Female Volleyball Players Are More Prone to Cortisol Anticipatory Stress Response than Sedentary Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
- irregular menstrual periods within the last 24 months or any history of pregnancy,
- presence of chronic disease, particularly cardiovascular disease or illness that involved disturbance of the HPA,
- previous/current mental disorders,
- heavy nicotine use (smoking >5 cigarettes per day, at least three times per week),
- heavy caffeine use (>300 mg/day) or feeling the side effects of caffeine withdrawal,
- using hormonal contraceptives,
- experiencing traumatic life events within the last six months,
- having had surgery under general anesthesia during the past year,
- underweight (body mass index (BMI) <18) or overweight (BMI >25),
- severe vision or hearing problems,
- for the control group only, moderate or high physical activity level measured by IPAQ [20].
2.2. Heart Rate Measurement
2.3. Salivary Cortisol Level Measurement
2.4. Psychological Tests
- TOC—task-oriented coping, which reflects undertaking attempts to manage the stressful situation, including cognitive reappraisal and information searching.
- EOC—emotion-oriented coping, manifesting as concentration on emotional experiences, and reducing emotional strain.
- AOC—avoidance-oriented coping, which can take two forms: engagement in alternative activities or social contacts to avoid confrontation with the source of stress.
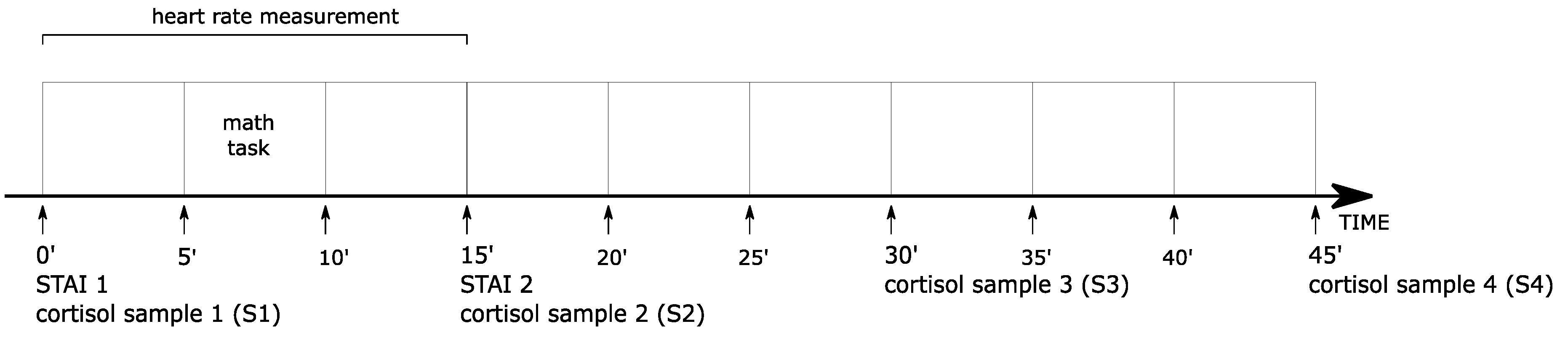
2.5. Study Design
2.6. Statistical Analysis
3. Results
3.1. Group Characteristics
3.2. State Anxiety
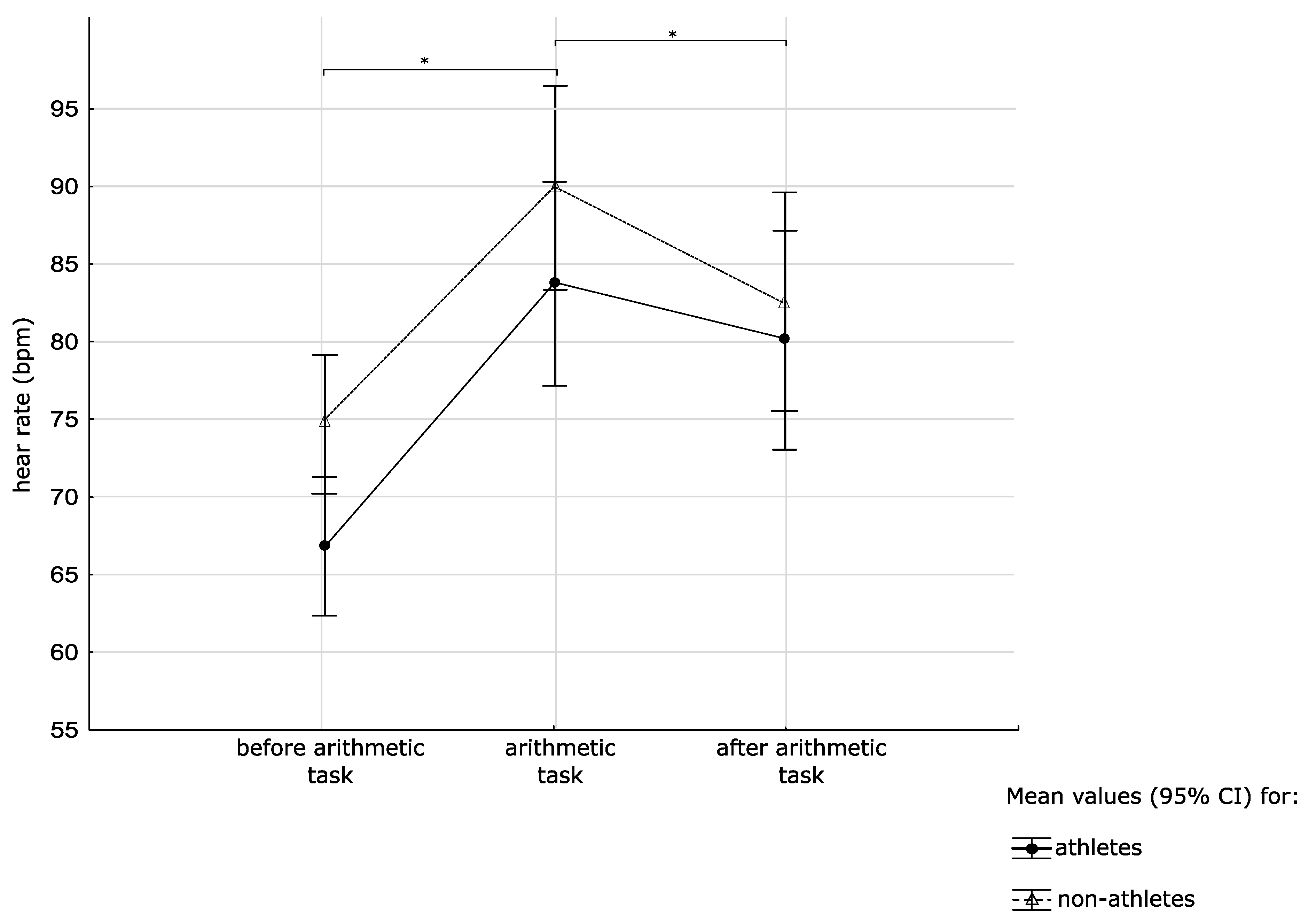
3.3. Heart Rate
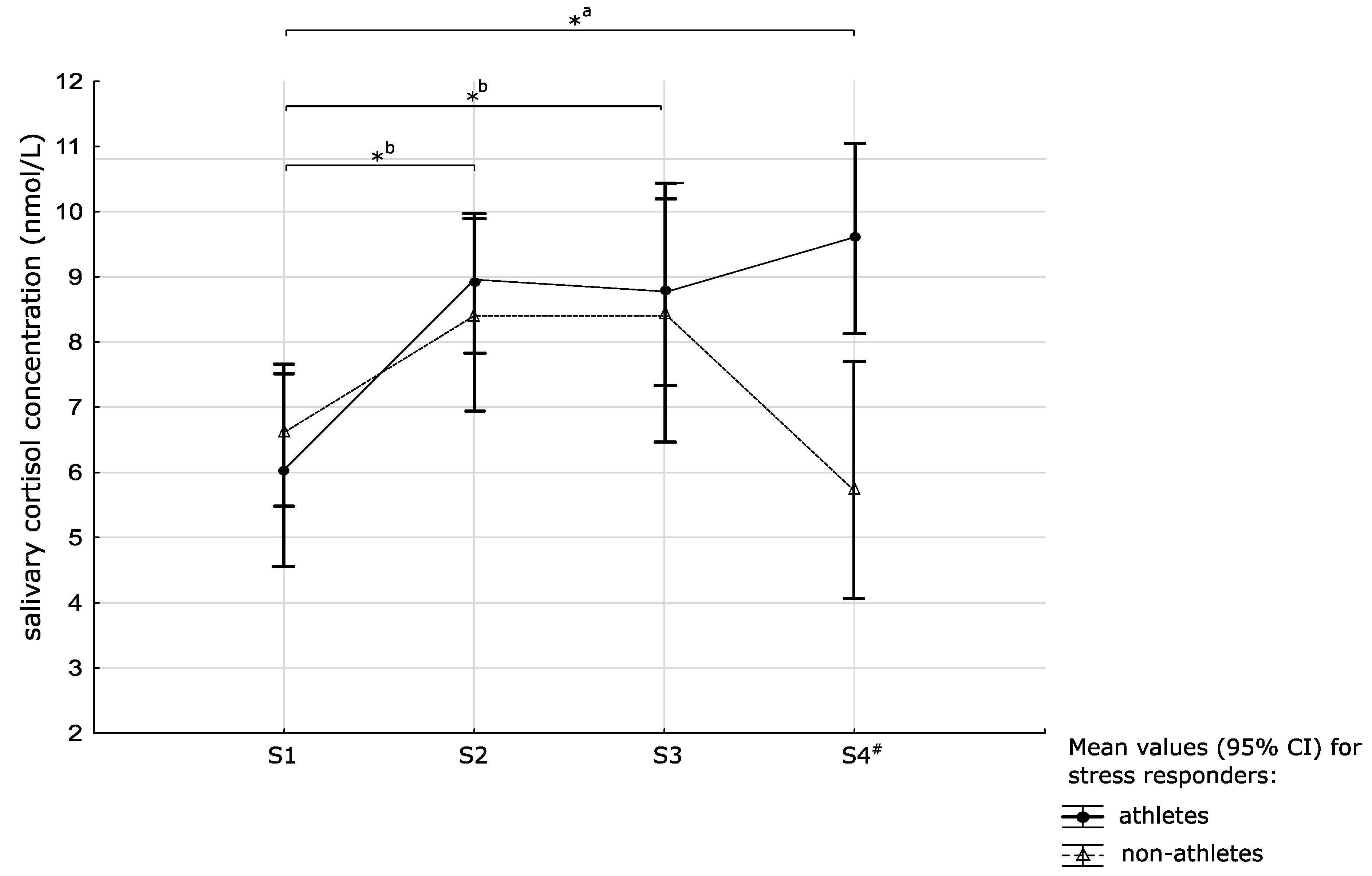
3.4. Salivary Cortisol
4. Discussion
4.1. Strengths and Limitations of the Study
4.2. Practical Applications
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Pero, R.; Cibelli, G.; Cortis, C.; Sbriccoli, P.; Capranica, L.; Piacentini, M.F. Stress related changes during TeamGym competition. J. Sports Med. Phys. Fitness 2016, 56, 639–647. [Google Scholar] [PubMed]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [PubMed]
