Influence of Sodium Citrate Supplementation after Dehydrating Exercise on Responses of Stress Hormones to Subsequent Endurance Cycling Time-Trial in the Heat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
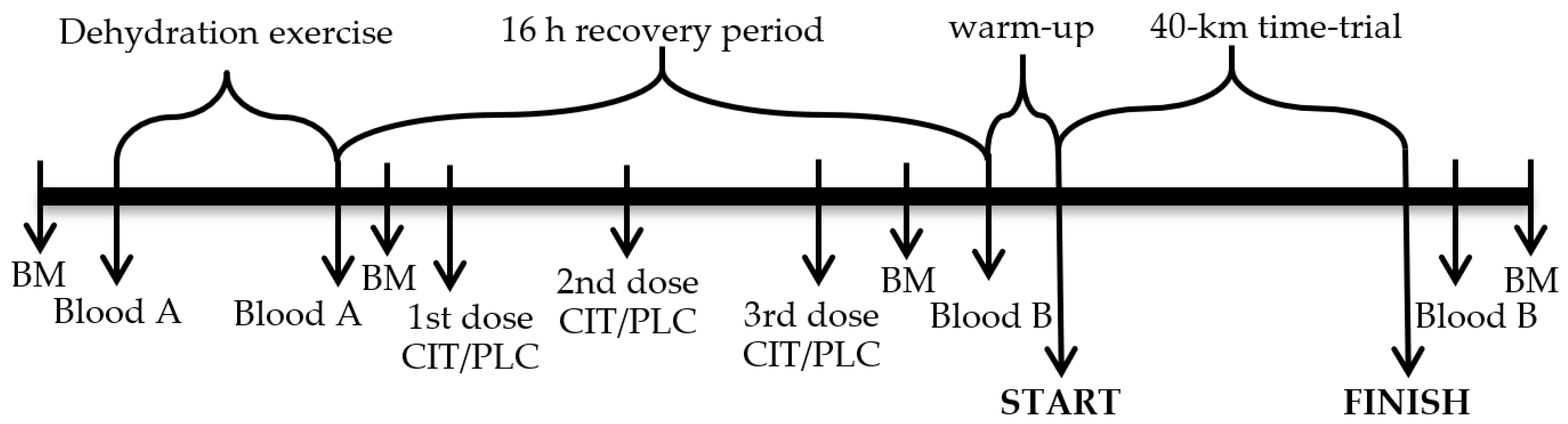
2.2. Study Design and Research Procedures
2.4. Blood Sampling and Analyses
2.5. Statistical Analysis
3. Results
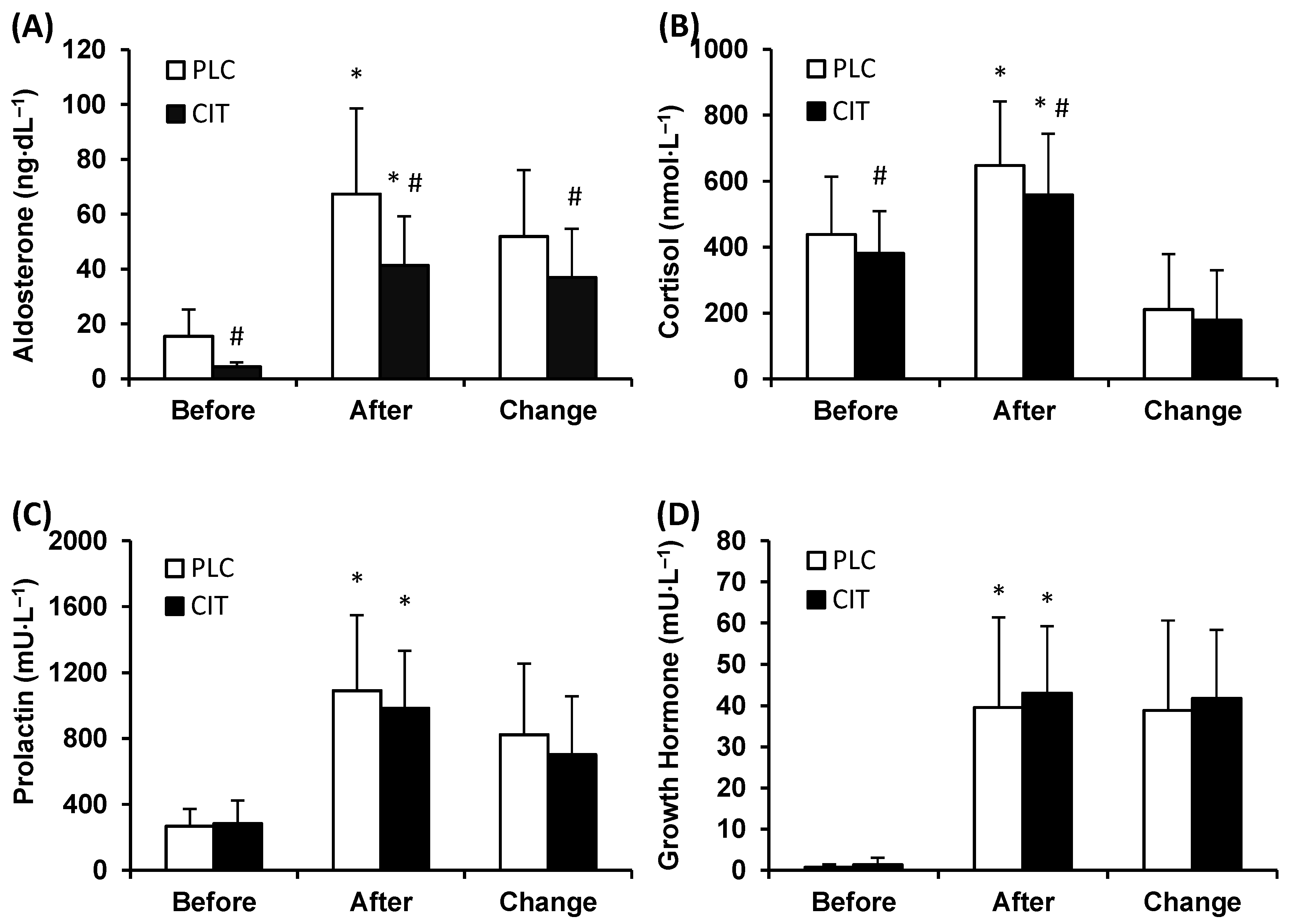
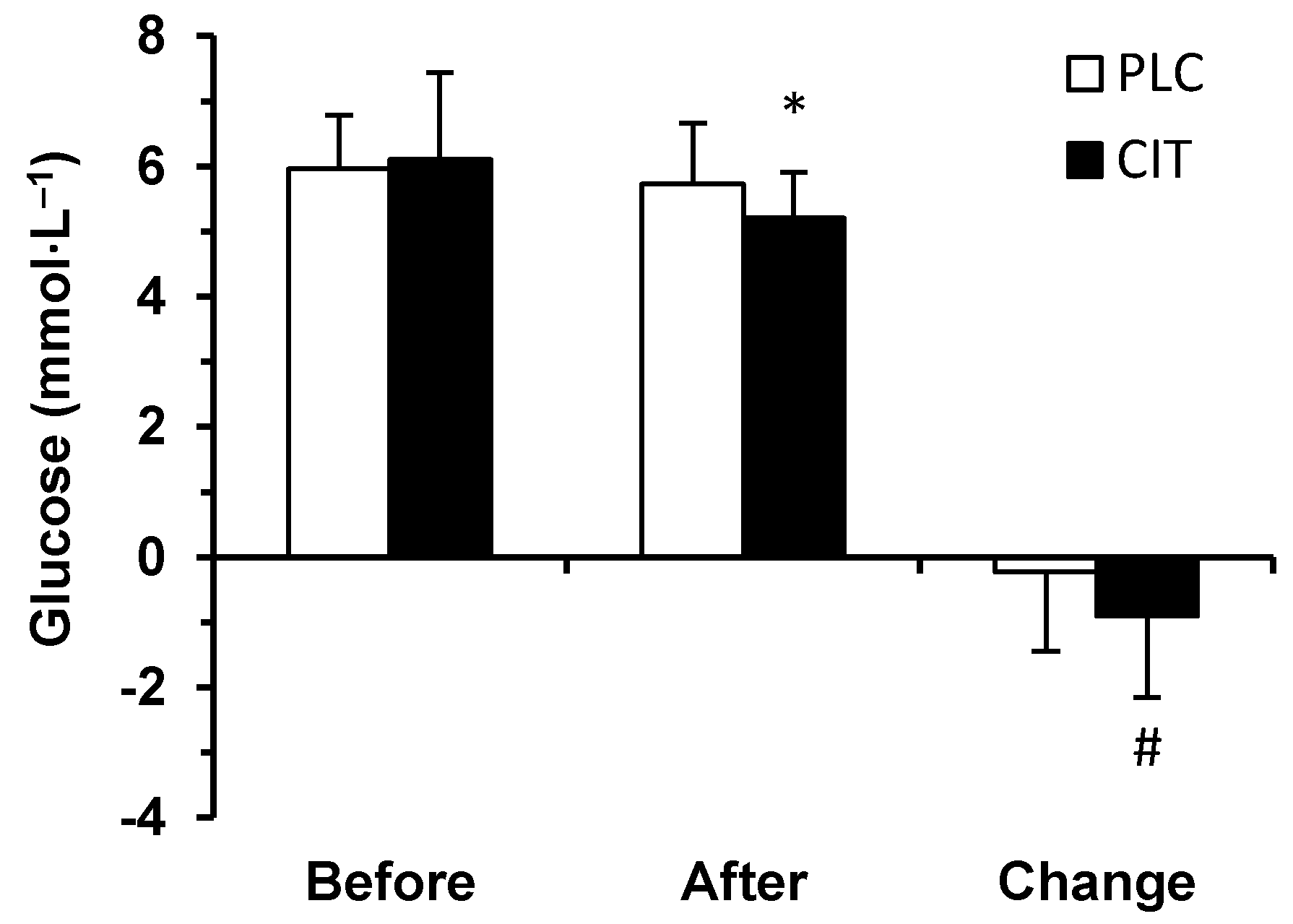
3.1. Blood Biochemical Parameters
3.2. Time-Trial Performance, Core Body Temperature and Plasma Volume
3.3. Correlations of Hormonal Parameters with Blood Lactate Concentration, Core Body Temperature, and Serum Glucose Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burke, L.; Cort, M.; Cox, G.; Crawford, R.; Desbrow, B.; Farthing, L.; Minehan, M.; Shaw, N.; Warnes, O. Supplements and sports foods. In Clinical Sports Nutrition, 3rd ed.; Burke, L., Deakin, V., Eds.; McGraw-Hill: Sydney, Australia, 2006; pp. 485–579. [Google Scholar]
- Carr, A.J.; Hopkins, W.G.; Gore, C.J. Effects of acute alkalosis and acidosis on performance. A meta-analysis. Sports Med. 2011, 41, 801–814. [Google Scholar] [CrossRef]
- Requena, B.; Zabala, M.; Padial, P.; Feriche, B. Sodium bicarbonate and sodium citrate: Ergogenic aids. J. Strength Cond. Res. 2005, 19, 213–224. [Google Scholar] [CrossRef]
- Bishop, D.; Edge, J.; Davis, C.; Goodman, C. Induced metabolic alkalosis affects muscle metabolism and repeated-sprint ability. Med. Sci. Sports Exerc. 2004, 36, 807–813. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, L.; Dalton, B.; Palmer, G. Sodium bicarbonate can be used as an ergogenic aid in high-intensity, competitive cycle ergometry of 1 h duration. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 80, 64–69. [Google Scholar] [CrossRef]
- Potteiger, J.A.; Nickel, G.L.; Webster, M.J.; Haub, M.D.; Palmer, R.J. Sodium citrate ingestion enhances 30 km cycling performance. Int. J. Sports Med. 1996, 17, 7–11. [Google Scholar] [CrossRef]
- Hollidge-Horvat, M.G.; Parolin, M.L.; Wong, D.; Jones, N.L.; Heigenhauser, G.J.F. Effect of induced metabolic alkalosis on human skeletal muscle metabolism during exercise. Am. J. Physiol. 2000, 278, E316–E329. [Google Scholar] [CrossRef]
