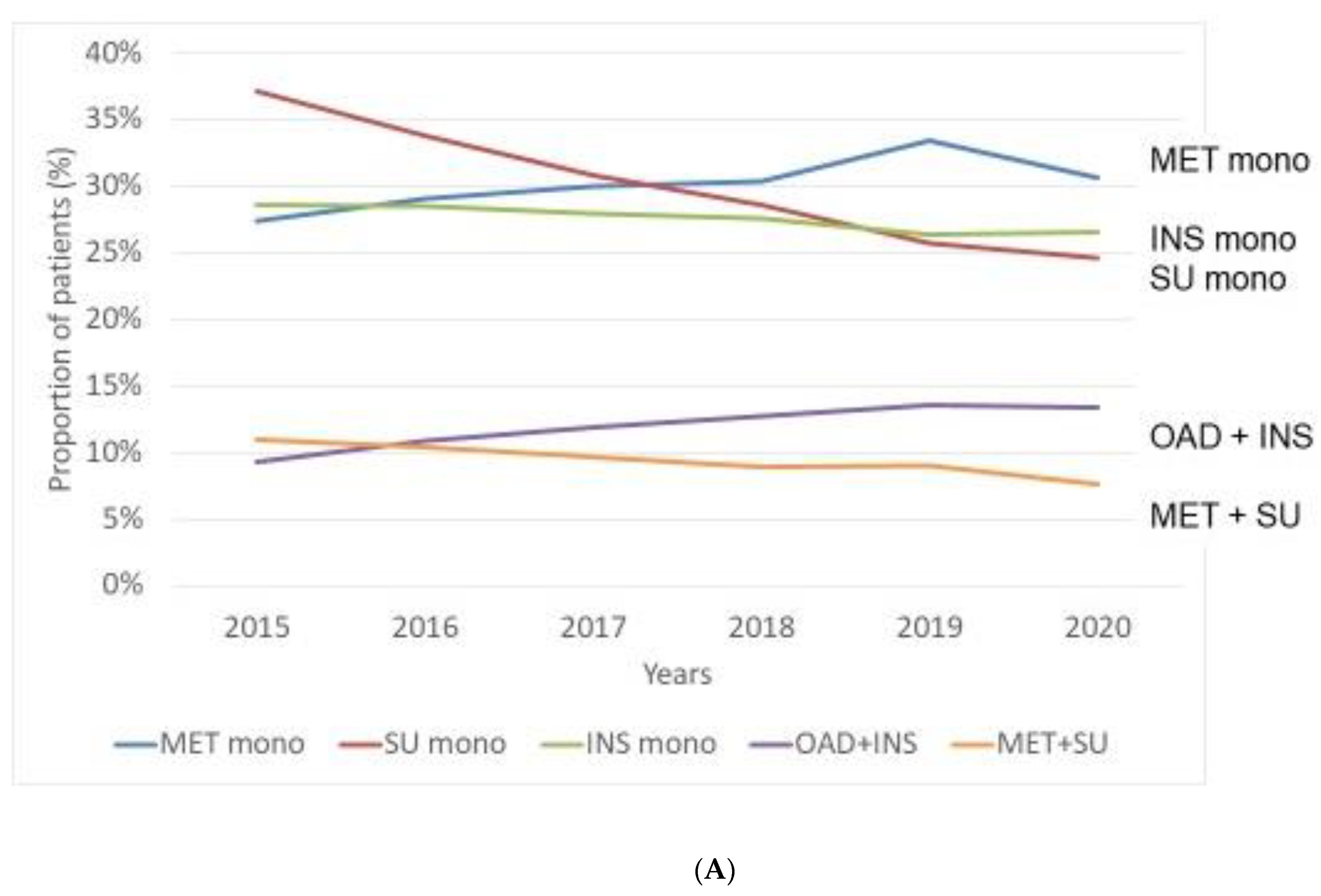
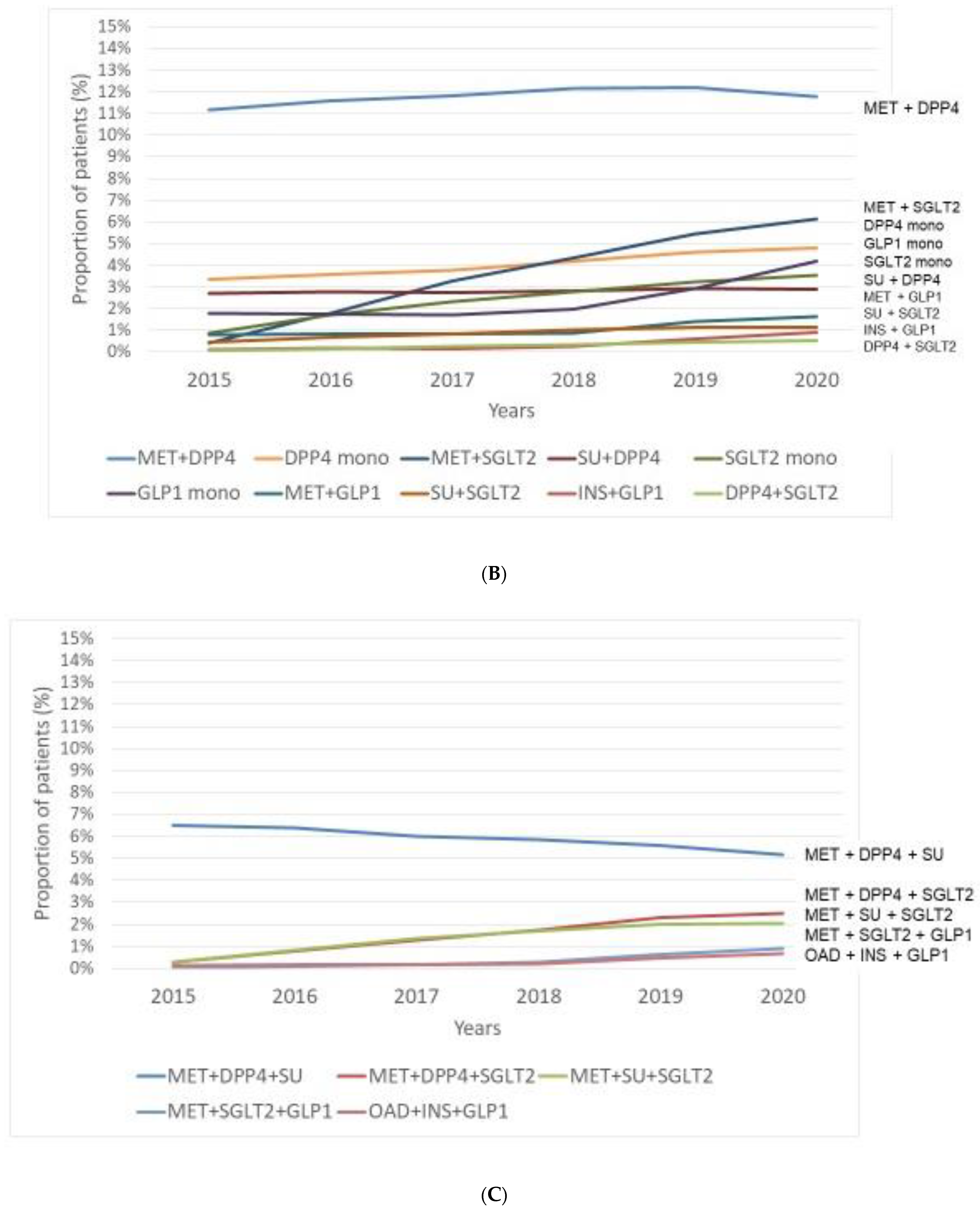
3.1. Prevalence of Patients with Different Treatment Patterns—Use of Antihyperglycaemic Agents in Monotherapy or in Combination between 2015 and 2020
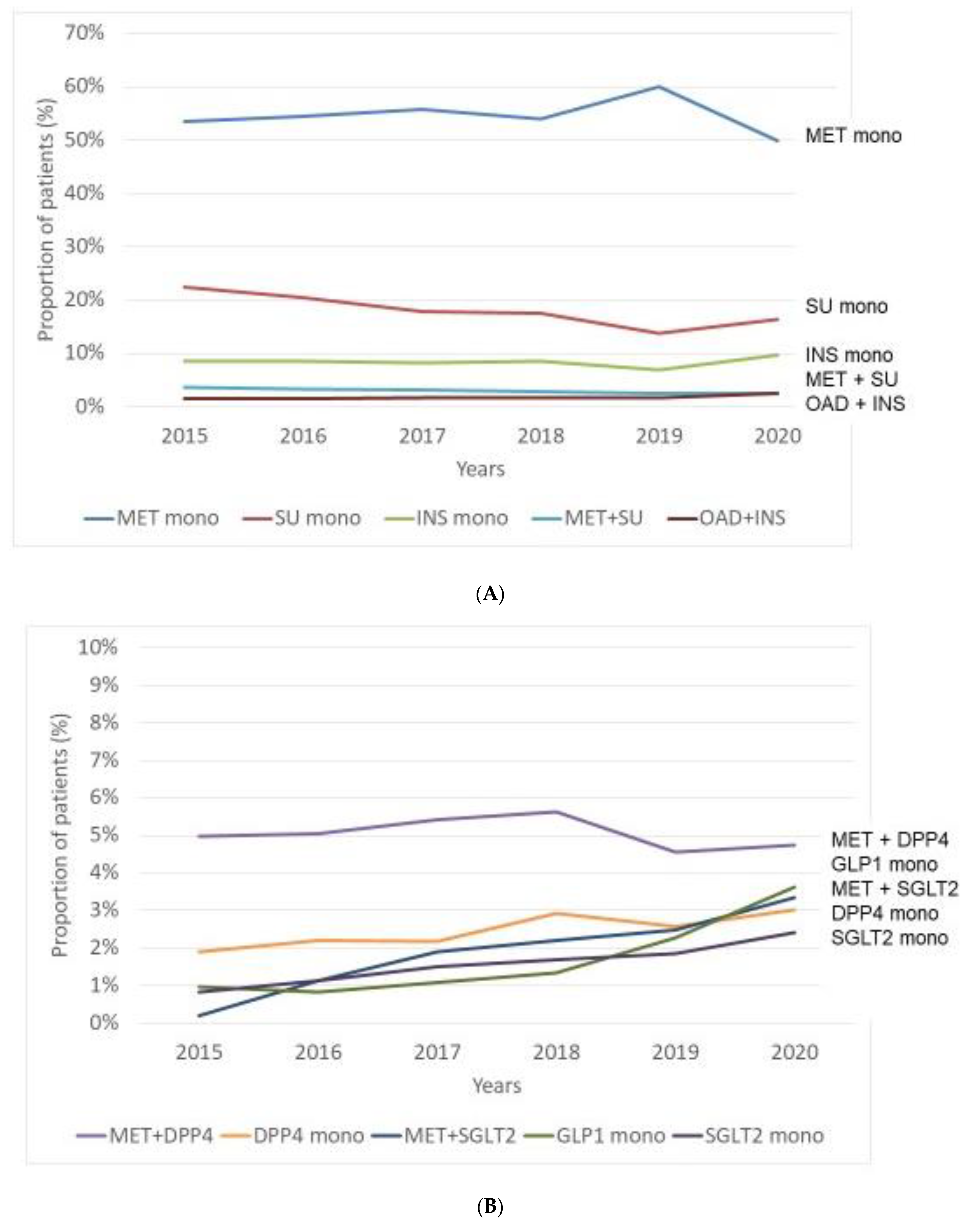
3.2. Incidence of Patients with Initial Therapy—Use of Antihyperglycaemic Agents as Initial Therapy in the Period of 2015–2020
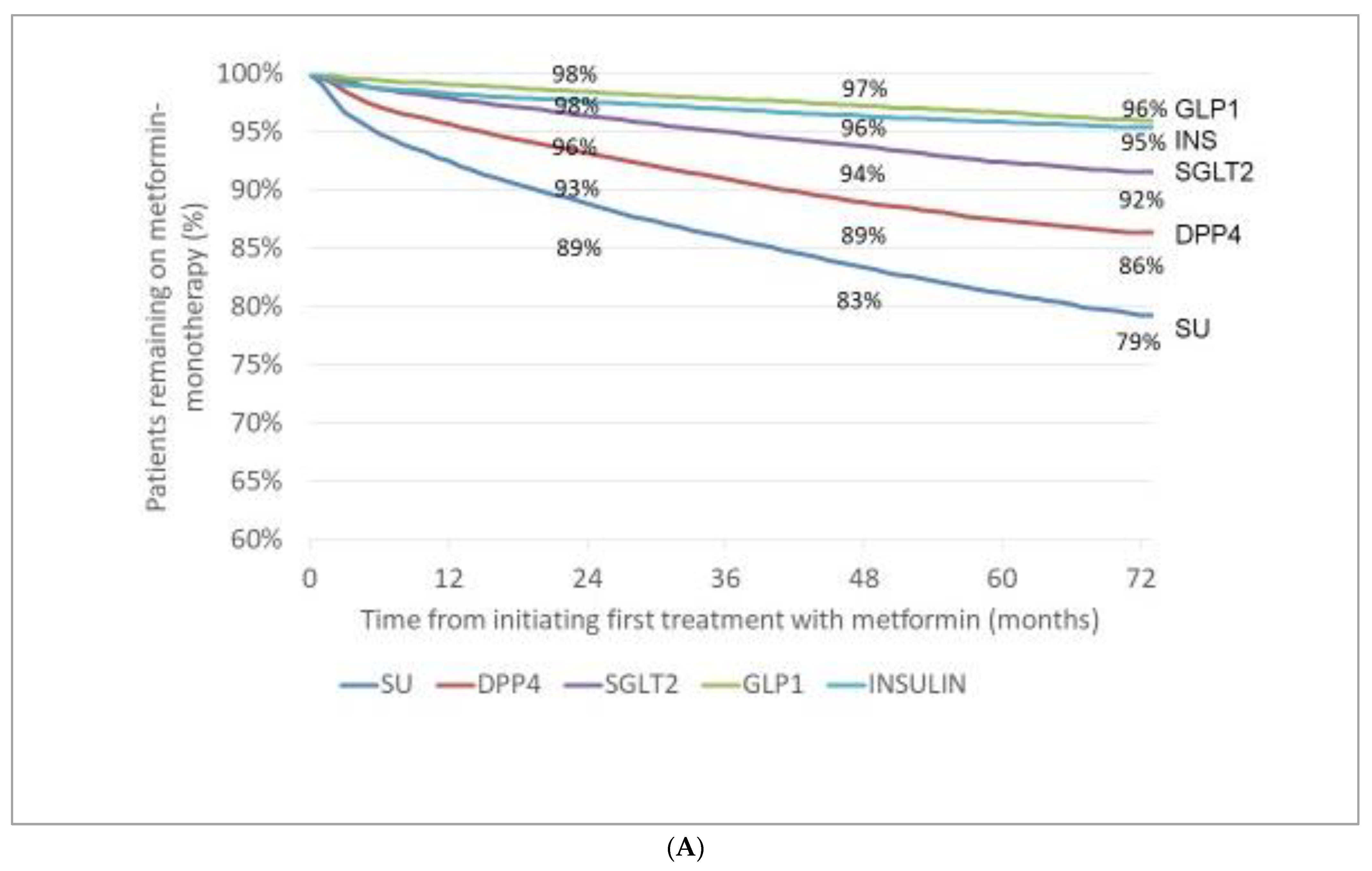
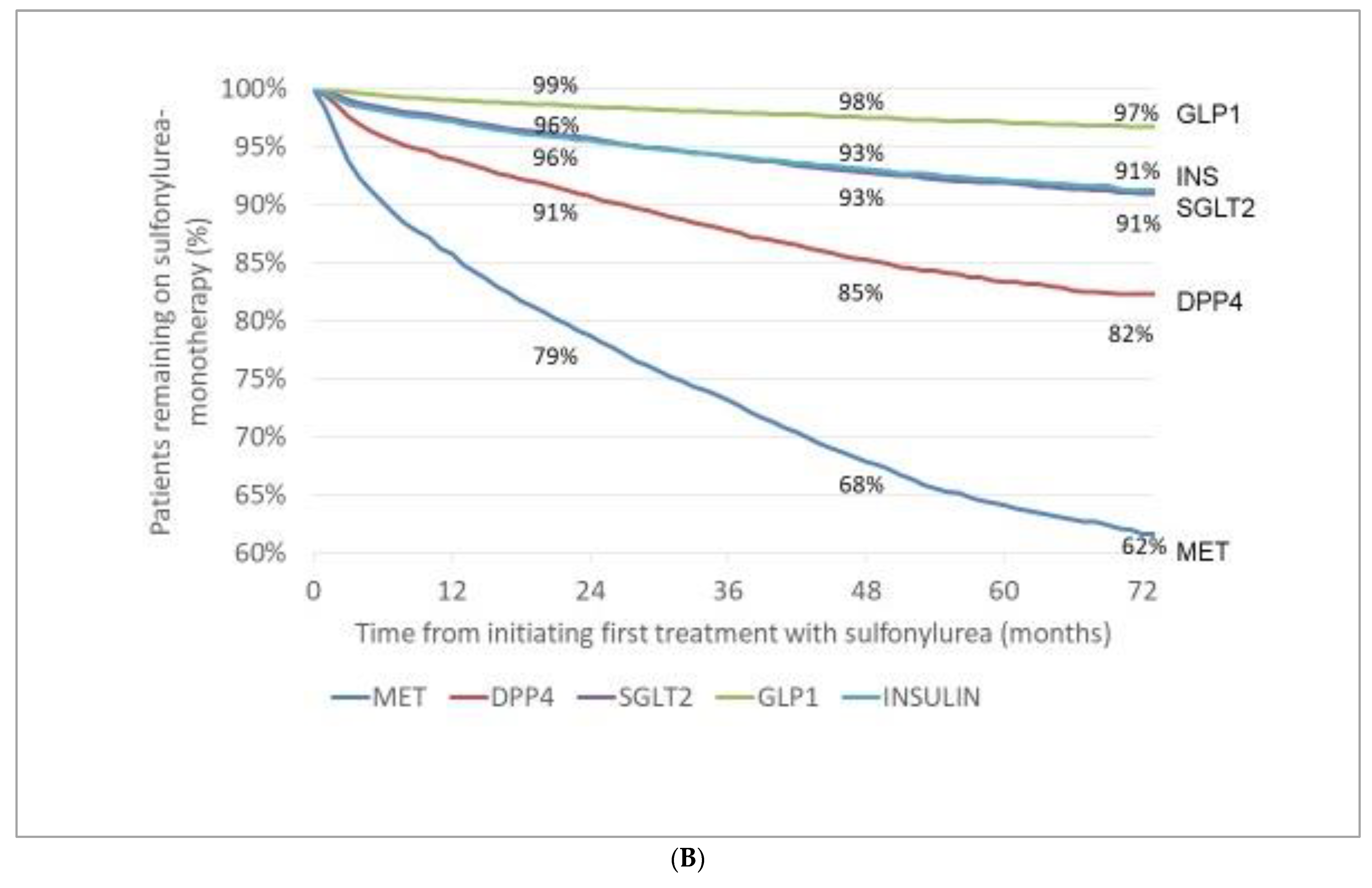