- McAllister, M.J.; Basham, S.A.; Waldman, H.S.; Smith, J.W.; Mettler, J.A.; Butawan, M.B.; Bloomer, R.J. Effects of psychological stress during exercise on markers of oxidative stress in young healthy, trained men. Physiol. Behav. 2019, 198, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Laufer, S.; Engel, S.; Knaevelsrud, C.; Schumacher, S. Cortisol and alpha-amylase assessment in psychotherapeutic intervention studies: A systematic review. Neurosci. Biobehav. Rev. 2018, 95, 235–262. [Google Scholar] [CrossRef] [PubMed]
- Kerémi, B.; Beck, A.; Fábián, T.; Fábián, G.; Szabó, G.; Nagy, Á.; Varga, G. Stress and Salivary Glands. Curr. Pharm. Des. 2017, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Slimani, M.; Baker, J.S.; Chéour, F.; Taylor, L.; Bragazzi, N.L. Steroid hormones and psychological responses to soccer matches: Insights from a systematic review and meta-analysis. PLOS ONE 2017, 12, e0186100. [Google Scholar] [CrossRef] [PubMed]
- Misra, M. Neuroendocrine mechanisms in athletes. Handb. Clin. Neurol. 2014, 124, 373–386. [Google Scholar] [PubMed]
- Smith, T.W.; Jordan, K.D. Interpersonal motives and social-evaluative threat: Effects of acceptance and status stressors on cardiovascular reactivity and salivary cortisol response. Psychophysiology 2015, 52, 269–276. [Google Scholar] [CrossRef]
- Tsigos, C.; Chrousos, G.P. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002, 53, 865–871. [Google Scholar] [CrossRef]
- Nakajima, M.; Allen, S.; al’Absi, M. Influences of the Menstrual Phase on Cortisol Response to Stress in Nicotine Dependent Women: A Preliminary Examination. Nicotine Tob. Res. 2019, 21, 617–622. [Google Scholar] [CrossRef]
- Nenke, M.A.; Zeng, A.; Meyer, E.J.; Lewis, J.G.; Rankin, W.; Johnston, J.; Kireta, S.; Jesudason, S.; Torpy, D.J. Differential Effects of Estrogen on Corticosteroid-Binding Globulin Forms Suggests Reduced Cleavage in Pregnancy. J. Endocr. Soc. 2017, 1, 202–210. [Google Scholar] [PubMed]
- Lee, D.Y.; Kim, E.; Choi, M.H. Technical and clinical aspects of cortisol as a biochemical marker of chronic stress. BMB Rep. 2015, 48, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Bozovic, D.; Račić, M.; Ivkovic, N. Salivary Cortisol Levels as a Biological Marker of Stress Reaction. Med. Arch. 2013, 67, 374. [Google Scholar] [CrossRef] [PubMed]
