Influence of Parenteral Nutrition Delivery Techniques on Growth and Neurodevelopment of Very Low Birth Weight Newborns: A Randomized Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
- Birth weight ≥750–<1500 g, anticipated PN duration no less than 5 days and written consent of both parents to participate in the trial.
- Newborns with chromosomal diseases, multiple dysplasia, coagulation disorders, and skin anomalies preventing catheterization; newborns in demand of central vein catheterization observed post-birth due to administered inotropic medicines; and newborns whose parents refused to participate in the trial.
2.2. Intervention
2.3. Data Collection
2.4. Outcome Measures
2.5. Statistical Methods
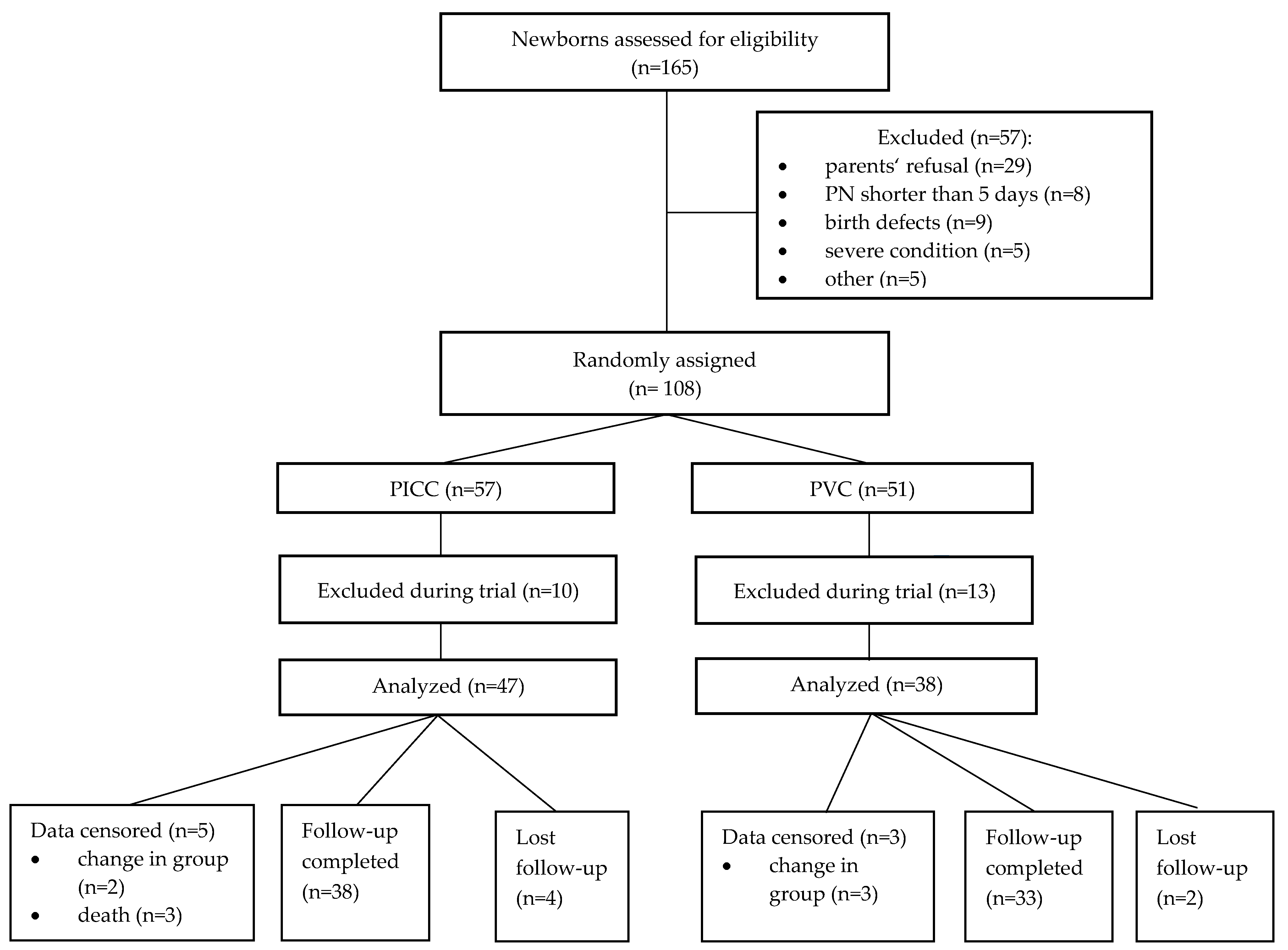
