Microstructure Evolution and Damage Mechanism of DD9 Single Crystal Superalloy-Thermal Barrier Coating System Under High Temperature Oxidation: A Comparative Study with DD6
Highlights
- The microstructural evolution and failure mechanisms of DD9-TBC system under 1050 °C high-temperature oxidation are systematically studied and the oxidation behavior between DD9-TBC and DD6-TBC systems is compared.
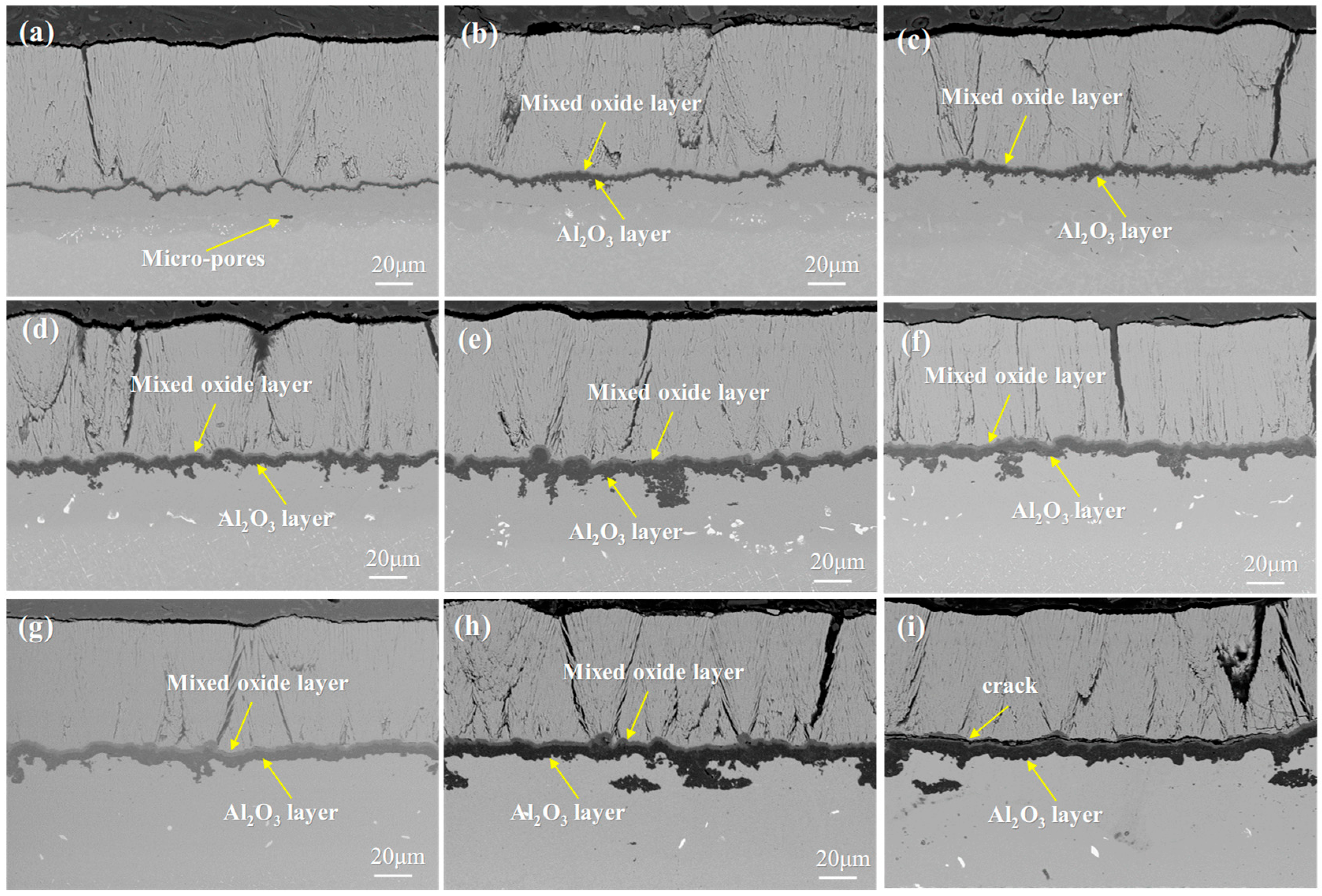
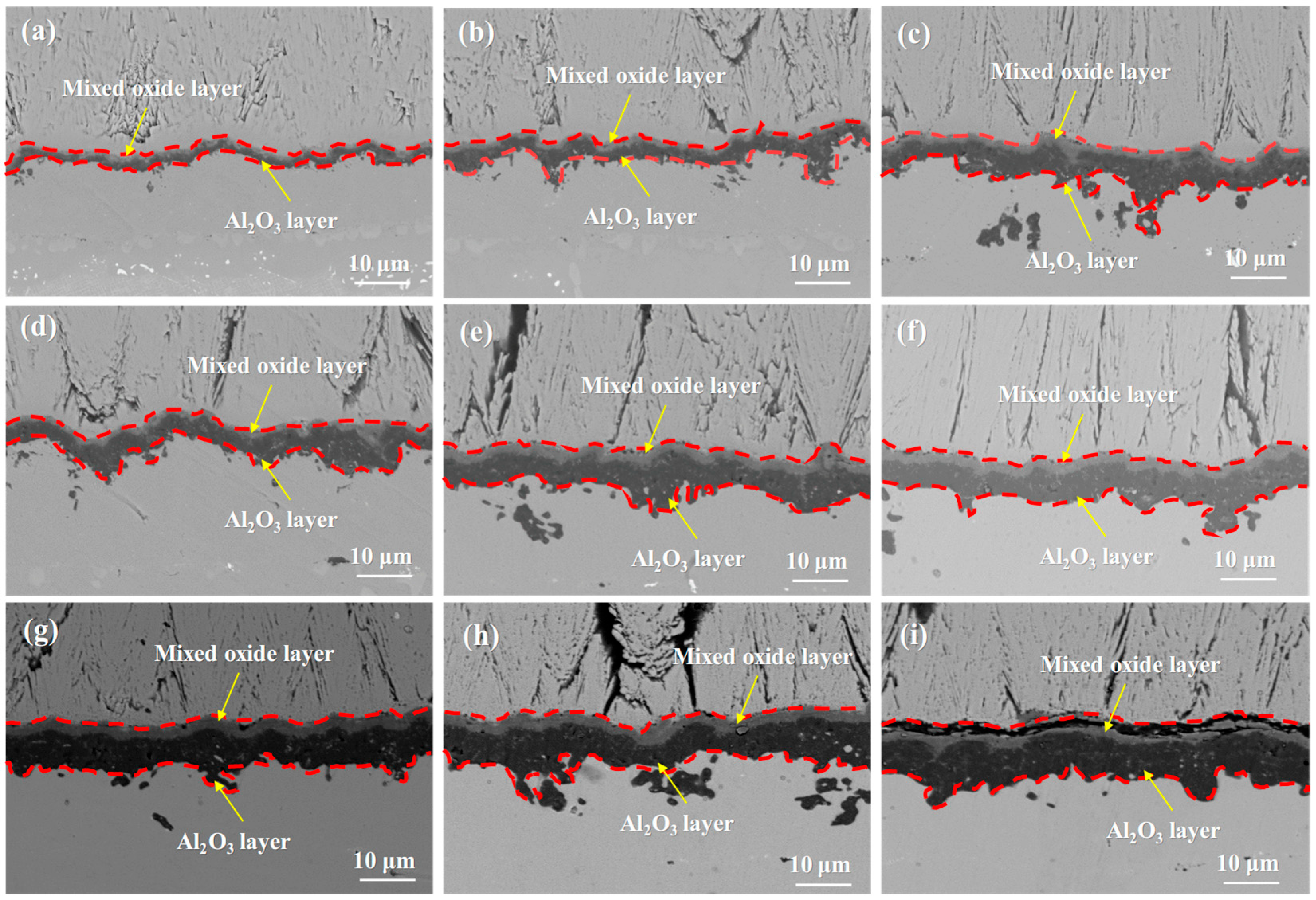
- Both systems show similar TGO growth mechanisms: TGO gradually forms a mixed oxide layer and an Al2O3 layer with prolonged oxidation, and its growth rate slows down in the later stage due to Al consumption in the bond coat.