- Percival, ME.; Martin, B.J.; Gillen, J.B.; Skelly, L.E.; MacInnis, M.J.; Green, A.E.; Tarnopolsky, M.A.; Gibala, M.J. Sodium bicarbonate ingestion augments the increase in PGC-1α mRNA expression during recovery from intense interval exercise in human skeletal muscle. J. Appl. Physiol. 2015, 119, 1303–1312. [Google Scholar] [CrossRef]
- Timpmann, S.; Burk, A.; Medijainen, L.; Tamm, M.; Kreegipuu, K.; Vähi, M.; Unt, E.; Ööpik, V. Dietary sodium citrate supplementation enhances rehydration and recovery from rapid body mass loss in trained wrestlers. Appl. Physiol. Nutr. Metab. 2012, 37, 1028–1037. [Google Scholar] [CrossRef]
- Ööpik, V.; Timpmann, S.; Hackney, A.C.; Kadak, K.; Medijainen, L.; Karelson, K. Ingestion of sodium citrate suppresses aldosterone level in blood at rest and during exercise. Appl. Physiol. Nutr. Metab. 2010, 35, 278–285. [Google Scholar] [CrossRef]
- Mora-Rodriguez, R.; Hamouti, N. Salt and fluid loading: Effects on blood volume and exercise performance. Med. Sports Sci. 2013, 59, 113–119. [Google Scholar]
- McEwen, B.S. Stress, definition and concepts. In Encyclopedia of Stress; Fink, G., Ed.; Academic Press: Millbrae, CA, USA, 2000; Volume 3, pp. 508–509. [Google Scholar]
- Kozlov, A.I.; Kozlova, M.A. Cortisol as a marker of stress. Hum. Physiol. 2014, 40, 224–236. [Google Scholar] [CrossRef]
- Glaser, R.; Kiecolt-Glaser, J.K. Stress-induced immune dysfunction: Implications for health. Nat. Rev. Immunol. 2005, 5, 243–251. [Google Scholar] [CrossRef]
- Hackney, A.C. Stress and the neuroendocrine system: The role of exercise as a stressor and modifier of stress. Expert. Rev. Endocrinol. Metab. 2006, 1, 783–792. [Google Scholar] [CrossRef]
- McMurray, R.G.; Hackney, A.C. Endocrine responses to exercise and training. In Exercise and Sport Science; Garrett, W.E., Kirkendall, D.T., Eds.; Lippincott Williams & Wilkins: Philadelphia, USA, 2000; pp. 135–161. [Google Scholar]
- Peart, D.J.; McNaughton, L.R.; Midgley, A.W.; Taylor, L.; Towlson, C.; Madden, L.A.; Vince, R.V. Pre-exercise alkalosis attenuates the heat shock protein 72 response to a single-bout of anaerobic exercise. J. Sci. Med. Sport. 2011, 14, 435–440. [Google Scholar] [CrossRef]
- Peart, D.J.; Kirk, R.J.; Madden, L.A.; Siegler, J.C.; Vince, R.V. The influence of exogenous carbohydrate provision and pre-exercise alkalosis on the heat shock protein response to prolonged interval cycling. Amino Acids 2013, 44, 903–910. [Google Scholar] [CrossRef]