3.3. Incidence (Appearance) of Novel Drugs with Cardiovascular/Renal Benefit (Either in Monotherapy or in Combination) after Initial Therapy with MET or SU
4. Discussion
- Regarding prevalence of patients with traditional antidiabetic drugs, we observed a relatively stable prevalence of patients with MET reaching the highest proportion among monotherapy in 2020. On the other hand, prevalent cases with SUs markedly and those of INS slightly decreased over time. Among novel drugs, DPP-4 inhibitors were the most frequently administered drugs. Although we found a clearly increasing rate of patients with SGLT-2 inhibitors and GLP-1-RAs, these drugs were used only in a small proportion of patients.
- As for initial therapy, patients with MET monotherapy had the highest incidence rate, while SUs—despite their steadily diminishing use—remained the second most frequently chosen initial monotherapy; incidence of patients with INS monotherapy did not change significantly over the entire period.
- After initial therapy with MET or SU, the incidence rates of patients with add-on GLP-1-RAs and those of add-on SGLT-2 inhibitors slowly increased in a relatively narrow range in the subsequent six years.
- Some abrupt alterations in the 6-year-long trends were observed in 2020, probably due to increasing rate of COVID-19 and the closedown in Hungary (from March 2020 onwards).
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IDF Diabetes Atlas—9th Edition. 2019. Available online: www.diabetesatlas.org (accessed on 17 July 2022).
- IDF Diabetes Atlas—10th Edition. 2021. Available online: www.diabetatlas.org (accessed on 17 July 2022).