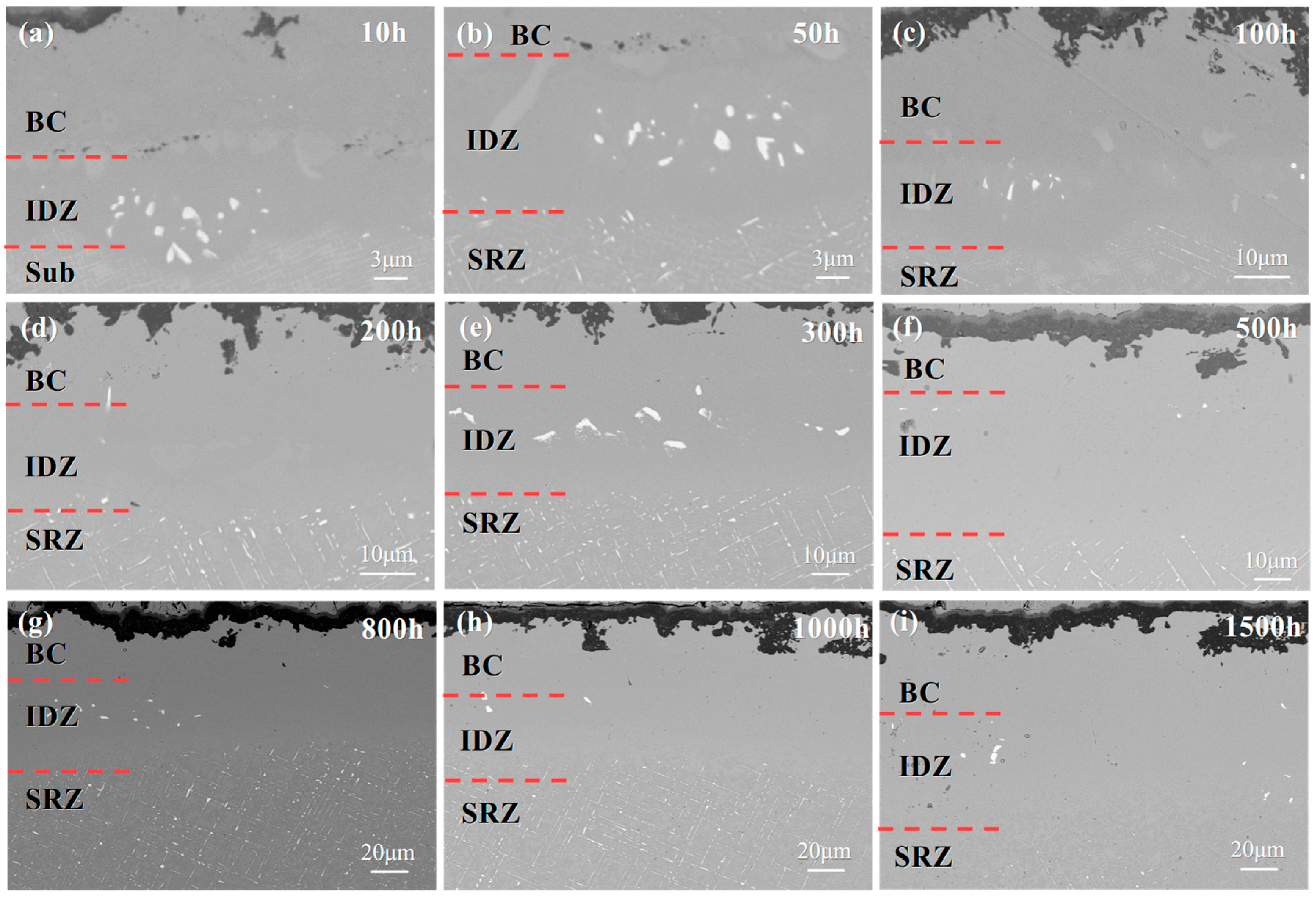
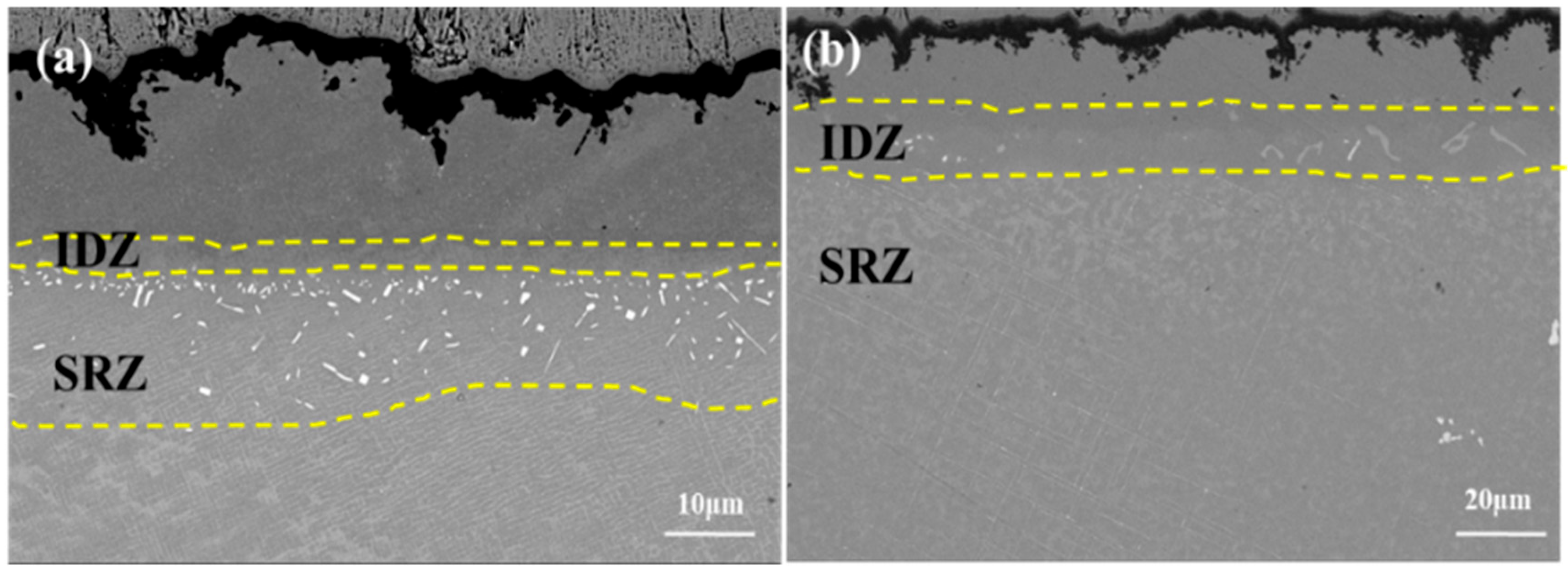
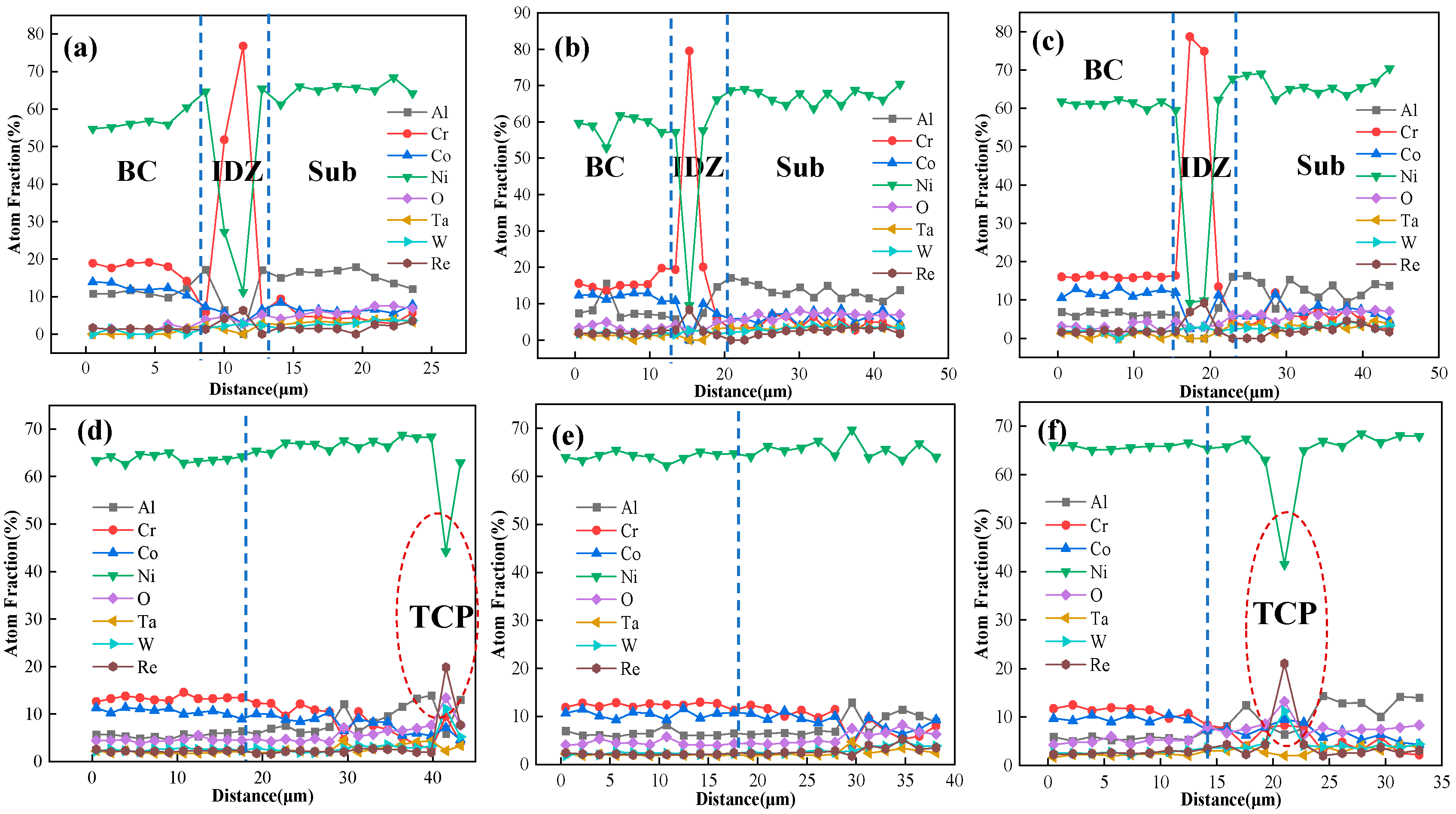
- The substrate significantly affects interfacial interdiffusion, with IDZ and SRZ continuously growing (SRZ at a faster rate); line-like TCP phases precipitate in SRZ and spread throughout the substrate, severely