- Bouissou, P.; Defer, G.; Guezennec, C.Y.; Estrade, P.Y.; Serrurier, B. Metabolic and blood catecholamine responses to exercise during alkalosis. Med. Sci. Sports. Exerc. 1988, 20, 228–232. [Google Scholar] [CrossRef]
- Bracken, R.M.; Linnane, D.M.; Brooks, S. Alkalosis and the plasma catecholamine response to high-intensity exercise in man. Med. Sci. Sports. Exerc. 2005, 37, 227–233. [Google Scholar] [CrossRef]
- Marx, J.O.; Gordon, S.E.; Vos, N.H.; Nindl, B.C.; Gómez, A.L.; Volek, J.S.; Pedro, J.; Ratamess, N.; Newton, R.U.; French, D.N.; et al. Effect of alkalosis on plasma epinephrine responses to high intensity cycle exercise in humans. Eur. J. Appl. Physiol. 2002, 87, 72–77. [Google Scholar] [CrossRef]
- Wahl, P.; Zinner, C.; Achtzen, S.; Bloch, W.; Mester, J. Effect of high and low intensity exercise and metabolic acidosis on levels of GH, IGF-I, IGFBP-3 and cortisol. Growth Horm. IGF Res. 2010, 20, 380–385. [Google Scholar] [CrossRef]
- Gordon, S.E.; Kraemer, W.J.; Vos, N.H.; Lynch, J.M.; Knuttgen, H.G. Effect of acid-base balance on the growth hormone response to acute high-intensity cycle exercise. J. Appl. Physiol. 1994, 76, 821–829. [Google Scholar] [CrossRef]
- Rojas Vega, S.; Strüder, H.K.; Wahrmann, B.V.; Bloch, W.; Hollmann, W. Bicarbonate reduces serum prolactin increase induced by exercise to exhaustion. Med. Sci. Sports. Exerc. 2006, 38, 675–680. [Google Scholar] [CrossRef]
- Sawka, M.N.; Leon, L.R.; Montain, S.J.; Sonna, L.A. Integrated physiological mechanisms of exercise performance, adaptation, and maladaptation to heat stress. Compr. Physiol. 2011, 1, 1883–1928. [Google Scholar]
- Cheshire, W.P. Thermoregulatory disorders and illness related to heat and cold stress. Auton. Neurosci. 2016, 196, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Polman, R.; Houlahan, K. A cumulative stress and training continuum model: a multidisciplinary approach to unexplained underperformance syndrome. Res. Sports Med. 2004, 12, 301–316. [Google Scholar] [CrossRef]
- Burk, A.; Timpmann, S.; Kreegipuu, K.; Tamm, M.; Unt, E.; Ööpik, V. Effects of heat acclimation on endurance capacity and prolactin response to exercise in the heat. Eur. J. Appl. Physiol. 2012, 112, 4091–4101. [Google Scholar] [CrossRef] [PubMed]
- Sparks, S.A.; Cable, N.T.; Doran, D.A.; Maclaren, D.P.M. The influence of environmental temperature on duathlon performance. Ergonomics 2005, 48, 1558–1567. [Google Scholar] [CrossRef]