- Jansson, S.P.; Fall, K.; Brus, O.; Magnuson, A.; Wändell, P.; Östgren, C.J.; Rolandsson, O. Prevalence and incidence of diabetes mellitus: A nationwide population-based pharmaco-epidemiological study in Sweden. Diabet. Med. 2015, 32, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Nazareth, I.; Petersen, I. Trends in incidence, prevalence and prescribing in type 2 diabetes mellitus between 2000 and 2013 in primary care: A retrospective cohort study. BMJ Open 2016, 6, e010210. [Google Scholar] [CrossRef] [PubMed]
- Norhammar, A.; Bodegård, J.; Nyström, T.; Thuresson, M.; Eriksson, J.W.; Nathanson, D. Incidence, prevalence and mortality of type 2 diabetes requiring glucose-lowering treatment, and associated risks of cardiovascular complications: A nationwide study in Sweden, 2006–2013. Diabetologia 2016, 59, 1692–1701. [Google Scholar] [CrossRef] [Green Version]
- Jermendy, G.; Kiss, Z.; Rokszin, G.; Abonyi-Tóth, Z.; Wittmann, I.; Kempler, P. Decreasing incidence of pharmacologically treated Type 2 diabetes in Hungary from 2001 to 2016: A nationwide cohort study. Diabetes Res. Clin. Pract. 2019, 155, 107788. [Google Scholar] [CrossRef] [PubMed]
- Magliano, D.J.; Chen, L.; Islam, R.M.; Carstensen, B.; Gregg, E.W.; Pavkov, M.E.; Andes, L.J.; Balicer, R.; Baviera, M.; Boersma-van Dam, E.; et al. Trends in the incidence of diagnosed diabetes: A multicountry analysis of aggregate data from 22 million diagnoses in high-income and middle-income settings. Lancet Diabetes Endocrinol. 2021, 9, 203–211. [Google Scholar] [CrossRef]
- Monami, M.; Ahrén, B.; Dicembrini, I.; Mannucci, E. Dipeptidyl peptidase-4 inhibitors and cardiovascular risk: A meta-analysis of randomized clinical trials. Diabetes Obes. Metab. 2013, 15, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; Norton, L.; Defronzo, R.A. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr. Rev. 2011, 32, 515–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheen, A.J. Cardiovascular effects of new oral glucose-lowering agents: DPP-4 and SGLT-2 inhibitors. Circ. Res. 2018, 122, 1439–1459. [Google Scholar] [CrossRef]
- Ahuja, V.; Chou, C.H. Novel therapeutics for diabetes: Uptake, usage trends, and comparative effectiveness. Curr. Diab. Rep. 2016, 16, 47. [Google Scholar] [CrossRef]
- Thrasher, J. Pharmacologic management of type 2 diabetes mellitus: Available therapies. Am. J. Med. 2017, 130, S4–S17. [Google Scholar] [CrossRef]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jermendy, G.; Kiss, Z.; Rokszin, G.; Abonyi-Tóth, Z.; Wittmann, I.; Kempler, P. Trends in antidiabetic treatment prescribed for patients with type 2 diabetes in Hungary between 2001 and 2014—Results from the database analysis of the National Health Insurance Fund. Orv. Hetil. 2017, 158, 770–778. (In Hungarian) [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carstensen, B.; Borch-Johnsen, K. Register-based studies of diabetes. Scand. J. Public Health 2011, 39 (Suppl. 7), 175–179. [Google Scholar] [CrossRef] [PubMed]
- Csatordai, M.; Benkő, R.; Matuz, M.; Bor, A.; Lengyel, C.; Doró, P. Use of glucose-lowering drugs in Hungary between 2008 and 2017: The increasing use of novel glucose-lowering drug groups. Diabet. Med. 2019, 36, 1612–1620. [Google Scholar] [CrossRef]
- Gęca, T.; Wojtowicz, K.; Guzik, P.; Góra, T. Increased risk of COVID-19 in patients with diabetes mellitus—Current challenges in pathophysiology, treatment and prevention. Int. J. Environ. Res. Public Health 2022, 19, 6555. [Google Scholar] [CrossRef]
- de Kreutzenberg, S.V. Telemedicine for the clinical management of diabetes; implications and considerations after COVID-19 experience. High Blood Press. Cardiovasc. Prev. 2022, 29, 319–326. [Google Scholar] [CrossRef]
- Jermendy, G.; Gaál, Z.; Gerő, L.; Hidvégi, T.; Jermendy, G.; Kempler, P.; Lengyel, C.; Várkonyi, T.; Winkler, G.; Wittmann, I. Clinical Practice Guideline—Diagnosis of diabetes, and antihyperglycaemic treatment and care of patients with diabetes in adulthood. Diabetol. Hung. 2020, 28, 119–204. (In Hungarian) [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [Green Version]