impairing the alloy’s mechanical properties.
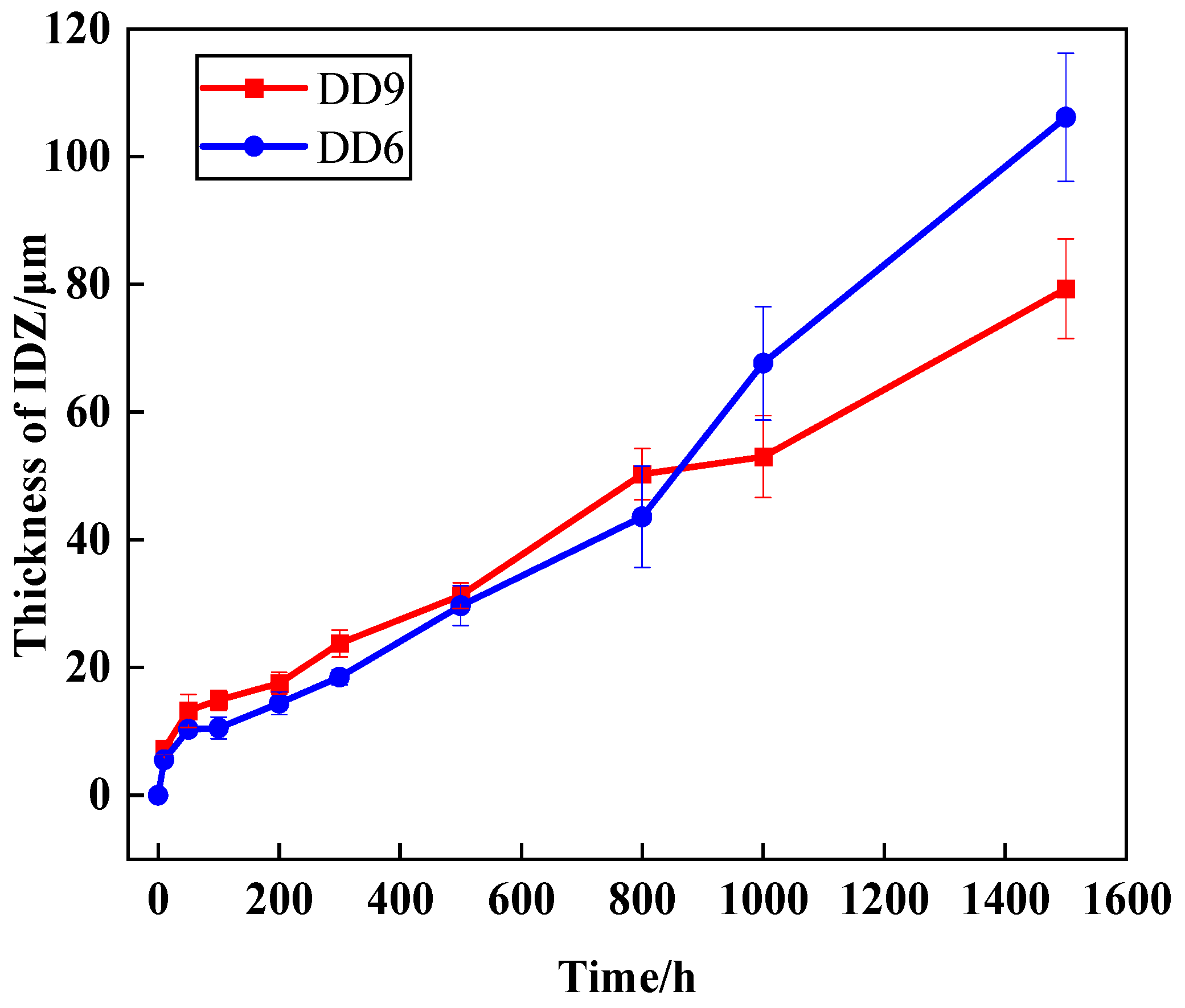
- DD9 exhibits faster IDZ growth than DD6 in the first 800 hours of oxidation, but the growth rate later becomes slower, which is related to the influence of alloying elements like Re on element diffusion and TCP phase precipitation.