3. Results
3.1. Baseline Characteristics of Subjects
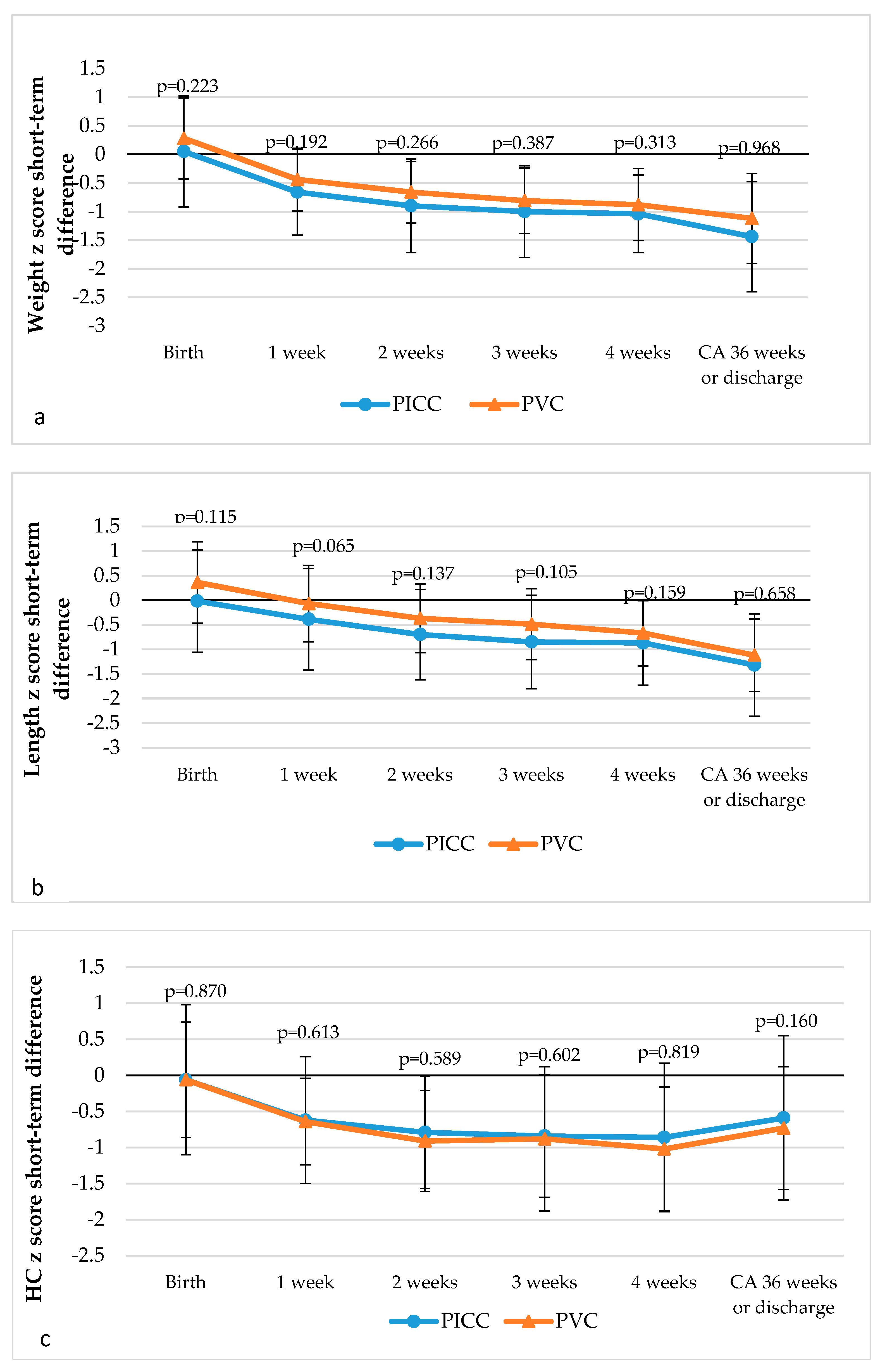
3.2. Short-Term Anthropometric Outcomes
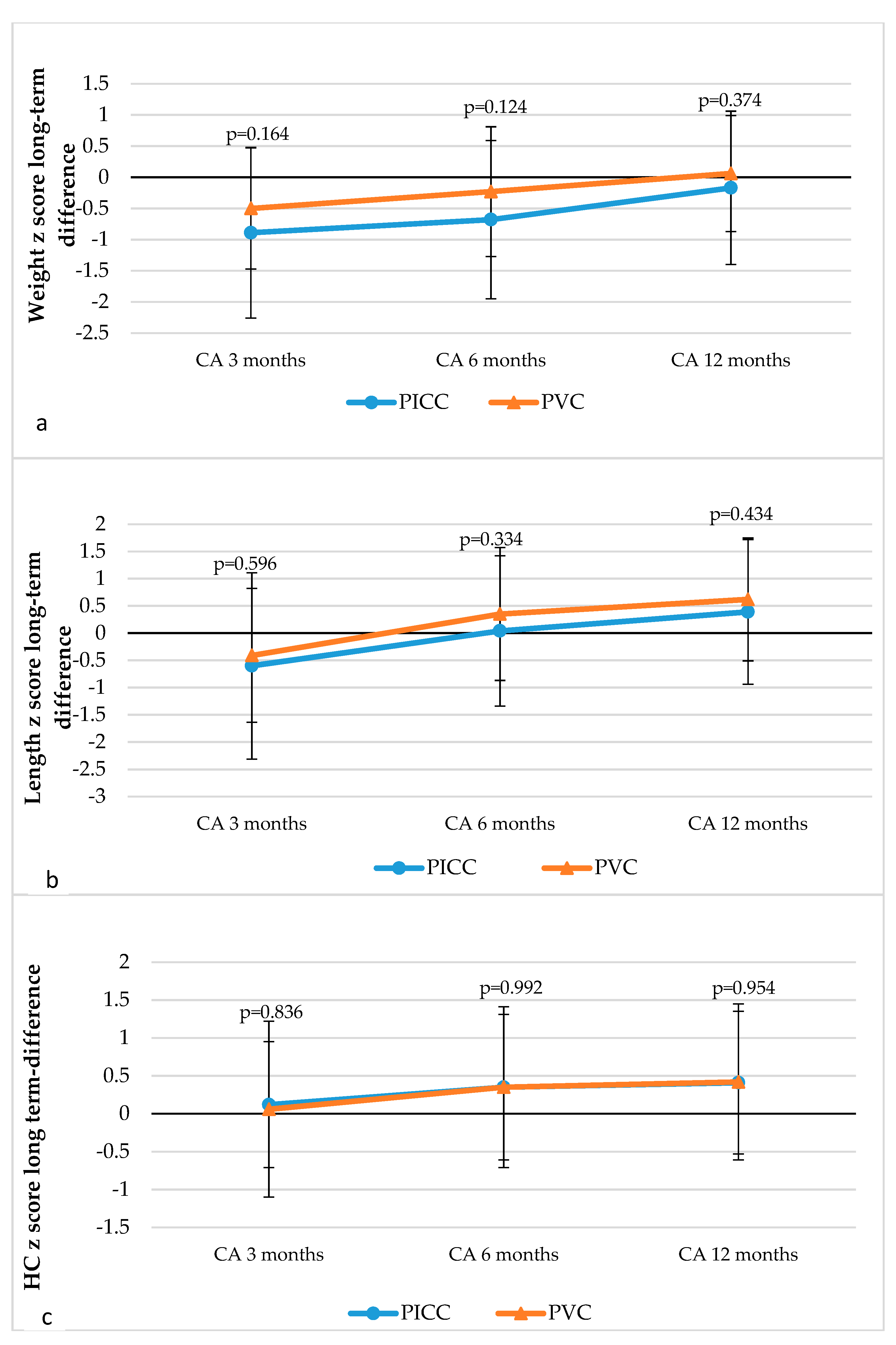
3.3. Long-Term Anthropometric Outcomes
3.4. Neurodevelopment Outcomes
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Euser, A.M.; de Wit, C.C.; Finken, M.J.; Rijken, M.; Wit, J.M. Growth of preterm born children. Horm. Res. Paediatr. 2008, 70, 319–328. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Shankaran, S.; Laptook, A.R.; Walsh, M.C.; Hale, E.C.; Newman, N.S.; Schibler, K.; Carlo, W.A.; et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010, 126, 443–456. [Google Scholar] [CrossRef]
- Moyses, H.E.; Johnson, M.J.; Leaf, A.A.; Cornelius, V.R. Early parenteral nutrition and growth outcomes in preterm infants: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2013, 97, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Kolacek, S.; Puntis, J.W.L.; Hojsak, I. ESPGHAN/ESPEN/ESPR/CSPEN working group on pediatric parenteral nutrition. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Venous access. Clin. Nutr. 2018, 37, 2379–2391. [Google Scholar] [CrossRef]
- Mactier, H.; Babarao, S.; Birch, J.; Johnson, M.; King, C.; Mahadevan-Bava, S.; Morgan, C.; Radbone, L.; Tomlin, S.; Nandi, L. The Provision of Parenteral Nutrition within Neonatal Services—A Framework for Practice. Available online: https://www.bapm.org/sites/default/files/files/Parenteral%20Nutrition%20April%202016.pdf (accessed on 12 February 2018).