- Bailey, C.J. Metformin: Historical overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Xie, H.; Liu, Y.; Gao, P.; Yang, X.; Shen, Z. Effect of metformin on all-cause and cardiovascular mortality in patients with coronary artery diseases: A systematic review and an updated meta-analysis. Cardiovasc. Diabetol. 2019, 18, 96. [Google Scholar] [CrossRef]
- Zhao, H.; Swanson, K.D.; Zheng, B. Therapeutic repurposing of biguanides in cancer. Trends Cancer 2021, 7, 714–730. [Google Scholar] [CrossRef] [PubMed]
- Heckman-Stoddard, B.M.; DeCensi, A.; Sahasrabuddhe, V.V.; Ford, L.G. Repurposing metformin for the prevention of cancer and cancer recurrence. Diabetologia 2017, 60, 1639–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajasurya, V.; Gunasekaran, K.; Surani, S. Interstitial lung disease and diabetes. World J. Diabetes 2020, 11, 351–357. [Google Scholar] [CrossRef]
- Lee, C.B.; Chae, S.U.; Jo, S.J.; Jerng, U.M.; Bae, S.K. The relationship between the gut microbiome and metformin as a key for treating type 2 diabetes mellitus. Int. J. Mol. Sci. 2021, 22, 3566. [Google Scholar] [CrossRef] [PubMed]
- Zangiabadian, M.; Nejadghaderi, S.A.; Zahmatkesh, M.M.; Hajikhani, B.; Mirsaeidi, M.; Nasiri, M.J. The efficacy and potential mechanisms of metformin in the treatment of COVID-19 in the diabetics: A systematic review. Front. Endocrinol. 2021, 12, 645194. [Google Scholar] [CrossRef] [PubMed]
- Soukas, A.A.; Hao, H.; Wu, L. Metformin as anti-aging therapy: Is it for everyone? Trends Endocrinol. Metab. 2019, 30, 745–755. [Google Scholar] [CrossRef]
- Henquin, J.C. The fiftieth anniversary of hypoglycaemic sulphonamides. How did the mother compound work? Diabetologia 1992, 35, 907–912. [Google Scholar] [CrossRef]
- Consoli, A.; Czupryniak, L.; Duarte, R.; Jermendy, G.; Kautzky-Willer, A.; Mathieu, C.; Melo, M.; Mosenzon, O.; Nobels, F.; Papanas, N.; et al. Positioning sulfonylureas in a modern treatment algorithm for patients with type 2 diabetes: Expert opinion from a European Consensus Panel. Diabetes Obes. Metab. 2020, 22, 1705–1713. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Rutty, C.J. Insulin therapy: The discovery that shaped a century. Can. J. Diabetes 2021, 45, 798–803. [Google Scholar] [CrossRef]
- Howey, D.C.; Bowsher, R.R.; Brunelle, L.R.; Woodworth, J.R. Lys(B28), Pro(B29)-human insulin: A rapidly absorbed analog of human insulin. Diabetes 1994, 43, 396–402. [Google Scholar] [CrossRef]
- Mosenzon, O.; Del Prato, S.; Schechter, M.; Leiter, L.A.; Ceriello, A.; DeFronzo, R.A.; Raz, I. From glucose lowering agents to disease/diabetes modifying drugs: A “SIMPLE” approach for the treatment of type 2 diabetes. Cardiovasc. Diabetol. 2021, 20, 92. [Google Scholar] [CrossRef] [PubMed]
- Jermendy, G. De-escalation of antihyperglycemic treatment in patients with type 2 diabetes—When less is more. Orv. Hetil. 2019, 160, 1207–1215. (In Hungarian) [Google Scholar] [CrossRef] [PubMed]
- Jude, E.B.; Malecki, M.T.; Gomez Huelgas, R.; Prazny, M.; Snoek, F.; Tankova, T.; Giugliano, D.; Khunti, K. Expert panel guidance and narrative review of treatment simplification of complex insulin regimens to improve outcomes in type 2 diabetes. Diabetes Ther. 2022, 13, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Green, J.B.; Bethel, M.A.; Armstrong, P.W.; Buse, J.B.; Engel, S.S.; Garg, J.; Josse, R.; Kaufman, K.D.; Koglin, J.; Korn, S.; et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2015, 373, 232–242. [Google Scholar] [CrossRef] [Green Version]
- Florentin, M.; Kostapanos, M.S.; Papazafiropoulou, A.K. Role of dipeptidyl peptidase 4 inhibitors in the new era of antidiabetic treatment. World J. Diabetes 2022, 13, 85–96. [Google Scholar] [CrossRef]
- Jermendy, G.; Gaál, Z.; Kempler, P.; Lengyel, C.; Rosta, L.; Várkonyi, T.; Wittmann, I. DPP-4-inhibitors for treating patients with type 2 diabetes—Updated guidelines, changing clinical practice. Diabetol. Hung. 2020, 28, 69–75. (In Hungarian) [Google Scholar] [CrossRef]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.L.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Furtado, R.H.M.; et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019, 393, 31–39. [Google Scholar] [CrossRef]