- Niess, A.M.; Fehrenbach, E.; Lehmann, R.; Opavsky, L.; Jesse, M.; Northoff, H.; Dickhuth, H.H. Impact of elevated ambient temperatures on the acute immune response to intensive endurance exercise. Eur. J. Appl. Physiol. 2003, 89, 344–351. [Google Scholar] [CrossRef]
- Ööpik, V.; Timpmann, S.; Kreegipuu, K.; Unt, E.; Tamm, M. Heat acclimation decreases the growth hormone response to acute constant-load exercise in the heat. Growth Horm. IGF Res. 2014, 24, 2–9. [Google Scholar] [CrossRef]
- Satarifard, S.; Gaeini, A.A.; Choobineh, S.; Neek, L.S. Effects of acute exercise on serum interleukin-17 concentrations in hot and neutral environments in trained males. J. Therm. Biol. 2012, 37, 402–407. [Google Scholar] [CrossRef]
- Tamm, M.; Jakobson, A.; Havik, M.; Timpmann, S.; Burk, A.; Ööpik, V.; Allik, J.; Kreegipuu, K. Effects of heat acclimation on time perception. Int. J. Psychophysiol. 2015, 95, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Akerman, A.P.; Lucas, S.J.E.; Katare, R.; Cotter, J.D. Heat and dehydration additively enhance cardiovascular outcomes following orthostatically-stressful calisthenics exercise. Front. Physiol. 2017, 8, 756. [Google Scholar] [CrossRef] [PubMed]
- Suvi, S.; Mooses, M.; Timpmann, S.; Medijainen, L.; Narõškina, D.; Unt, E.; Ööpik, V. Impact of sodium citrate ingestion during recovery after dehydrating exercise on rehydration and subsequent 40-km cycling time-trial performance in the heat. Appl. Physiol. Nutr. Metab. 2018, 43, 571–579. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, L. Sodium citrate and anaerobic performance: Implications of dosage. Eur. J. Appl. Physiol. 1990, 61, 392–397. [Google Scholar] [CrossRef]
- Dill, D.B.; Costill, D.L. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J. Appl. Physiol. 1974, 37, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Augustinsson, O.; Jonasson, H.; Junkergård, J. Aldosterone secretion during acute metabolic and respiratory alkalosis in the goat. Acta Physiol. Scand. 1989, 137, 143–149. [Google Scholar] [CrossRef]
- Bollag, W.B. Regulation of aldosterone synthesis and secretion. Compr. Physiol. 2014, 4, 1017–1055. [Google Scholar]
- El Ghorayeb, N.; Bourdeau, I.; Lacroix, A. Role of ACTH and other hormones in the regulation of aldosterone production in primary aldosteronism. Front. Endocrinol. 2016, 7, 72. [Google Scholar] [CrossRef]