- Murphy, J.; Browne, C.; Doolan, A.; Zidaru, A.; Fallon, W.; McDermott, C.; Moroney, K.; a Bryce-Smith, A.; O’Reilly-Marshall, A.; Duddy, P. Guideline on the Use of Parenteral Nutrition in Neonatal and Paediatric Units. Available online: https://www.hse.ie/eng/services/publications/clinical-strategy-and-programmes/guideline-on-the-use-of-parenteral-nutrition-in-neonatal-and-paediatric-units.pdf (accessed on 12 February 2018).
- Jarvis, C.; Hooley, A.; Budge, H.; Ojha, S. Available online: https://www.nuh.nhs.uk/download.cfm?doc=docm93jijm4n961 (accessed on 12 February 2018).
- Bolisetty, S. Parenteral Nutrition in Newborns. Available online: https://www.seslhd.health.nsw.gov.au/sites/default/files/migration/RHW/Newborn_Care/Guidelines/Medical/nccparenteralnutrition.pdf (accessed on 21 March 2019).
- Uygun, I.; Okur, M.H.; Otcu, S.; Ozturk, H. Peripherally inserted central catheters in the neonatal period. Acta Cir. Bras. 2011, 26, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Milstone, A.M.; Reich, N.G.; Advani, S.; Yuan, G.; Bryant, K.; Coffin, S.E.; Huskins, W.C.; Livingston, R.; Saiman, L.; Smith, P.B.; et al. Catheter dwell time and CLABSIs in neonates with PICCs: A multicenter cohort study. Pediatrics 2013, 132, e1609–e1615. [Google Scholar] [CrossRef]
- Unbeck, M.; Forberg, U.; Ygge, B.M.; Ehrenberg, A.; Petzold, M.; Johansson, E. Peripheral venous catheter related complications are common among paediatric and neonatal patients. Acta Paediatr. 2015, 104, 566–574. [Google Scholar] [CrossRef]
- Ainsworth, S.B.; Furness, J.; Fenton, A.C. Randomized comparative trial between percutaneous longlines and peripheral cannulae in the delivery of neonatal parenteral nutrition. Acta Paediatr. 2001, 90, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- van Veenendaal, N.R.; van der Schoor, S.R.D.; Limpens, J.; van Kempen, A.A.M.W.; van Goudoever, J.B. Effect of single family rooms for preterm infants on neurodevelopment: Study protocol for a systematic review. BMJ Open 2017, 7, e015818. [Google Scholar] [CrossRef]
- Ranger, M.; Zwicker, J.G.; Chau, C.M.; Park, M.T.; Chakravarthy, M.M.; Poskitt, K.; Miller, S.P.; Bjornson, B.H.; Tam, E.W.; Chau, V.; et al. Neonatal Pain and Infection Relate to Smaller Cerebellum in Very Preterm Children at School Age. J. Pediatr. 2015, 167, 292–298. [Google Scholar] [CrossRef]
- Ohki, Y.; Maruyama, K.; Harigaya, A.; Kohno, M.; Arakawa, H. Complications of peripherally inserted central venous catheter in Japanese neonatal intensive care units. Pediatr. Int. 2013, 55, 185–189. [Google Scholar] [CrossRef]
- Bulbul, A.; Okan, F.; Nuhoglu, A. Percutaneously inserted central catheters in the newborns: A center’s experience in Turkey. J. Matern. Fetal Neonatal Med. 2010, 23, 529–535. [Google Scholar] [CrossRef]
- Arnts, I.J.; Bullens, L.M.; Groenewoud, J.M.; Liem, K.D. Comparison of complication rates between umbilical and peripherally inserted central venous catheters in newborns. J. Obstet. Gynecol. Neonatal Nurs. 2014, 43, 205–215. [Google Scholar] [CrossRef]