- Kristensen, S.L.; Rørth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Køber, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785. [Google Scholar] [CrossRef]
- Janez, A.; Muzurovic, E.; Stoian, A.P.; Haluzik, M.; Guja, C.; Czupryniak, L.; Duvnjak, L.; Lalic, N.; Tankova, T.; Bogdanski, P.; et al. Translating results from the cardiovascular outcomes trials with glucagon-like peptide-1 receptor agonists into clinical practice: Recommendations from a Eastern and Southern Europe diabetes expert group. Int. J. Cardiol. 2022. [CrossRef]
- Mosenzon, O.; Alguwaihes, A.; Leon, J.L.A.; Bayram, F.; Darmon, P.; Davis, T.M.E.; Dieuzeide, G.; Eriksen, K.T.; Hong, T.; Kaltoft, M.S.; et al. CAPTURE: A multinational, cross-sectional study of cardiovascular disease prevalence in adults with type 2 diabetes across 13 countries. Cardiovasc. Diabetol. 2021, 20, 154. [Google Scholar] [CrossRef]
- Khunti, K.; Gomes, M.B.; Pocock, S.; Shestakova, M.V.; Pintat, S.; Fenici, P.; Hammar, N.; Medina, J. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: A systematic review. Diabetes Obes. Metab. 2018, 20, 427–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeFronzo, R.A. Banting Lecture. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, D.R.; Paldánius, P.M.; Proot, P.; Chiang, Y.; Stumvoll, M.; Del Prato, S.; VERIFY study group. Glycaemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): A 5-year, multicentre, randomised, double-blind trial. Lancet 2019, 394, 1519–1529. [Google Scholar] [CrossRef]
- Cho, Y.K.; Lee, J.; Kim, H.S.; Park, J.Y.; Jung, C.H.; Lee, W.J. Clinical efficacy of quadruple oral therapy for type 2 diabetes in real-world practice: A retrospective observational study. Diabetes Ther. 2020, 11, 2029–2039. [Google Scholar] [CrossRef]
- Ekström, N.; Svensson, A.M.; Miftaraj, M.; Andersson Sundell, K.; Cederholm, J.; Zethelius, B.; Eliasson, B.; Gudbjörnsdottir, S. Durability of oral hypoglycemic agents in drug naïve patients with type 2 diabetes: Report from the Swedish National Diabetes Register (NDR). BMJ Open Diabetes Res. Care 2015, 3, e000059. [Google Scholar] [CrossRef] [Green Version]
- Liatis, S.; Dafoulas, G.E.; Kani, C.; Politi, A.; Litsa, P.; Sfikakis, P.P.; Makrilakis, K. The prevalence and treatment patterns of diabetes in the Greek population based on real-world data from the nation-wide prescription database. Diabetes Res. Clin. Pract. 2016, 118, 162–167. [Google Scholar] [CrossRef]
- Roberto, G.; Barone-Adesi, F.; Giorgianni, F.; Pizzimenti, V.; Ferrajolo, C.; Tari, M.; Bartolini, C.; Da Cas, R.; Maggini, M.; Spila-Alegiani, S.; et al. Patterns and trends of utilization of incretin-based medicines between 2008 and 2014 in three Italian geographic areas. BMC Endocr. Disord. 2019, 19, 18. [Google Scholar] [CrossRef] [Green Version]
- Moreno Juste, A.; Menditto, E.; Orlando, V.; Monetti, V.M.; Gimeno Miguel, A.; González Rubio, F.; Aza-Pascual-Salcedo, M.M.; Cahir, C.; Prados Torres, A.; Riccardi, G. Treatment patterns of diabetes in Italy: A population-based study. Front. Pharmacol. 2019, 10, 870. [Google Scholar] [CrossRef] [Green Version]
- Bang, C.; Mortensen, M.B.; Lauridsen, K.G.; Bruun, J.M. Trends in antidiabetic drug utilization and expenditure in Denmark: A 22-year nationwide study. Diabetes Obes. Metab. 2020, 22, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Bucsa, C.; Farcas, A.; Iaru, I.; Mogosan, C.; Rusu, A. Drug utilisation study of antidiabetic medication during 2012–2019 in Romania. Int. J. Clin. Pract. 2021, 75, e14770. [Google Scholar] [CrossRef]
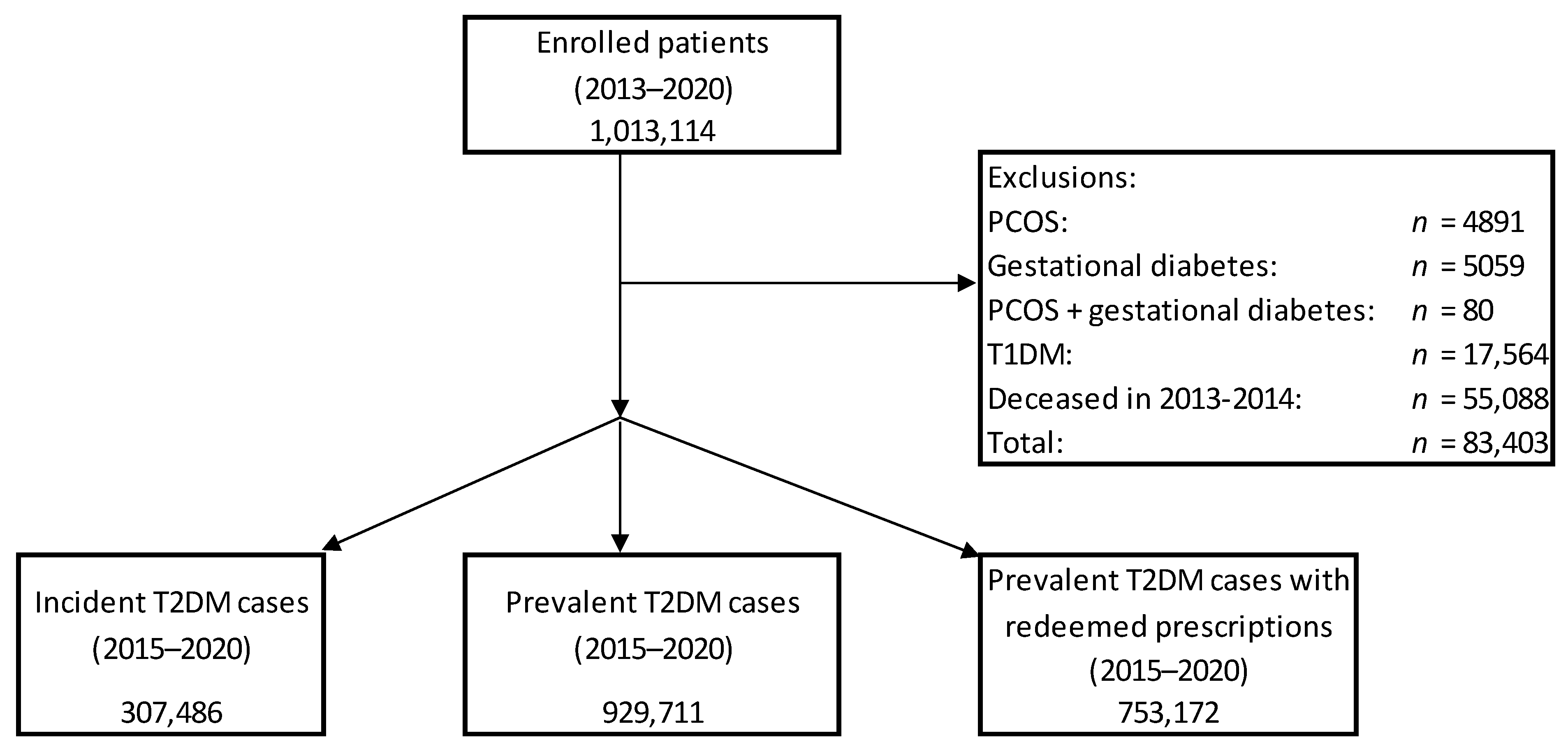
| Incident Cases (n) | Prevalent Cases (n) | Prevalent Cases with Redeemed Prescriptions of Antidiabetic Drugs (n) | |
|---|---|---|---|
| 2015 | 60,049 | 682,274 | 526,076 |
| 2016 | 57,726 | 706,617 | 535,220 |
| 2017 | 54,059 | 727,071 | 541,072 |
| 2018 | 52,506 | 743,585 | 543,494 |
| 2019 | 53,279 | 760,032 | 560,142 |