- El-Farhan, N.; Rees, D.A.; Evans, C. Measuring cortisol in serum, urine and saliva - are our assays good enough? Ann. Clin. Biochem. Int. J. Lab. Med. 2017, 54, 308–322. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, E.; Bali, A.; Singh, N.; Jaggi, A.S. Cross stress adaptation: Phenomenon of interactions between homotypic and heterotypic stressors. Life Sci. 2015, 137, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Mücke, M.; Ludyga, S.; Colledge, F.; Gerber, M. Influence of Regular Physical Activity and Fitness on Stress Reactivity as Measured with the Trier Social Stress Test Protocol: A Systematic Review. Sports Med. 2018, 48, 2607–2622. [Google Scholar] [CrossRef]
- Van Paridon, K.N.; A Timmis, M.; Nevison, C.M.; Bristow, M. The anticipatory stress response to sport competition; a systematic review with meta-analysis of cortisol reactivity. BMJ Open Sport Exerc. Med. 2017, 3, e000261. [Google Scholar] [CrossRef] [PubMed]
- Siart, B.; Nimmerichter, A.; Vidotto, C.; Wallner, B. Status, Stress and Performance in Track and Field Athletes during the European Games in Baku (Azerbaijan). Sci. Rep. 2017, 7, 6076. [Google Scholar] [CrossRef]
- Maki, P.M.; Mordecai, K.L.; Rubin, L.H.; Sundermann, E.; Savarese, A.; Eatough, E.; Drogos, L. Menstrual cycle effects on cortisol responsivity and emotional retrieval following a psychosocial stressor. Horm. Behav. 2015, 74, 201–208. [Google Scholar] [CrossRef]
- Keaney, L.C.; Kilding, A.E.; Merien, F.; Dulson, D.K. The impact of sport related stressors on immunity and illness risk in team-sport athletes. J. Sci. Med. Sport 2018, 21, 1192–1199. [Google Scholar] [CrossRef]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research – Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front. Psychol. 2017, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, K.; Larentzakis, A.V.; Khamis, N.N.; Alsuhaibani, G.I.; Alaska, Y.A.; Giallafos, E.J. Can Wearable Devices Accurately Measure Heart Rate Variability? A Systematic Review. Folia Med. 2018, 60, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Szafranski, D.D.; Barrera, T.L.; Norton, P.J. Test anxiety inventory: 30 years later. Anxiety, Stress. Coping 2012, 25, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Bergier, J.; Kapka-Skrzypczak, L.; Biliński, P.; Paprzycki, P.; Wojtyła, A. Physical activity of Polish adolescents and young adults according to IPAQ: A population based study. Ann. Agric. Environ. Med. 2012, 19, 109–115. [Google Scholar] [PubMed]