Abstract
1. Introduction
2. Materials and Experiments
2.1. Materials Preparation
2.2. Oxidation Experiments
2.3. Materials Characterization
3. Oxidation Behavior of the DD9 Superalloy-Coating System
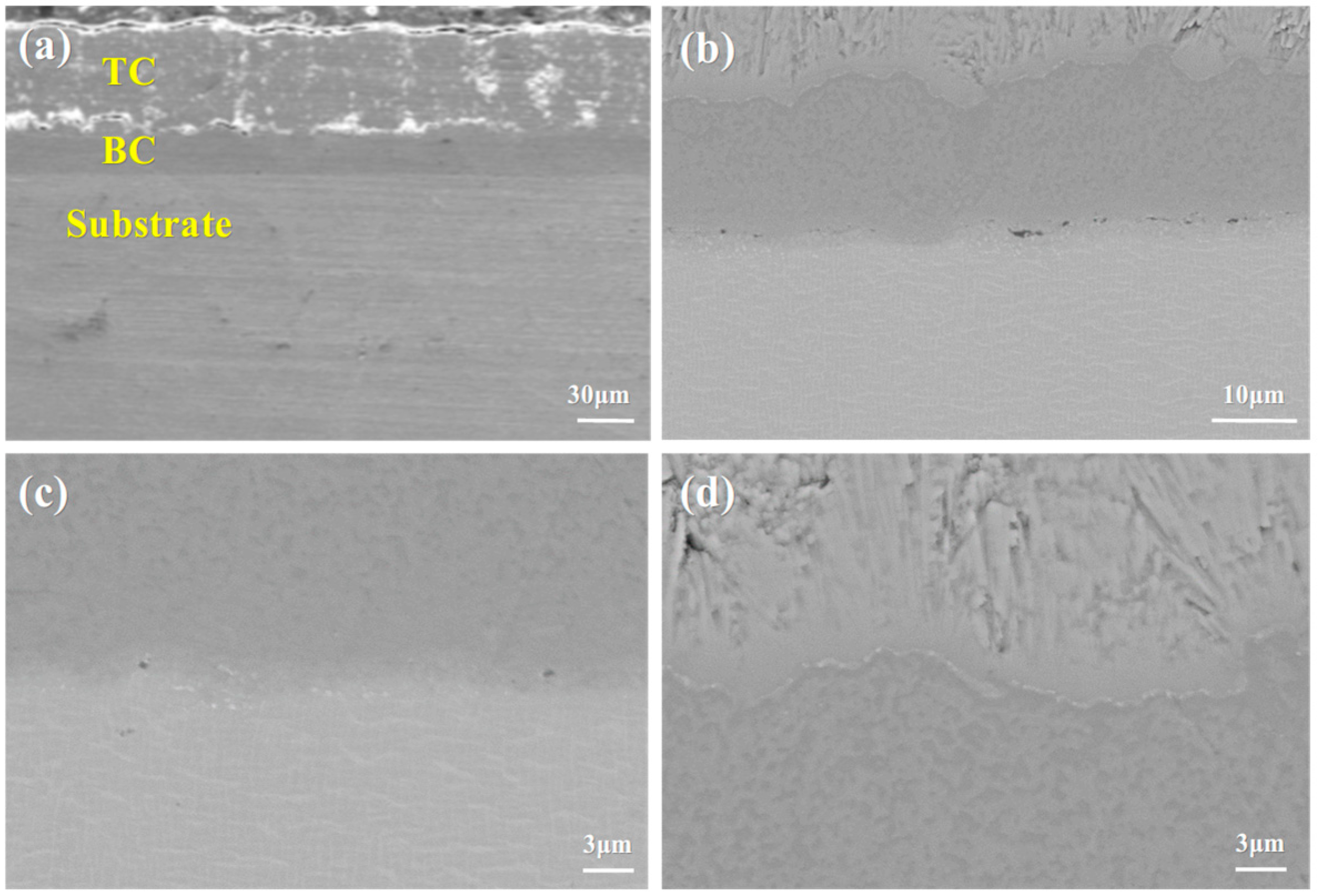
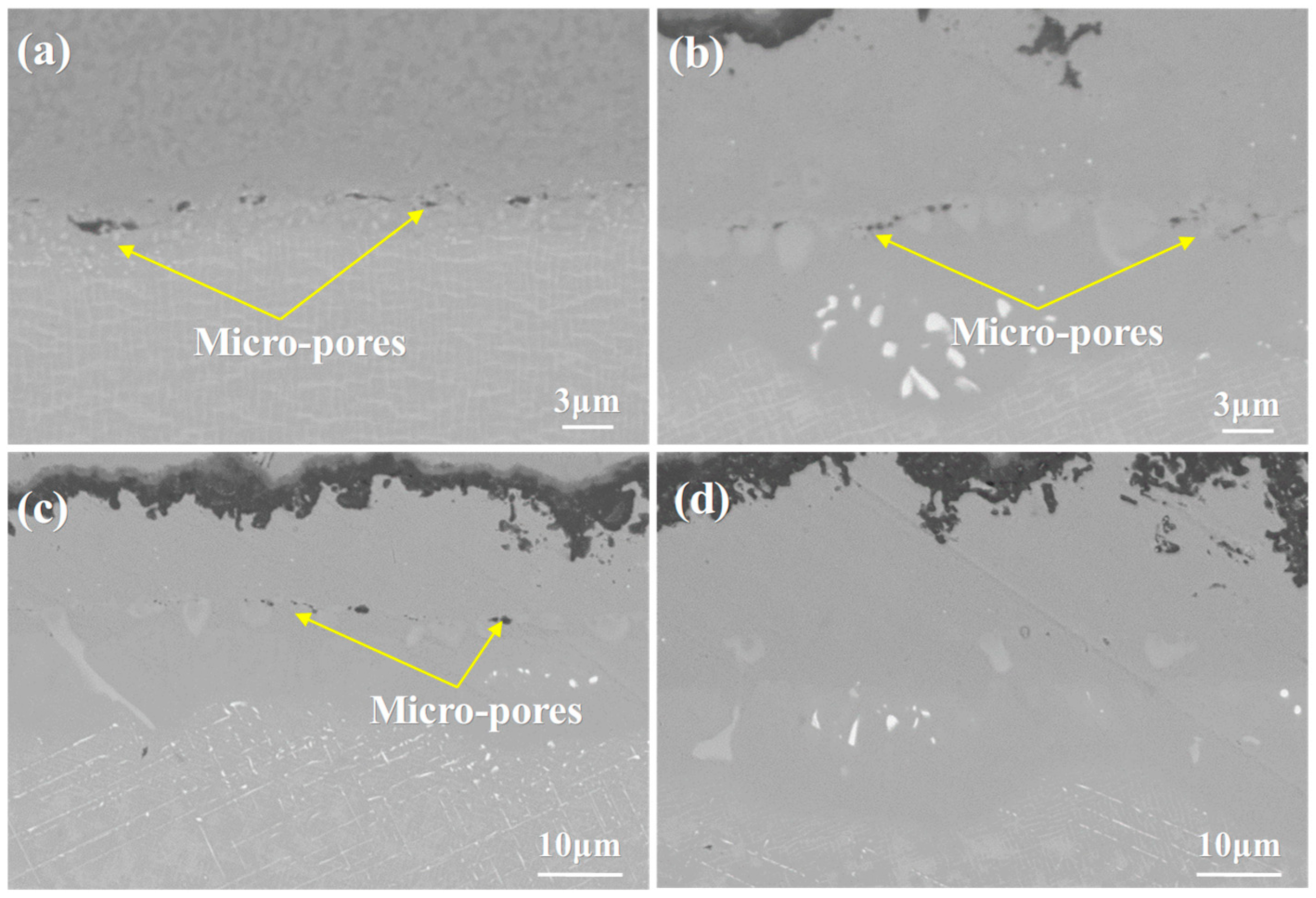
3.1. Microstructure of the As-Prepared DD9 Superalloy-Coating System