- Singh, A.; Bajpai, M.; Panda, S.S.; Jana, M. Complications of peripherally inserted central venous catheters in neonates: Lesson learned over 2 years in a tertiary care centre in India. Afr. J. Paediatr. Surg. 2014, 11, 242–247. [Google Scholar]
- Webster, J.; Osborne, S.; Rickard, C.M.; New, K. Clinically-indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst. Rev. 2013, 8, CD007798. [Google Scholar] [CrossRef]
- Wojkowska-Mach, J.; Gulczynska, E.; Nowiczewski, M.; Borszewska-Kornacka, M.; Domanska, J.; Merritt, T.A.; Helwich, E.; Kordek, A.; Pawlik, D.; Gadzinowski, J.; et al. Late-onset bloodstream infections of Very-Low-Birth-Weight infants: Data from the Polish Neonatology Surveillance Network in 2009–2011. BMC Infect. Dis. 2014, 14, 339. [Google Scholar] [CrossRef]
- Geffers, C.; Gastmeier, A.; Schwab, F.; Groneberg, K.; Ruden, H.; Gastmeier, P. Use of central venous catheter and peripheral venous catheter as risk factors for nosocomial bloodstream infection in very-low-birth-weight infants. Infect. Control Hosp. Epidemiol. 2010, 31, 395–401. [Google Scholar] [CrossRef]
- Olsen, A.L.; Reinholdt, J.; Jensen, A.M.; Andersen, L.P.; Jensen, E.T. Nosocomial infection in a Danish Neonatal Intensive Care Unit: A prospective study. Acta Paediatr. 2009, 98, 1294–1299. [Google Scholar] [CrossRef]
- Ofek Shlomai, N.; Reichman, B.; Lerner-Geva, L.; Boyko, V.; Bar-Oz, B. Population-based study shows improved postnatal growth in preterm very-low-birthweight infants between 1995 and 2010. Acta Paediatr. 2014, 103, 498–503. [Google Scholar] [CrossRef]
- Cao, W.; Zhang, Y.H.; Zhao, D.Y.; Xia, H.P.; Zhu, T.W.; Xie, L.J. Risk factors for extrauterine growth restriction in preterm infants with gestational age less than 34 weeks. Chin. J. Contempor. Paediatr. 2015, 17, 453–458. [Google Scholar]
- Tsai, M.H.; Hsu, J.F.; Chu, S.M.; Lien, R.; Huang, H.R.; Chiang, M.C.; Fu, R.H.; Lee, C.W.; Huang, Y.C. Incidence, clinical characteristics and risk factors for adverse outcome in neonates with late-onset sepsis. Pediatr. Infect. Dis. J. 2014, 33, e7–e13. [Google Scholar] [CrossRef]
- Bright, H.R.; Babata, K.; Allred, E.N.; Erdei, C.; Kuban, K.C.K.; Joseph, R.M.; O’Shea, T.M.; Leviton, A.; Dammann, O. Neurocognitive Outcomes at 10 Years of Age in Extremely Preterm Newborns with Late-Onset Bacteremia. J. Pediatr. 2017, 187, 43–49. [Google Scholar] [CrossRef]
- Ainsworth, S.B.; Clerihew, L.; McGuire, W. Percutaneous central venous catheters versus peripheral cannulae for delivery of parenteral nutrition in neonates. Cochrane Database Syst. Rev. 2004, CD004219. [Google Scholar] [CrossRef]
- Ainsworth, S.B.; Clerihew, L.; McGuire, W. Percutaneous central venous catheters versus peripheral cannulae for delivery of parenteral nutrition in neonates. Cochrane Database Syst. Rev. 2007, 18, CD004219. [Google Scholar]
- Ainsworth, S.; McGuire, W. Percutaneous central venous catheters versus peripheral cannulae for delivery of parenteral nutrition in neonates. Cochrane Database Syst. Rev. 2015, CD004219. [Google Scholar] [CrossRef]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 1–15. [Google Scholar] [CrossRef]
- WHO. Child Growth Standarts. 2006. Available online: http://www.who.int/childgrowth/standards/en/ (accessed on 8 September 2018).