- Qi, M.; Gao, H.; Guan, L.; Liu, G.; Yang, J. Subjective Stress, Salivary Cortisol, and Electrophysiological Responses to Psychological Stress. Front. Psychol. 2016, 7, 229. [Google Scholar] [CrossRef] [PubMed]
- Engert, V.; Efanov, S.I.; Duchesne, A.; Vogel, S.; Corbo, V.; Pruessner, J.C. Differentiating anticipatory from reactive cortisol responses to psychosocial stress. Psychoneuroendocrinology 2013, 38, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Maruo, K.; Partlett, C.; Riley, R.D. A random effects meta-analysis model with Box-Cox transformation. BMC Med Res. Methodol. 2017, 17, 109. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.; Plessow, F.; Kirschbaum, C.; Stalder, T. Classification criteria for distinguishing cortisol responders from nonresponders to psychosocial stress: evaluation of salivary cortisol pulse detection in panel designs. Psychosom. Med. 2013, 75, 832–840. [Google Scholar] [CrossRef]
- Woody, A.; Hooker, E.D.; Zoccola, P.M.; Dickerson, S.S. Social-evaluative threat, cognitive load, and the cortisol and cardiovascular stress response. Psychoneuroendocrinology 2018, 97, 149–155. [Google Scholar] [CrossRef]
- Nikolaidis, P.T.; Ziv, G.; Arnon, M.; Lidor, R. Physical Characteristics and Physiological Attributes of Female Volleyball Players—The Need for Individual Data. J. Strength Cond. Res. 2012, 26, 2547–2557. [Google Scholar] [CrossRef]
- Nikolaidis, P.T.; Busko, K.; Afonso, J.; Chtourou, H.; Padulo, J.; Goudas, K.; Heller, J. The Effect Of Maturity On Heart Rate Responses During Training And Testing In Postpubescent Female Volleyball Players. Fiziol. Cheloveka 2015, 41, 78–85. [Google Scholar] [PubMed]
- D’Souza, A.; Sharma, S.; Boyett, M.R. CrossTalk opposing view: Bradycardia in the trained athlete is attributable to a downregulation of a pacemaker channel in the sinus node. J. Physiol. 2015, 593, 1749–1751. [Google Scholar] [CrossRef] [PubMed]