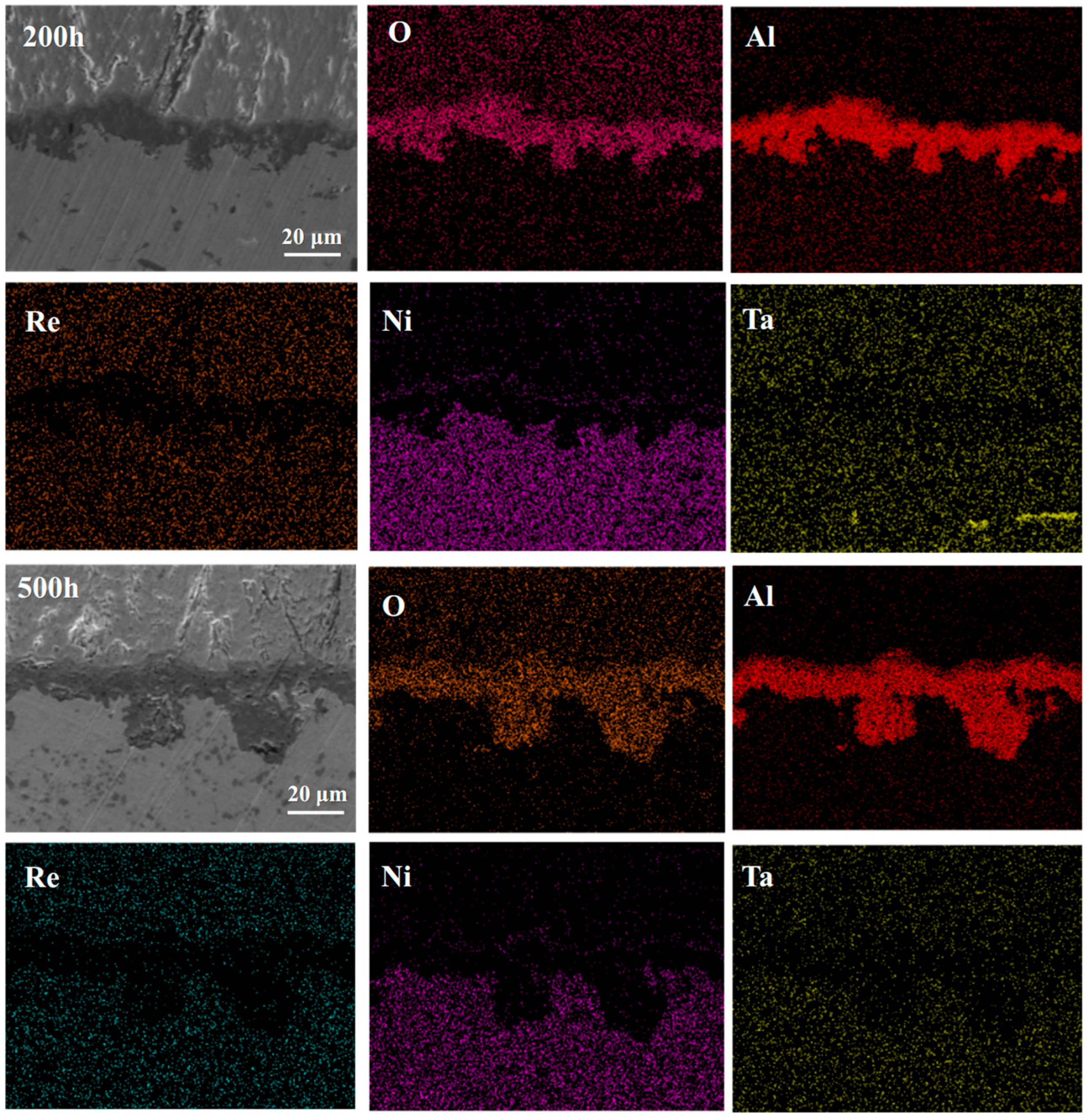
3.2. Microstructural Evolution of the Single Crystal-Coating System
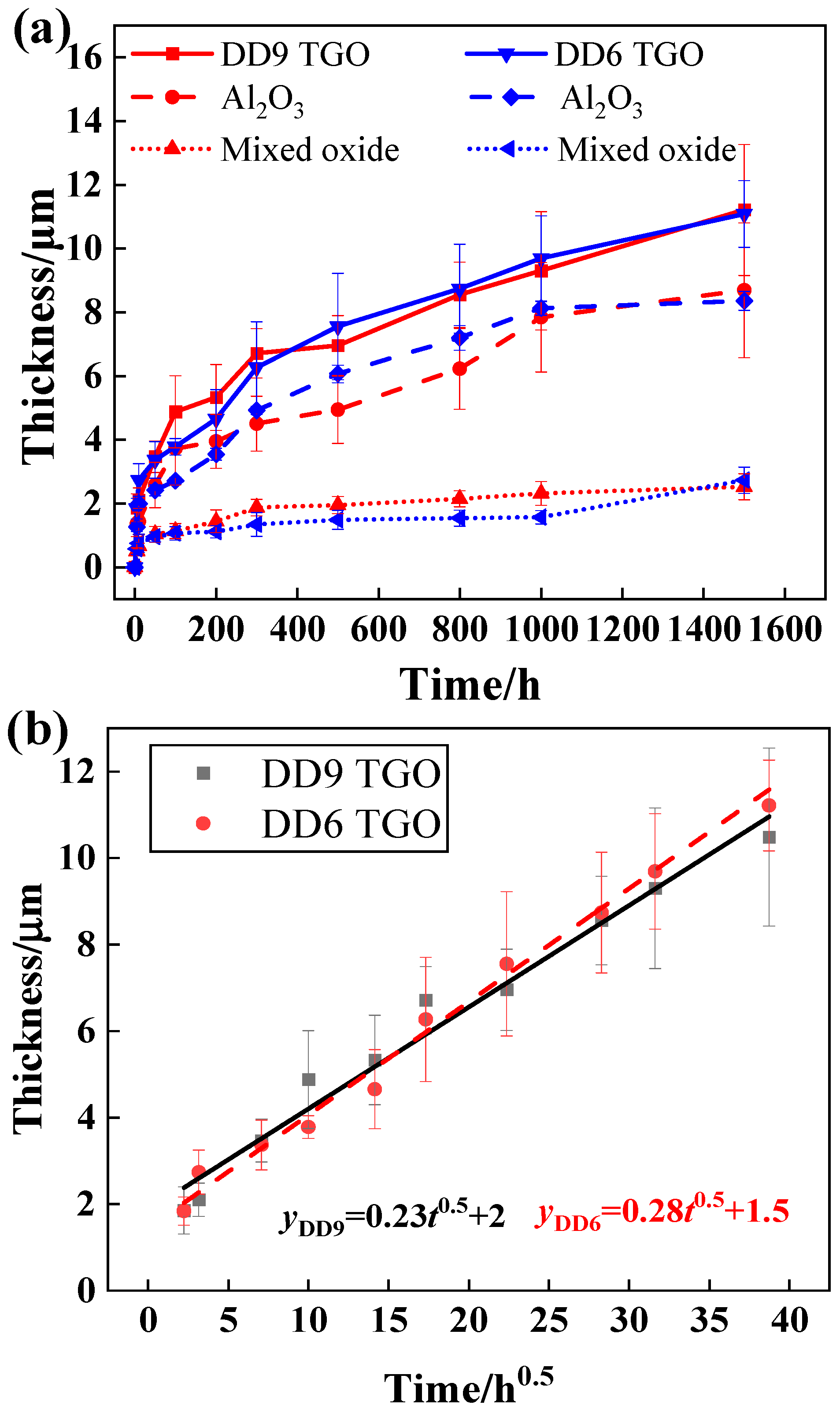
3.3. Isothermal Oxidation Kinetic Model of the DD9 Superalloy-Coating System
4. Microstructure Evolution of the DD9 Superalloy-Coating Interface
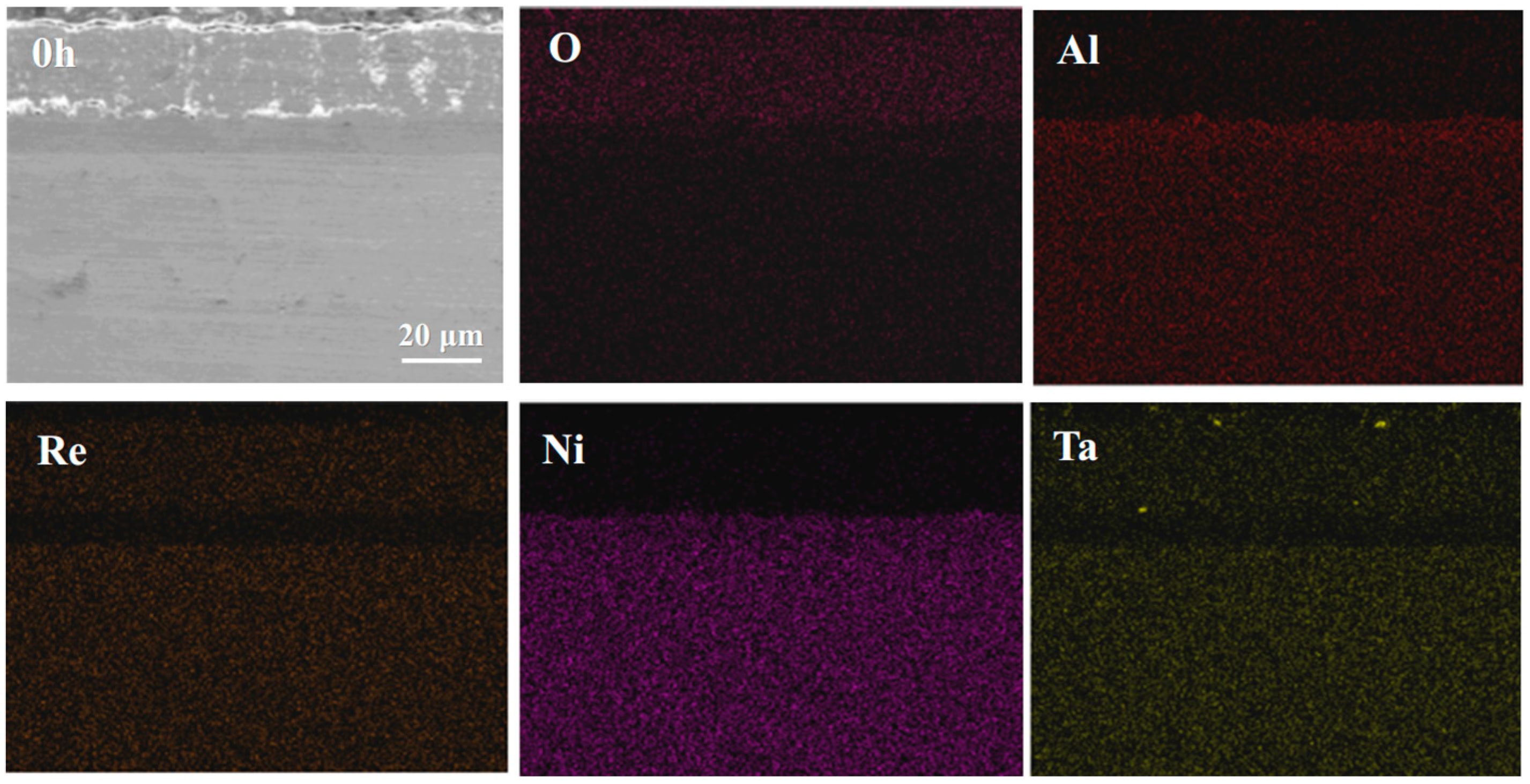
