Salivary and Serum Interferon-Gamma/Interleukin-4 Ratio in Oral Lichen Planus Patients: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Eligibility Criteria
2.4. Data Extraction
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
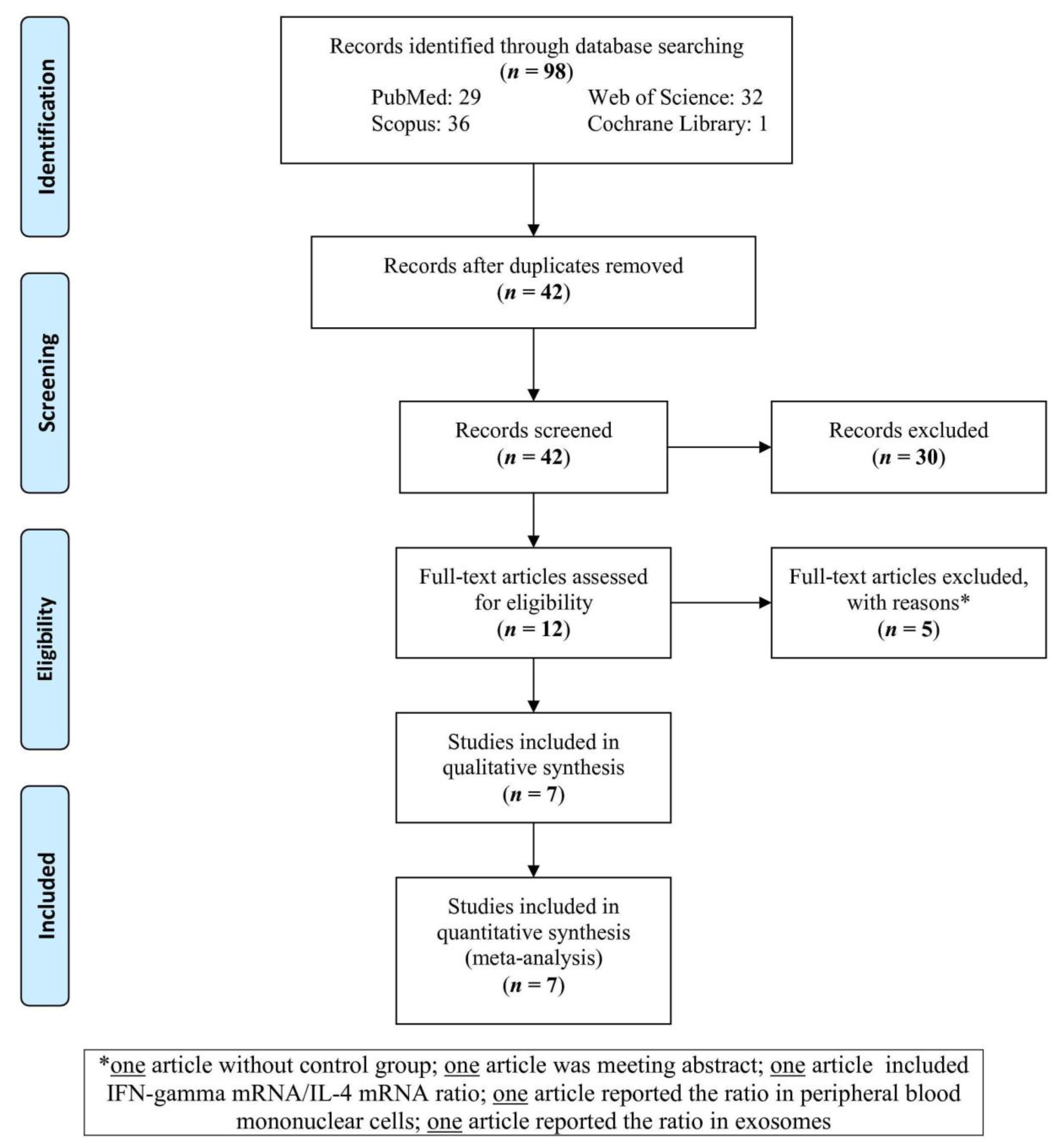
3.1. Study Selection
3.2. Study Characteristics
3.3. Meta-Analysis Report
3.3.1. OLP vs. Control (Serum)
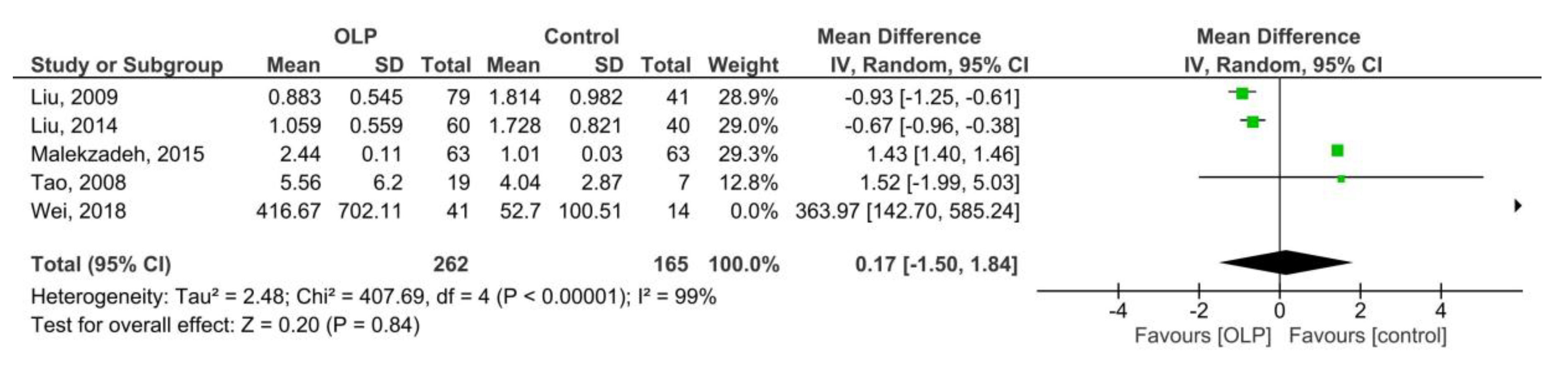
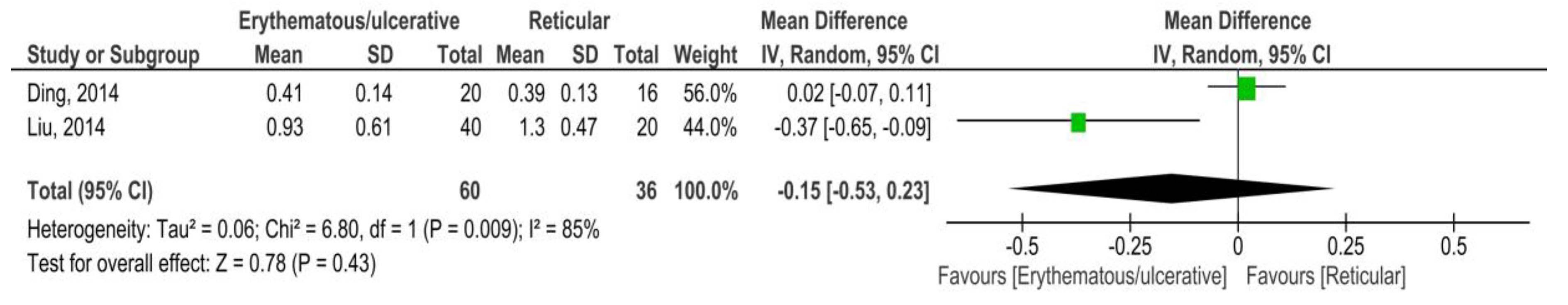
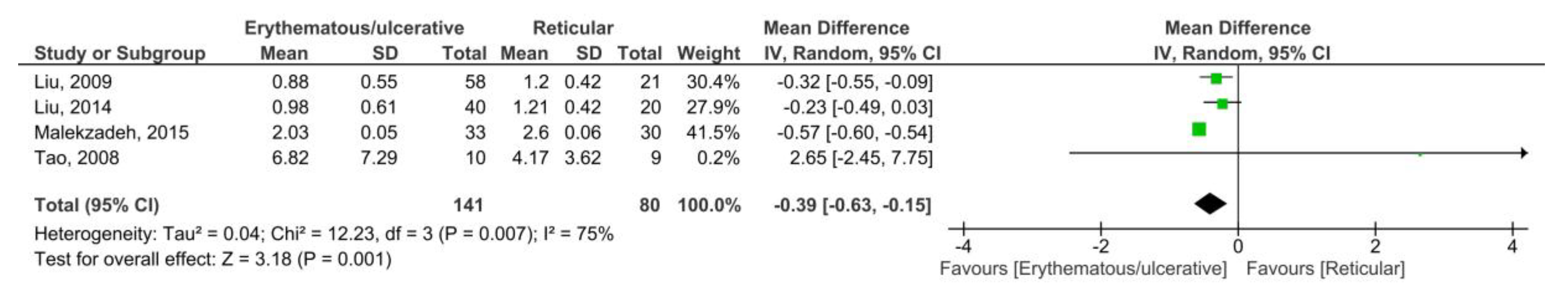
3.3.2. OLP vs. Control (Saliva)
3.4. Sensitivity Analysis
3.5. Quality Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sugerman, P.B.; Savage, N.W.; Walsh, L.J.; Zhao, Z.Z.; Zhou, X.J.; Khan, A.; Seymour, G.J.; Bigby, M. The pathogenesis of oral lichen planus. Crit. Rev. Oral. Biol. Med. 2002, 13, 350–365. [Google Scholar] [CrossRef] [PubMed]
- Alrashdan, M.S.; Cirillo, N.; McCullough, M. Oral lichen planus: A literature review and update. Arch. Dermatol. Res. 2016, 308, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Lodi, G.; Scully, C.; Carrozzo, M.; Griffiths, M.; Sugerman, P.B.; Thongprasom, K. Current controversies in oral lichen planus: Report of an international consensus meeting. Part 2. Clinical management and malignant transformation. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2005, 100, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Sun, Q.; Deng, Y.; Wang, Y.; Du, G.; Song, C.; Li, C.; Zhu, M.; Chen, G.; Tang, G. Mixed and inhomogeneous expression profile of Th1/Th2 related cytokines detected by cytometric bead array in the saliva of patients with oral lichen planus. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2018, 126, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Krupaa, R.J.; Sankari, S.L.; Masthan, K.M.; Rajesh, E. Oral lichen planus: An overview. J. Pharm. Bioallied. Sci. 2015, 7, S158–S161. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, C.; Mihai, L.; Parlatescu, I.; Tovaru, S. Association of oral lichen planus with chronic C hepatitis. Review of the data in literature. Maedica (Buchar) 2014, 9, 98–103. [Google Scholar] [PubMed]
- Salgado, D.S.; Jeremiah, F.; Cappella, M.V.; Onofre, M.A.; Massucato, E.M.S.; Orrico, S.R.P. Plaque control improves the painful symptoms of oral lichen planus gingival lesions. A short-term study. J. Oral. Pathol. Med. 2013, 42, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Rhodus, N.L.; Cheng, B.; Ondrey, F. Th1/Th2 cytokine ratio in tissue transudates from patients with oral lichen planus. Mediators Inflamm. 2007, 2007, 19854. [Google Scholar] [CrossRef] [PubMed]
- Gray, P.W.; Goeddel, D.V. Structure of human immune interferone gene. Nature 1982, 298, 859–863. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwon, J.Y.; Kim, S.Y.; Jung, K.; Cho, M.L. Interferon-gamma regulates inflammatory cell death by targeting necroptosis in experimental autoimmune arthritis. Sci. Rep. 2017, 7, 10133. [Google Scholar] [CrossRef]
- Schoenborn, J.R.; Wilson, C.B. Regulation of interferon-gamma during innate and adaptive immune response. Adv. Immunol. 2007, 96, 41–101. [Google Scholar] [PubMed]