- Hermann, R.; Biallas, B.; Predel, H.G.; Petrowski, K. Physical versus psychosocial stress: effects on hormonal, autonomic, and psychological parameters in healthy young men. Stress 2019, 22, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Gulliver, A.; Griffiths, K.M.; MacKinnon, A.; Batterham, P.J.; Stanimirovic, R. The mental health of Australian elite athletes. J. Sci. Med. Sport 2015, 18, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-J.; Webb, H.E.; Zourdos, M.C.; Acevedo, E.O. Cardiovascular reactivity, stress, and physical activity. Front. Physiol. 2013, 4, 314. [Google Scholar] [CrossRef] [PubMed]
- Wunsch, K.; Wurst, R.; Von Dawans, B.; Strahler, J.; Kasten, N.; Fuchs, R. Habitual and acute exercise effects on salivary biomarkers in response to psychosocial stress. Psychoneuroendocrinology 2019, 106, 216–225. [Google Scholar] [CrossRef]
- Zanstra, Y.J.; Johnston, D.W. Cardiovascular reactivity in real life settings: Measurement, mechanisms and meaning. Boil. Psychol. 2011, 86, 98–105. [Google Scholar] [CrossRef]
- Moreira, A.; Freitas, C.G.; Nakamura, F.Y.; Drago, G.; Drago, M.; Aoki, M.S. Effect of Match Importance on Salivary Cortisol and Immunoglobulin A Responses in Elite Young Volleyball Players. J. Strength Cond. Res. 2013, 27, 202–207. [Google Scholar] [CrossRef]
- Luger, A.; Deuster, P.A.; Kyle, S.B.; Gallucci, W.T.; Montgomery, L.C.; Gold, P.W.; Loriaux, D.L.; Chrousos, G.P. Acute Hypothalamic–Pituitary–Adrenal Responses to the Stress of Treadmill Exercise. New Engl. J. Med. 1987, 316, 1309–1315. [Google Scholar] [CrossRef]
- Wood, C.J.; Clow, A.; Hucklebridge, F.; Law, R.; Smyth, N. Physical fitness and prior physical activity are both associated with less cortisol secretion during psychosocial stress. Anxiety Stress Coping 2018, 31, 135–145. [Google Scholar] [CrossRef]
- Hill, E.E.; Zack, E.; Battaglini, C.; Viru, M.; Viru, A.; Hackney, A.C. Exercise and circulating Cortisol levels: The intensity threshold effect. J. Endocrinol. Investig. 