- Liossis, G.; Bardin, C.; Papageorgiou, A. Comparison of risks from percutaneous central venous catheters and peripheral lines in infants of extremely low birth weight: A cohort-controlled study of infants <1000 g. J. Matern. Fetal Neonatal Med. 2003, 13, 171–174. [Google Scholar]
- Xia, B.; Tang, J.; Xiong, Y.; Li, X.H.; Mu, D.Z. Peripherally inserted central catheters and the incidence of candidal sepsis in VLBW and ELBW infants: Is sepsis increased? World J. Pediatr. 2010, 6, 154–157. [Google Scholar] [CrossRef]
- Lima, P.A.; Carvalho, M.; Costa, A.C.; Moreira, M.E. Variables associated with extra uterine growth restriction in very low birth weight infants. J. Pediatr. 2014, 90, 22–27. [Google Scholar] [CrossRef]
- Avila-Alvarez, A.; Solar Boga, A.; Bermudez-Hormigo, C.; Fuentes Carballal, J. Extrauterine growth restriction among neonates with a birthweight less than 1500 grams. An. Pediatr. 2018, 89, 325–332. [Google Scholar] [CrossRef]
- Genoni, G.; Binotti, M.; Monzani, A.; Bernascone, E.; Stasi, I.; Bona, G.; Ferrero, F. Nonrandomised interventional study showed that early aggressive nutrition was effective in reducing postnatal growth restriction in preterm infants. Acta Paediatr. 2017, 106, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Belfort, M.B.; Rifas-Shiman, S.L.; Sullivan, T.; Collins, C.T.; McPhee, A.J.; Ryan, P.; Kleinman, K.P.; Gillman, M.W.; Gibson, R.A.; Makrides, M. Infant growth before and after term: Effects on neurodevelopment in preterm infants. Pediatrics 2011, 128, e899–e906. [Google Scholar] [CrossRef]
- Rover, M.M.; Viera, C.S.; Silveira, R.C.; Guimaraes, A.T.; Grassiolli, S. Risk factors associated with growth failure in the follow-up of very low birth weight newborns. J. Pediatr. 2016, 92, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.B.; de Mello, R.R.; Morsch, D.S.; Meio, M.D.; da Silva, K.S. Mental performance of very low birth weight preterm infants: Assessment of stability in the first two years of life and factors associated with mental performance. Rev. Bras. Epidemiol. 2012, 15, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.T.; Chen, C.H.; Lin, M.C.; Wang, T.M.; Hsu, Y.C. Correction: Post-discharge body weight and neurodevelopmental outcomes among very low birth weight infants in Taiwan: A nationwide cohort study. PLoS ONE 2018, 13, e0198310. [Google Scholar] [CrossRef]
- Belfort, M.B.; Anderson, P.J.; Nowak, V.A.; Lee, K.J.; Molesworth, C.; Thompson, D.K.; Doyle, L.W.; Inder, T.E. Breast Milk Feeding, Brain Development, and Neurocognitive Outcomes: A 7-Year Longitudinal Study in Infants Born at Less Than 30 Weeks’ Gestation. J. Pediatr. 2016, 177, 133–139. [Google Scholar] [CrossRef]
- Cejas, G.; Gomez, Y.; Roca Mdel, C.; Dominguez, F. Neurodevelopment of very low birth weight infants in the first two years of life in a Havana tertiary care hospital. MEDICC Rev. 2015, 17, 14–17. [Google Scholar] [PubMed]
| Characteristics | PICC Group (n = 47) | PVC Group (n = 38) | p |
|---|---|---|---|
| Gestation, median (IQR), weeks | 28.0 (27; 30) | 28.5 (27; 29) | 0.936 |