- Anovazzi, G.; Medeiros, M.C.; Pigossi, S.C.; Finoti, L.S.; Souza Moreira, T.M.; Mayer, M.P.; Zanelli, C.F.; Valentini, S.R.; Rossa-Junior, C.; Scarel-Caminaga, R.M. Functionality and opposite roles of two interleukin 4 haplotypes in immune cells. Genes Immun. 2017, 18, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Hershey, G.K.; Friedrich, M.F.; Esswein, L.A.; Thomas, M.L.; Chatila, T.A. The association of atopy with a gain-of-function mutation in the alpha subunit of the interlukin-4 receptor. N. Engl. J. Med. 1997, 337, 1720–1750. [Google Scholar] [CrossRef] [PubMed]
- Rengarajan, J.; Szabo, S.J.; Glimcher, L.H. Transcriptional regulation of Th1/Th2 polarization. Immunol. Today 2000, 21, 479–483. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.; Fu, S.; Wang, C.; Zhou, B. A Study of Association Between Oral Lichen Planus and Immune Balance of Th1/Th2 Cells. Inflammation 2015, 38, 1874–1879. [Google Scholar] [CrossRef] [PubMed]
- Kalogerakou, F.; Albanidou-Farmaki, E.; Markopoulos, A.K.; Antoniades, D.Z. Detection of T cells secreting type 1 and type 2 cytokines in the peripheral blood of patients with oral lichen planus. Hippokratia 2008, 12, 230–235. [Google Scholar] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Robertson, J.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-Randomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2011. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 12 January 2016).