- Lieu, F.K.; Lin, C.Y.; Wang, P.S.; Jian, C.Y.; Yeh, Y.H.; Chen, Y.A.; Wang, K.L.; Lin, Y.C.; Chang, L.L.; Wang, G.J.; et al. Effect of swimming on the production of aldosterone in rats. PLoS ONE 2013, 9, e87080. [Google Scholar] [CrossRef]
- Lindinger, M.I.; Franklin, T.W.; Lands, L.C.; Pedersen, P.K.; Welsh, D.G.; Heigenhauser, G.J. NaHCO3 and KHCO3 ingestion rapidly increases renal electrolyte excretion in humans. J. Appl. Physiol. 2000, 88, 540–550. [Google Scholar] [CrossRef]
- Shier, D.; Butler, J.; Lewis, R. Hole’s Human Anatomy and Physiology, 7th ed.; Wm. C. Brown Publishers: Dubuquae, IA, USA, 1996; pp. 488–528. [Google Scholar]
- Grant, S.M.; Green, H.J.; Phillips, S.M.; Enns, D.L.; Sutton, J.R. Fluid and electrolyte hormonal responses to exercise and acute plasma volume expansion. J. Appl. Physiol. 1996, 81, 2386–2392. [Google Scholar] [CrossRef]
- Roy, B.D.; Green, H.J.; Grant, S.M.; Tarnopolsky, M.A. Acute plasma volume expansion in the untrained alters the hormonal response to prolonged moderate-intensity exercise. Horm. Metab. Res. 2001, 33, 238–245. [Google Scholar] [CrossRef]
- Staufenbiel, S.M.; Penninx, B.W.; Spijker, A.T.; Elzinga, B.M.; van Rossum, E.F. Hair cortisol, stress exposure, and mental health in humans: A systematic review. Psychoneuroendocrinology 2013, 38, 1220–1235. [Google Scholar] [CrossRef]
- Robergs, R.A.; Ghiasvand, F.; Parker, D. Biochemistry of exercise-induced metabolic acidosis. Am. J. Physiol. 2004, 287, R502–R516. [Google Scholar] [CrossRef]
- Cooper, E.S.; Berry, M.P.; McMurray, R.G.; Hosick, P.A.; Hackney, A.C. Core temperature influences on the relationship between exercise-induced leukocytosis and cortisol or TNF-alpha. Aviat. Space. Environ. Med. 2010, 81, 460–466. [Google Scholar] [CrossRef]
- Rhind, S.G.; Gannon, G.A.; Shephard, R.J.; Buguet, A.; Shek, P.N.; Radomski, M.V. Cytokine induction during exertional hyperthermia is abolished by core temperature clamping: neuroendocrine regulatory mechanisms. Int. J. Hyperth. 2004, 20, 503–516. [Google Scholar] [CrossRef]
- Hackney, A.C.; Smith-Ryan, A.E. Methodological considerations in exercise endocrinology. In Endocrinology of Physical Activity and Sport, 2nd ed.; Constantini, N., Hackney, A.C., Eds.; Humana Press: New York, NY, USA, 2013; pp. 1–19. [Google Scholar]
- Scheen, A.J.; Buxton, O.M.; Jison, M.; Van Reeth, O.; Leproult, R.; L’Hermite-Baleriaux, M.; Van Cauter, E. Effects of exercise on neuroendocrine secretions and glucose regulation at different times of day. Am. J. Physiol. 1998, 274, E1040–E1049. [Google Scholar] [CrossRef]