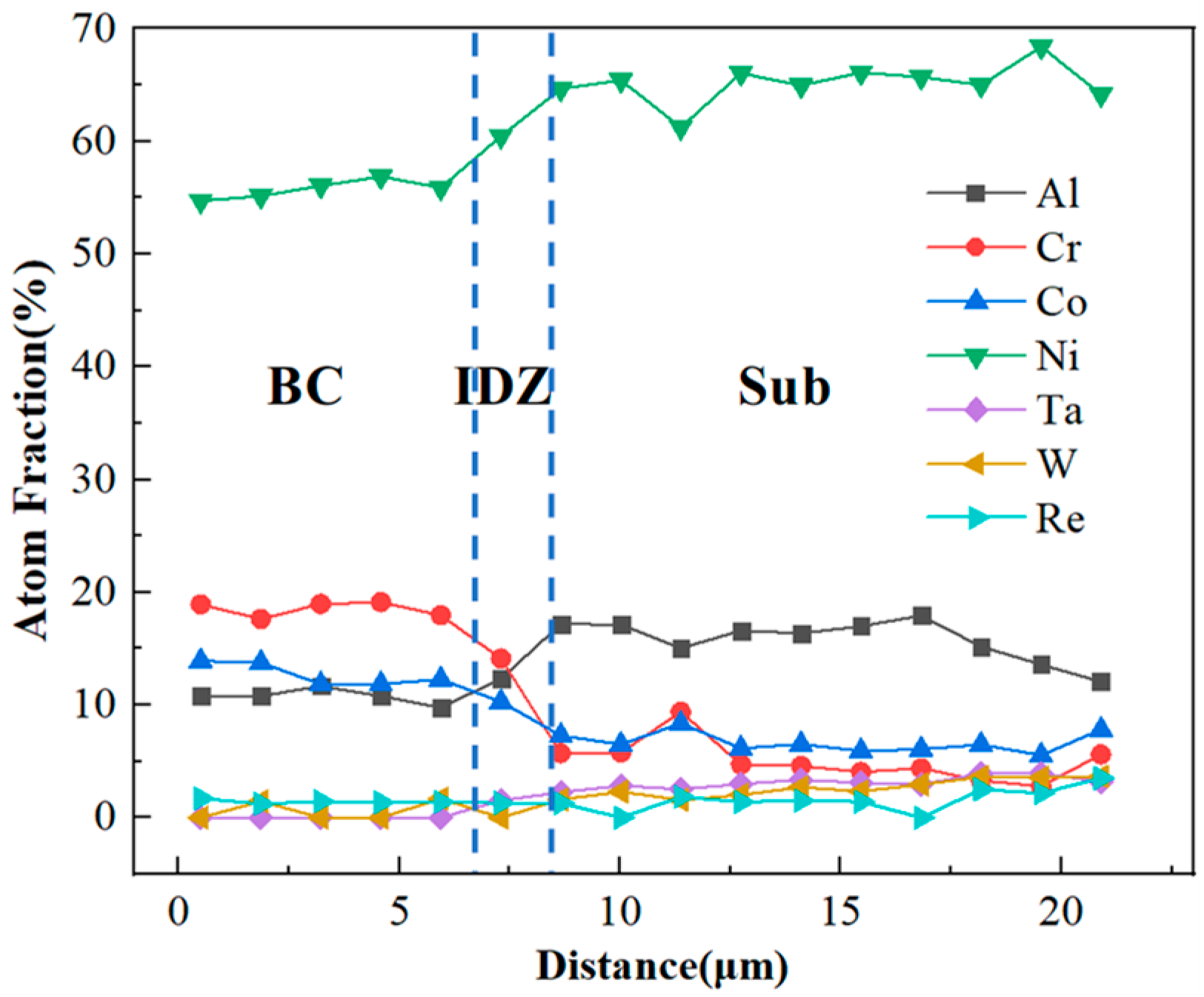
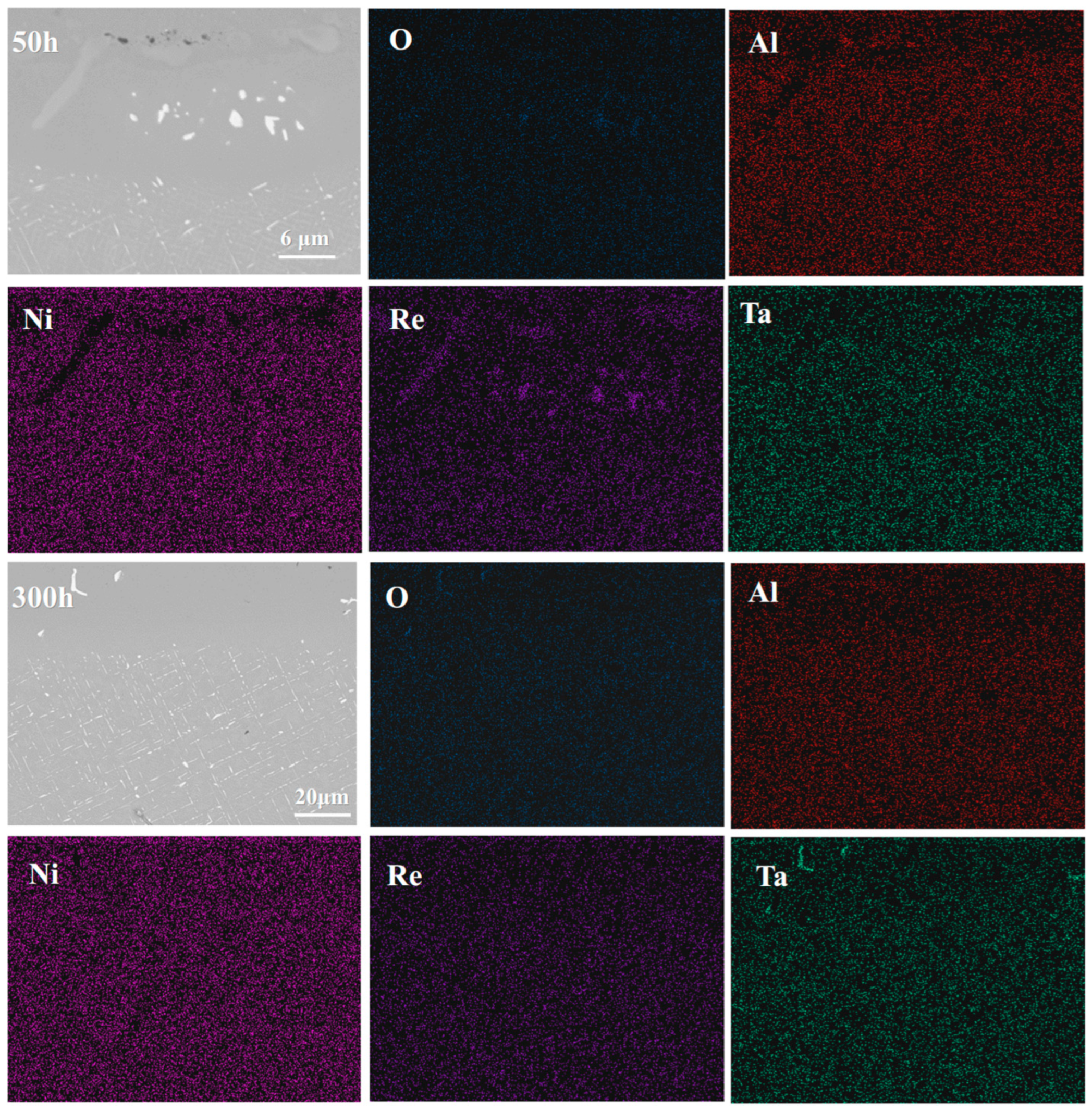
4.1. Microstructural Evolution of the IDZ
4.2. Elements Interdiffusion Behaviors
5. Conclusions
- (1)
- The oxidation mechanisms of DD9 superalloy-coating system and DD6 superalloy-coating system are quite similar. The rapid growth of the TGO leads to the rapid consumption of Al elements in the bond coat, with slower TGO growth in the later stages of oxidation. As oxidation progresses, the TGO develops into two distinct layers: a mixed oxide layer and an Al2O3 layer. This study provides a theoretical basis for the optimized design of domestic third-generation single-crystal superalloy-thermal barrier coating (TBC) systems.