| Birth weight, median (IQR), g | 1086 (900; 1270) | 1196 (987.75; 1280.5) | 0.265 |
| Sex, n (%) | 0.355 | ||
| Male | 27 (57.4) | 18 (47.4) | |
| Female | 20 (42.6) | 20 (52.6) | |
| SGA, n (%) | 5 (10.6) | 1 (2.6) | 0.218 |
| Length, median (IQR), cm | 37 (35; 38) | 37 (36; 39) | 0.183 |
| Head circumference, median (IQR), cm | 26 (24; 27) | 26 (24.75; 27) | 0.875 |
| Apgar 1 min, median (IQR) | 7 (5; 8) | 7 (5; 7) | 0.356 |
| Apgar 5 min, median (IQR) | 8 (7; 8) | 8 (7; 8) | 0.507 |
| Characteristics | PICC Group (n = 45) | PVC Group (n = 38) | p |
|---|---|---|---|
| PN duration, median (IQR), days | 8 (6; 9) | 7(6; 9) | 0.331 |
| Nutrients | |||
| Amino acids, median (IQR), g/kg/d | 2.51 (2.24; 2.76) | 2.45 (2.20; 2.81) | 0.593 |
| Carbohydrates, median (IQR), g/kg/d | 7.29 (6.46; 8.20) | 7.25 (5.88; 8.41) | 0.309 |
| Lipids, median (IQR), g/kg/d | 1.93 (1.63; 2.15) | 1.93 (1.60; 2.04) | 0.919 |
| Energy, median (IQR), kcal/kg/d | 53.1 (47.3; 58.4) | 51.2 (44.9; 57.8) | 0.536 |
| Morbidity and mortality, n (%) | |||
| RDS | 41 (91.1) | 33 (94.3) | 0.691 |
| BPD | 6 (13.3) | 8 (22.9) | 0.266 |
| PDA | 22 (48.9) | 18 (51.4) | 0.822 |
| Early onset sepsis | 9 (20.0) | 11 (31.4) | 0.242 |
| Late onset sepsis | 8 (17.8) | 7 (20.0) | 0.801 |
| IVH III | 1 (2.2) | 1 (2.9) | 1.000 |
| IVH IV | 4 (8.9) | 0 (0) | 0.127 |
| PVL | 14 (30.4) | 10 (28.6) | 1.00 |
| Mortality before discharge | 3 (6.4) | 0 (0) | 0.25 |
| MDI | PICC, n (%) | PVC, n (%) | Fisher p | χ2 |
|---|---|---|---|---|
| <70 (significant delay) | 4 (10.5) | 0 (0.0) | 0.118 | 3.68 |
| <85 (total delay) | 10 (26.3) | 7 (21.2) | 0.781 | 0.25 |
| PDI | PICC, n (%) | PVC, n (%) | Fisher p | χ2 |
|---|---|---|---|---|
| <70 (significant delay) | 5 (13.2) | 0 (0.0) | 0.057 | 4.67 |
| <85 (delay) | 15 (39.5) | 18 (54.5) | 0.239 | 1.61 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldakauskienė, I.; Tamelienė, R.; Marmienė, V.; Rimdeikienė, I.; Šmigelskas, K.; Kėvalas, R. Influence of Parenteral Nutrition Delivery Techniques on Growth and Neurodevelopment of Very Low Birth Weight Newborns: A Randomized Trial. Medicina 2019, 55, 82. https://doi.org/10.3390/medicina55040082
Aldakauskienė I, Tamelienė R, Marmienė V, Rimdeikienė I, Šmigelskas K, Kėvalas R. Influence of Parenteral Nutrition Delivery Techniques on Growth and Neurodevelopment of Very Low Birth Weight Newborns: A Randomized Trial. Medicina. 2019; 55(4):82. https://doi.org/10.3390/medicina55040082
Chicago/Turabian StyleAldakauskienė, Ilona, Rasa Tamelienė, Vitalija Marmienė, Inesa Rimdeikienė, Kastytis Šmigelskas, and Rimantas Kėvalas. 2019. "Influence of Parenteral Nutrition Delivery Techniques on Growth and Neurodevelopment of Very Low Birth Weight Newborns: A Randomized Trial" Medicina 55, no. 4: 82. https://doi.org/10.3390/medicina55040082
APA StyleAldakauskienė, I., Tamelienė, R., Marmienė, V., Rimdeikienė, I., Šmigelskas, K., & Kėvalas, R. (2019). Influence of Parenteral Nutrition Delivery Techniques on Growth and Neurodevelopment of Very Low Birth Weight Newborns: A Randomized Trial. Medicina, 55(4), 82. https://doi.org/10.3390/medicina55040082