| 2020 | 29,865 | 752,367 | 540,607 |
| 2015–2020 | 307,486 | 929,711 | 753,172 |
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | |
|---|---|---|---|---|---|---|
| Total number of patients with redeemed prescriptions | 526,076 | 535,220 | 541,072 | 543,494 | 560,142 | 540,607 |
|
Number of patients with traditional agents (monotherapy or combination) | ||||||
| MET monotherapy | 144,044 | 155,563 | 162,311 | 165,088 | 187,110 | 165,779 |
| INS monotherapy | 150,659 | 152,503 | 151,282 | 149,983 | 147,666 | 143,500 |
| SU monotherapy | 195,355 | 181,033 | 166,961 | 155,466 | 144,375 | 133,283 |
| OAD + INS combination | 48,916 | 58,037 | 64,580 | 69,059 | 75,875 | 72,357 |
| MET + SU | 57,918 | 55,566 | 52,230 | 48,497 | 50,596 | 41,428 |
|
Number of patients with novel agents (monotherapy or dual combination) | ||||||
| MET + DPP-4 inhibitor | 58,700 | 62,108 | 63,934 | 66,083 | 68,331 | 63,734 |
| MET + SGLT-2 inhibitor | 2148 | 9578 | 17,770 | 23,640 | 30,544 | 33,144 |
| DPP-4 inhibitor | 17,696 | 19,170 | 20,476 | 22,840 | 25,907 | 25,940 |
| GLP-1-RAs | 9349 | 9305 | 9357 | 10,717 | 16,495 | 22,683 |
| SGLT-2- inhibitor | 4651 | 9121 | 12,579 | 15,087 | 18,150 | 19,163 |
| SU + DPP-4 inhibitor | 14,226 | 14,790 | 14,846 | 15,306 | 16,417 | 15,746 |
| MET + GLP-1-RA | 4136 | 4367 | 4427 | 4634 | 7395 | 8541 |
| SU + SGLT-2 inhibitor | 2286 | 3640 | 4489 | 5297 | 5968 | 6039 |
| INS + GLP-1-RA | 673 | 926 | 833 | 1374 | 3172 | 4745 |
| DPP-4 inhibitor + SGLT-2 inhibitor | 323 | 838 | 1381 | 1816 | 2434 | 2870 |
|
Number of patients with novel agents (triple combination) | ||||||
| MET + DPP-4 inhibitor + SU | 34,136 | 34,113 | 32,575 | 31,833 | 31,282 | 27,948 |
| MET + DPP-4 inhibitor + SGLT-2 inhibitor | 1499 | 4104 | 6705 | 9098 | 12,133 | 13,078 |
| MET + SU + SGLT-2 inhibitor | 1438 | 4237 | 7118 | 8974 | 10,555 | 10,751 |
| MET + SGLT-2 inhibitor + GLP-1-RA | 171 | 486 | 944 | 1588 | 3261 | 4673 |
| OAD + INS + GLP-1-RA | 652 | 900 | 850 | 1081 | 2539 | 3531 |
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | |
|---|---|---|---|---|---|---|
| Total number of patients with initial redeemed prescriptions | 43,115 | 42,023 | 39,236 | 37,250 | 42,151 | 25,496 |
| Number of patients with initial traditional agents (monotherapy or combination) | ||||||
| MET monotherapy | 23,060 | 22,901 | 21,889 | 20,128 | 25,278 | 12,710 |
| SU monotherapy | 9686 | 8611 | 7006 | 6512 | 5781 | 4194 |
| INS monotherapy | 3712 | 3599 | 3197 | 3203 | 2916 | 2481 |
| MET + SU | 1587 | 1376 | 1209 | 1054 | 1065 | 653 |
| OAD + insulin | 622 | 655 | 653 | 650 | 728 | 631 |
|
Number of patients with initial novel agents (monotherapy or combination) | ||||||
| MET + DPP-4 inhibitor | 2149 | 2119 | 2125 | 2095 | 1924 | 1208 |
| GLP-1-RA | 412 | 346 | 427 | 502 | 953 | 921 |
| MET + SGLT-2 inhibitor | 85 | 470 | 745 | 822 | 1045 | 851 |
| DPP-4 inhibitor | 814 | 926 | 857 | 1086 | 1089 | 769 |
| SGLT-2- inhibitor | 358 | 470 | 590 | 631 | 781 | 616 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jermendy, G.; Kiss, Z.; Rokszin, G.; Abonyi-Tóth, Z.; Lengyel, C.; Kempler, P.; Wittmann, I. Changing Patterns of Antihyperglycaemic Treatment among Patients with Type 2 Diabetes in Hungary between 2015 and 2020—Nationwide Data from a Register-Based Analysis. Medicina 2022, 58, 1382. https://doi.org/10.3390/medicina58101382
Jermendy G, Kiss Z, Rokszin G, Abonyi-Tóth Z, Lengyel C, Kempler P, Wittmann I. Changing Patterns of Antihyperglycaemic Treatment among Patients with Type 2 Diabetes in Hungary between 2015 and 2020—Nationwide Data from a Register-Based Analysis. Medicina. 2022; 58(10):1382. https://doi.org/10.3390/medicina58101382
Chicago/Turabian StyleJermendy, György, Zoltán Kiss, György Rokszin, Zsolt Abonyi-Tóth, Csaba Lengyel, Péter Kempler, and István Wittmann. 2022. "Changing Patterns of Antihyperglycaemic Treatment among Patients with Type 2 Diabetes in Hungary between 2015 and 2020—Nationwide Data from a Register-Based Analysis" Medicina 58, no. 10: 1382. https://doi.org/10.3390/medicina58101382