- Tao, X.A.; Li, C.Y.; Rhodus, N.L.; Xia, J.; Yang, X.P.; Cheng, B. Simultaneous detection of IFN-gamma and IL-4 in lesional tissues and whole unstimulated saliva from patients with oral lichen planus. J. Oral. Pathol. Med. 2008, 37, 83–87. [Google Scholar] [CrossRef]
- Liu, W.; Dan, H.; Wang, Z.; Jiang, L.; Zhou, Y.; Zhao, M.; Chen, Q.; Zeng, X. IFN-gamma and IL-4 in saliva of patients with oral lichen planus: A study in an ethnic Chinese population. Inflammation 2009, 32, 176–181. [Google Scholar] [CrossRef]
- Ding, M.; Zeng, J.; Sroussi, H.; Yu, J.; Xu, J.; Cheng, X.; Fan, Y. Interactions between Golli-MBP and Th1/Th2 cytokines in patients with oral lichen planus. Oral Dis. 2014, 20, 205–211. [Google Scholar] [CrossRef]
- Liu, W.Z.; He, M.J.; Long, L.; Mu, D.L.; Xu, M.S.; Xing, X.; Zeng, X.; Liao, G.; Dan, H.X.; Chen, Q.M. Interferon-γ and interleukin-4 detected in serum and saliva from patients with oral lichen planus. Int. J. Oral. Sci. 2014, 6, 22–26. [Google Scholar] [CrossRef]
- Malekzadeh, H.; Robati, M.; Yousefimanesh, H.; Ghafourian Boroujerdnia, M.; Nadripour, R. Salivary Interferon Gamma and Interleukin-4 Levels in Patients Suffering from Oral Lichen Planus. Cell J. 2015, 17, 554–558. [Google Scholar]
- Edwards, P.C.; Kelsch, R. Oral lichen planus: Clinical presentation and management. J. Can. Dent. Assoc. 2002, 68, 494–499. [Google Scholar]
- Gomez, D.; Correa, P.A.; Gomez, L.M.; Cadena, J.; Molina, J.F.; Anaya, J.M. Th1/Th2 cytokines in patients with systemic lupus erythematosus: Is tumor necrosis factor alpha protective? Semin. Arthritis Rheum. 2004, 33, 404–413. [Google Scholar] [CrossRef]
- Sommer, F.; Meixner, M.; Mannherz, M.; Ogilvie, A.L.; Rollinghoff, M.; Lohoff, M. Analysis of cytokine patterns produced by individual CD4+ lymph node cells during experimental murine leishmaniasis in resistant and susceptible mice. Int. Immunol. 1998, 10, 1853–1861. [Google Scholar] [CrossRef]
- Lenschow, D.J.; Herold, K.C.; Rhee, L.; Patel, B.; Koons, A.; Qin, H.Y.; Fuchs, E.; Singh, B.; Thompson, C.B.; Bluestone, J.A. CD28⁄B7 regulation of Th1 and Th2 subsets in the development of autoimmune diabetes. Immunity 1996, 5, 285–293. [Google Scholar] [CrossRef]
- Mozaffari, H.R.; Sharifi, R.; Hayati, M.; Imani, M.M.; Lopez-Jornet, P.; Golshah, A.; Moradpoor, H.; Rezaei, R.; Sadeghi, M. Evaluation of serum and salivary interferon-γ levels in patients with oral lichen planus: A systematic review and meta-analysis of case-control studies. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2019, 127, 210–217. [Google Scholar] [CrossRef]
- Zhou, G.; Zhang, J.; Ren, X.W.; Hu, J.Y.; Du, G.F.; Xu, X.Y. Increased B7-H1 expression on peripheral blood T cells in oral lichen planus correlated with disease severity. J. Clin. Immunol. 2012, 32, 794–801. [Google Scholar] [CrossRef]
- Pekiner, F.N.; Demirel, G.Y.; Borahan, M.O.; Ozbayrak, S. Cytokine profiles in serum of patients with oral lichen planus. Cytokine 2012, 60, 701–706. [Google Scholar] [CrossRef]
- Haukim, N.; Bidwell, J.L.; Smith, A.J.; Keen, L.J.; Gallagher, G.; Kimberley, R.; Huizinga, T.; McDermott, M.F.; Oksenberg, J.; McNicholl, J.; et al. Cytokine gene polymorphism in human disease: On-line databases, supplement 2. Genes Immun. 2002, 3, 313–330. [Google Scholar] [CrossRef]