- Rojas Vega, S.; Hollmann, W.; Strüder, H.K. Influences of exercise and training on the circulating concentration of prolactin in humans. J. Neuroendocrinol. 2012, 24, 395–402. [Google Scholar] [CrossRef]
- Mündel, T.; Cox, J.P.; Jones, D.A. Exercise, heat stress and the interleukin-6 response: support for temperature-mediated neuroendocrine regulatory mechanisms. Med. Sport. 2010, 14, 96–102. [Google Scholar] [CrossRef]
- Brisson, G.R.; Audet, A.; Ledoux, M.; Matton, P.; Pellerin-Massicotte, J.; Peronnet, F. Exercise-induced blood prolactin variations in trained adult males: A thermic stress more than an osmotic stress. Horm. Res. 1986, 23, 200–206. [Google Scholar] [CrossRef]
- Pitsiladis, Y.P.; Strachan, A.T.; Davidson, I.; Maughan, R.J. Hyperprolactinaemia during prolonged exercise in the heat: Evidence for a centrally mediated component of fatigue in trained cyclists. Exp. Physiol. 2002, 87, 215–226. [Google Scholar] [CrossRef]
- De Meirleir, K.L.; Baeyens, L.; L’Hermite-Baleriaux, M.; L’Hermite, M.; Hollmann, W. Exercise-induced prolactin release is related to anaerobiosis. J. Clin. Endocrinol. Metab. 1985, 60, 1250–1252. [Google Scholar] [CrossRef] [PubMed]
- Luger, A.; Watschinger, B.; Deuster, P.; Svoboda, T.; Clodi, M.; Chrousos, G.P. Plasma growth hormone and prolactin responses to graded levels of acute exercise and to a lactate infusion. Neuroendocrinology 1992, 56, 112–117. [Google Scholar] [CrossRef]
- Schulte, S.; Schiffer, T.; Sperlich, B.; Knicker, A.; Podlog, L.W.; Strüder, H.K. The impact of increased blood lactate on serum S100B and prolactin concentrations in male adult athletes. Eur. J. Appl. Physiol. 2013, 113, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Bridge, M.W.; Weller, A.S.; Rayson, M.; Jones, D.A. Ambient temperature and the pituitary hormone response to exercise in humans. Exp. Physiol. 2003, 88, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Brisson, G.R.; Péronnet, F.; Perrault, H.; Boisvert, P.; Massicotte, D.; Gareau, R. Prolactinotrophic effect of endogenous and exogenous heat loads in human male adults. J. Appl. Physiol. 1991, 70, 1351–1355. [Google Scholar] [CrossRef] [PubMed]