2008, 31, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.T.; Matthews, T.D.; Murray, M.; Van Raalte, J.; E Jensen, B. Psychological Correlates of Performance in Female Athletes During a 12-Week Off-Season Strength and Conditioning Program. J. Strength Cond. Res. 2010, 24, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.W.; Hartley, H.L.; Kotchen, T.A.; Mougey, E.H.; Ricketts, P.T.; Jones, L.G. Plasma Cortisol and Norepinephrine Responses in Anticipation of Muscular Exercise. Psychosom. Med. 1973, 35, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.J.; Jones, M.V.; Sheffield, D.; Cross, S.L. Cardiovascular indices of challenge and threat states predict competitive performance. Int. J. Psychophysiol. 2012, 86, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Trotman, G.P.; Williams, S.E.; Quinton, M.L.; Van Zanten, J.J.V. Challenge and threat states: examining cardiovascular, cognitive and affective responses to two distinct laboratory stress tasks. Int. J. Psychophysiol. 2018, 126, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Biondi, M.; Picardi, A. Psychological Stress and Neuroendocrine Function in Humans: The Last Two Decades of Research. Psychother. Psychosom. 1999, 68, 114–150. [Google Scholar] [CrossRef] [PubMed]
- Monasterio, E.; Mei-Dan, O.; Hackney, A.C.; Lane, A.R.; Zwir, I.; Rozsa, S.; Cloninger, C.R. Stress reactivity and personality in extreme sport athletes: The psychobiology of BASE jumpers. Physiol. Behav. 2016, 167, 289–297. [Google Scholar] [CrossRef]
- Robazza, C.; Izzicupo, P.; D’Amico, M.A.; Ghinassi, B.; Crippa, M.C.; Di Cecco, V.; Ruiz, M.C.; Bortoli, L.; Di Baldassarre, A. Psychophysiological responses of junior orienteers under competitive pressure. PLOS ONE 2018, 13, e0196273. [Google Scholar] [CrossRef] [PubMed]
- Balsalobre-Fernández, C.; Tejero-González, C.M.; Del Campo-Vecino, J. Relationships between Training Load, Salivary Cortisol Responses and Performance during Season Training in Middle and Long Distance Runners. PLOS ONE 2014, 9, e106066. [Google Scholar] [CrossRef][Green Version]
- Nikolaidis, P.T.; Gkoudas, K.; Afonso, J.; Clemente-Suarez, V.J.; Knechtle, B.; Kasabalis, S.; Kasabalis, A.; Douda, H.; Tokmakidis, S.; Torres-Luque, G. Who jumps the highest? Anthropometric and physiological correlations of vertical jump in youth elite female volleyball players. J. Sports. Med. Phys. Fitness 2017, 57, 802–810. [Google Scholar] [CrossRef]

| Variable | Athletes | Non-Athletes | p | ||||
|---|---|---|---|---|---|---|---|