| The First Author, Year | Country | Mean Age (OLP/Control) | Male:Female (OLP/Control) | No. of OLP Patients | No. of Controls | Method | Sample |
|---|---|---|---|---|---|---|---|
| Tao, 2008 [19] | China | 46.5/26.9 | 12:7/4:3 | 19 | 7 | ELISA kit (eBioscience Inc., San Diego, CA, USA) | Saliva |
| Liu, 2009 [20] | China | 46/41 | 37:42/20:21 | 79 | 41 | ELISA kit (R&D Systems Inc., Minneapolis, MN, USA) | Saliva |
| Ding, 2014 [21] | China | 45/43 | 15:21/7:12 | 36 | 19 | ELISA kit (BioLegend, Inc., San Diego, CA, USA) | Serum |
| Liu, 2014 [22] | China | 45/42 | 25:35/19:21 | 60 | 40 | ELISA kit (R&D Systems Inc., Minneapolis, MN, USA) | Serum and Saliva |
| Malekzadeh, 2015 [23] | Iran | 41.5/37 | 25:38/30:33 | 63 | 63 | ELISA kit (eBioscience Inc., San Diego, CA, USA) | Saliva |
| Wang, 2015 [15] | China | 53/54 | 4:31/4:31 | 35 | 35 | ELISA kit (R&D Systems Inc., Minneapolis, MN, USA) | Serum |
| Wei, 2018 [4] | China | 56.3/51.2 | 9:32/6:8 | 41 | 14 | BD™ CBA Human Enhanced Sensitivity Flex Sets | Saliva |
| Subgroup | Omitted Study | Removed Reason | Z | p | Heterogeneity | MD | 95%CI (min, max) |
|---|---|---|---|---|---|---|---|
| OLP vs. Control (saliva) | Wei, 2018 [4] | Outlier study | 0.17 | 0.86 | 99% | 0.15 | −1.51, 1.80 |
| Erythematous/ulcerative vs. Reticular (saliva) | Tao, 2008 [19] | Outlier study | 3.31 | 0.0009 | 81% | −0.40 | −0.64, −0.16 |
| The First Author (year) | Selection | Comparability | Exposure | Total Points |
|---|---|---|---|---|
| Tao, 2008 [19] | *** | * | *** | 7 |
| Liu, 2009 [20] | **** | ** | *** | 9 |
| Ding, 2014 [21] | *** | ** | *** | 8 |
| Liu, 2014 [22] | *** | * | *** | 7 |
| Malekzadeh, 2015 [23] | *** | ** | *** | 8 |
| Wang, 2015 [15] | *** | ** | *** | 8 |
| Wei, 2018 [4] | *** | * | *** | 7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mozaffari, H.R.; Molavi, M.; Lopez-Jornet, P.; Sadeghi, M.; Safaei, M.; Imani, M.M.; Sharifi, R.; Moradpoor, H.; Golshah, A.; Jamshidy, L. Salivary and Serum Interferon-Gamma/Interleukin-4 Ratio in Oral Lichen Planus Patients: A Systematic Review and Meta-Analysis. Medicina 2019, 55, 257. https://doi.org/10.3390/medicina55060257
Mozaffari HR, Molavi M, Lopez-Jornet P, Sadeghi M, Safaei M, Imani MM, Sharifi R, Moradpoor H, Golshah A, Jamshidy L. Salivary and Serum Interferon-Gamma/Interleukin-4 Ratio in Oral Lichen Planus Patients: A Systematic Review and Meta-Analysis. Medicina. 2019; 55(6):257. https://doi.org/10.3390/medicina55060257
Chicago/Turabian StyleMozaffari, Hamid Reza, Maryam Molavi, Pia Lopez-Jornet, Masoud Sadeghi, Mohsen Safaei, Mohammad Moslem Imani, Roohollah Sharifi, Hedaiat Moradpoor, Amin Golshah, and Ladan Jamshidy. 2019. "Salivary and Serum Interferon-Gamma/Interleukin-4 Ratio in Oral Lichen Planus Patients: A Systematic Review and Meta-Analysis" Medicina 55, no. 6: 257. https://doi.org/10.3390/medicina55060257
APA StyleMozaffari, H. R., Molavi, M., Lopez-Jornet, P., Sadeghi, M., Safaei, M., Imani, M. M., Sharifi, R., Moradpoor, H., Golshah, A., & Jamshidy, L. (2019). Salivary and Serum Interferon-Gamma/Interleukin-4 Ratio in Oral Lichen Planus Patients: A Systematic Review and Meta-Analysis. Medicina, 55(6), 257. https://doi.org/10.3390/medicina55060257