- Strachan, A.T.; Leiper, J.B.; Maughan, R.J. Paroxetine administration to influence human exercise capacity, perceived effort or hormone responses during prolonged exercise in a warm environment. Exp. Physiol. 2004, 89, 657–664. [Google Scholar] [CrossRef]
- Laing, S.J.; Jackson, A.R.; Walters, R.; Lloyd-Jones, E.; Whitham, M.; Maassen, N.; Wlash, N.P. Human blood neutrophil responses to prolonged exercise with and without a thermal clamp. J. Appl. Physiol. 2008, 104, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.E.; Jorgensen, O.L.; Moller, N.; Orskov, H. Characterization of growth hormone release in response to external heating. Comparison to exercise induced release. Acta Endocrinol. (Copenhagen) 1984, 107, 295–301. [Google Scholar] [CrossRef]
- Wheldon, A.; Savine, R.L.; Sönksen, P.H.; Holt, R.I.G. Exercising in the cold inhibits growth hormone secretion by reducing the rise in core body temperature. Growth Horm. IGF Res. 2006, 16, 125–131. [Google Scholar] [CrossRef]
- Ftaiti, F.; Jemni, M.; Kacem, A.; Zaouali, M.A.; Tabka, Z.; Zbidi, A.; Grelot, L. Effect of hyperthermia and physical activity on circulating growth hormone. Appl. Physiol. Nutr. Metab. 2008, 33, 880–887. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suvi, S.; Mooses, M.; Timpmann, S.; Medijainen, L.; Unt, E.; Ööpik, V. Influence of Sodium Citrate Supplementation after Dehydrating Exercise on Responses of Stress Hormones to Subsequent Endurance Cycling Time-Trial in the Heat. Medicina 2019, 55, 103. https://doi.org/10.3390/medicina55040103
Suvi S, Mooses M, Timpmann S, Medijainen L, Unt E, Ööpik V. Influence of Sodium Citrate Supplementation after Dehydrating Exercise on Responses of Stress Hormones to Subsequent Endurance Cycling Time-Trial in the Heat. Medicina. 2019; 55(4):103. https://doi.org/10.3390/medicina55040103
Chicago/Turabian StyleSuvi, Silva, Martin Mooses, Saima Timpmann, Luule Medijainen, Eve Unt, and Vahur Ööpik. 2019. "Influence of Sodium Citrate Supplementation after Dehydrating Exercise on Responses of Stress Hormones to Subsequent Endurance Cycling Time-Trial in the Heat" Medicina 55, no. 4: 103. https://doi.org/10.3390/medicina55040103
APA StyleSuvi, S., Mooses, M., Timpmann, S., Medijainen, L., Unt, E., & Ööpik, V. (2019). Influence of Sodium Citrate Supplementation after Dehydrating Exercise on Responses of Stress Hormones to Subsequent Endurance Cycling Time-Trial in the Heat. Medicina, 55(4), 103. https://doi.org/10.3390/medicina55040103