| n = 25 | n = 30 | ||||||
| Me | Q1 | Q3 | Me | Q1 | Q3 | ||
| Age (years) | 22.0 | 20.0 | 23.0 | 23.0 | 21.0 | 24.0 | 0.200 |
| Height | 177.3 | 172.1 | 180.1 | 173.1 | 169.8 | 175.9 | 0.034 |
| Weight | 73.5 | 68.5 | 76.1 | 71.2 | 67.4 | 74.9 | 0.081 |
| BMI (kg/m2) | 23.5 | 22.25 | 24.76 | 24.44 | 23.24 | 25.14 | 0.060 |
| Menstrual cycle length (days) | 28.0 | 27.0 | 29.0 | 28.0 | 28.0 | 29.0 | 0.143 |
| Years of education | 14.0 | 13.0 | 14.0 | 14.0 | 14.0 | 16.0 | 0.055 |
| Time from awakening (min) | 192.0 | 154.0 | 210.0 | 175.0 | 155.0 | 213.0 | 0.888 |
| Physical activity (MET min/week) | 2700.0 | 2250.0 | 3700.0 | 420.0 | 340.0 | 550.0 | <0.001 |
| Psychometric variables: | |||||||
| State anxiety (points) | 38.0 | 36.0 | 39.0 | 38.0 | 36.0 | 40.0 | 0.994 |
| Coping strategies | |||||||
| Task-oriented coping (points) | 59.0 | 57.0 | 62.0 | 60.0 | 55.0 | 63.0 | 0.927 |
| Emotion-oriented coping (points) | 46.0 | 41.0 | 54.0 | 47.0 | 40.0 | 59.0 | 0.668 |
| Avoidance-oriented coping (points) | 36.0 | 32.0 | 41.0 | 39.0 | 28.0 | 44.0 | 0.778 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziembowska, I.; Wójcik, M.; Hołyńska-Iwan, I.; Litwic-Kaminska, K.; Słomka, A.; Żekanowska, E. Female Volleyball Players Are More Prone to Cortisol Anticipatory Stress Response than Sedentary Women. Medicina 2019, 55, 258. https://doi.org/10.3390/medicina55060258
Dziembowska I, Wójcik M, Hołyńska-Iwan I, Litwic-Kaminska K, Słomka A, Żekanowska E. Female Volleyball Players Are More Prone to Cortisol Anticipatory Stress Response than Sedentary Women. Medicina. 2019; 55(6):258. https://doi.org/10.3390/medicina55060258
Chicago/Turabian StyleDziembowska, Inga, Małgorzata Wójcik, Iga Hołyńska-Iwan, Kamila Litwic-Kaminska, Artur Słomka, and Ewa Żekanowska. 2019. "Female Volleyball Players Are More Prone to Cortisol Anticipatory Stress Response than Sedentary Women" Medicina 55, no. 6: 258. https://doi.org/10.3390/medicina55060258
APA StyleDziembowska, I., Wójcik, M., Hołyńska-Iwan, I., Litwic-Kaminska, K., Słomka, A., & Żekanowska, E. (2019). Female Volleyball Players Are More Prone to Cortisol Anticipatory Stress Response than Sedentary Women. Medicina, 55(6), 258. https://doi.org/10.3390/medicina55060258