- (2)
- The substrate has a significant impact on the interdiffusion at the substrate-bond coat interface. As oxidation time increases, both the IDZ and SRZ continue to grow, with the SRZ growing at a faster rate. Line-like TCP phases precipitate within the SRZ, quickly spreading throughout the entire substrate, which can negatively affect the high-temperature mechanical properties of the single-crystal alloy.
- (3)
- In the early stages of oxidation, the growth rate of the TGO and IDZ in DD9 is faster than that in DD6. However, as time progresses, the growth rate of DD6 gradually exceeds that of DD9. This study provides experimental support for the service life evaluation of coated turbine blades.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goucem, M. Influence of the Ambient Temperature on the Efficiency of Gas Turbines. Fluid. Dyn. Mater. Process. 2024, 20, 2265–2279. [Google Scholar] [CrossRef]
- Snyder, T.; Davis, D.; McKinney, R. 1 Aero Gas Turbines. In Renewable Fuels: Sources, Conversion, and Utilization; Cambridge University Press: Cambridge, UK, 2022; pp. 3–34. Available online: https://www.cambridge.org/core/books/abs/renewable-fuels/aero-gas-turbines/EBB9E85F293D7D909840D6F5BC3A702F (accessed on 11 September 2025).
- Zhang, C.; Yang, B.; Wang, Y.; Tu, G. Preliminary Research on a High Thrust-to-Weight Ratio of Double-Sided Composite Impeller Microturbine Engine. Int. J. Aerosp. Eng. 2021, 2021, 9931701. [Google Scholar]
- Saraçyakupoğlu, T. Energy Savings from New Materials and Processes in Aviation. In Sustainable Materials and Manufacturing Techniques in Aviation; Springer Nature: Cham, Switzerland, 2024; pp. 1–26. [Google Scholar]
- Song, J.; Qi, H.; Li, S.; Shi, D.; Yang, X. Computational method for the analysis of erosion-induced stress and damage in thermal barrier coatings. Surf. Coat. Technol. 2019, 380, 125089. [Google Scholar]
- Xia, W.; Zhao, X.; Yue, L.; Zhang, Z. A review of composition evolution in Ni-based single crystal superalloys. J. Mater. Sci. Technol. 2020, 44, 76–95. [Google Scholar] [CrossRef]
- Liu, Y.; Ru, Y.; Zhang, H.; Pei, Y.; Li, S.; Gong, S. Coating-assisted deterioration mechanism of creep resistance at a nickel-based single-crystal superalloy. Surf. Coat. Technol. 2021, 406, 126668. [Google Scholar] [CrossRef]
- Clarke, D.R.; Phillpot, S.R. Thermal barrier coating materials. Mater. Today 2005, 8, 22–29. [Google Scholar] [CrossRef]
- Padture, N.P.; Gell, M.; Jordan, E.H. Thermal barrier coatings for gas-turbine engine applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef]
- Shi, Z.X.; Wang, X.G.; Liu, S.Z.; Li, J.R. Rotary bending high cycle fatigue properties of DD9 single crystal superalloy at 800 C. Mater. Mech. Eng. 2016, 40, 16–19. [Google Scholar]
- Wang, X.; Li, J.; Yu, J.; Liu, S.Z.; Shi, Z.X.; Yue, X.D. Tensile anisotropy of single crystal superalloy DD9. Acta Metall. Sin. 2015, 51, 1253–1260. [Google Scholar]
- Yang, W.P.; Li, J.R.; Liu, S.Z.; Shi, Z.X.; Zhao, J.Q.; Wang, X.G. Orientation dependence of transverse tensile properties of nickel-based third generation single crystal superalloy DD9 from 760 to 1100 C. Trans. Nonferrous Met. Soc. China 2019, 29, 558–568. [Google Scholar] [CrossRef]
- Li, J.R.; Liu, S.Z.; Wang, X.G.; Shi, Z.X.; Zhao, J.Q. Development of a Low-Cost Third Generation Single Crystal Superalloy DD9. In Superalloys 2016, Proceedings of the 13th Intenational Symposium of Superalloys, Burlington, MA, USA, 11–15 September 2016; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 55–63. [Google Scholar]
- Li, J.R.; Zhong, Z.G.; Tang, D.Z.; Liu, S.Z.; Wei, P.; Wu, Z.T.; Huang, D.; Han, M. A Low-cost second geneution single crystal superalloy DD6. Superalloys 2000, 1, 777–783. [Google Scholar]
- Li, J.R.; Zhao, J.Q.; Liu, S.Z.; Han, M. Effects of low angle boundaries on the mechanical properties of single crystal superalloy DD6. Superalloys 2008, 2008, 443–451. [Google Scholar]
- Zhu, Z.; Basoalto, H.; Warnken, N.; Reed, R.C. A model for the creep deformation behaviour of nickel-based single crystal superalloys. Acta Mater. 2012, 60, 4888–4900. [Google Scholar] [CrossRef]
- Li, P.; Jin, X.; Zhao, J.; Lu, P.; Hu, N.; Liu, D.L.; Dong, J.M.; Fan, X.L. Oxidation behaviors and compressive strength evolution of DD6 Ni-based single-crystal superalloy at 1100 °C. Corros. Sci. 2022, 208, 110684. [Google Scholar] [CrossRef]
- Sumoyama, D.; Thosin, K.Z.; Nishimoto, T.; Yoshioka, T.; Izumi, T.; Hayashi, S.; Narita, T. Formation of a Rhenium-base Diffusion-barrier-coating System on Ni-base Single Crystal Superalloy and its Stability at 1423 K. Oxid. Met. 2007, 68, 313–329. [Google Scholar] [CrossRef]
- Suzuki, A.; Kawagishi, K.; Yokokawa, T.; Harada, H. Effect of Cr on microstructural evolution of aluminized fourth generation Ni-base single crystal superalloys. Surf. Coat. Technol. 2012, 206, 2769–2773. [Google Scholar] [CrossRef]
- Zhou, L.; Mehta, A.; Cho, K.; Sohn, Y. Composition-dependent interdiffusion coefficient, reduced elastic modulus and hardness in γ-, γ′- and β-phases in the Ni-Al system. J. Alloy. Compd. 2017, 727, 153–162. [Google Scholar] [CrossRef]
- Guo, H.; Gong, S.; Khor, K.A.; Xu, H. Effect of thermal exposure on the microstructure and properties of EB-PVD gradient thermal barrier coatings. Surf. Coat. Technol. 2003, 168, 23–29. [Google Scholar] [CrossRef]
- Xie, S.; Lin, S.; Shi, Q.; Wang, W.; Song, C.; Xu, W.; Dai, M. A study on the mechanical and thermal shock properties of MCrAlY coating prepared by arc ion plating. Surf. Coat. Technol. 2021, 413, 127092. [Google Scholar] [CrossRef]
- Kopec, M.; Kukla, D.; Yuan, X.; Rejmer, W.; Kowalewski, Z.L.; Senderowski, C. Aluminide thermal barrier coating for high temperature performance of MAR 247 nickel based superalloy. Coatings 2021, 11, 48. [Google Scholar] [CrossRef]
- Yang, H.Z.; Zou, J.P.; Shi, Q.; Lin, S.S.; Dai, M.J.; Wang, D. Recent advances for interface diffusion behavior in MCrAlY coatings at elevated temperature oxidation. Rare Met. Mat. Eng. 2020, 49, 2240–2249. [Google Scholar]
- Texier, D.; Monceau, D.; Hervier, Z.; Andrieu, E. Effect of interdiffusion on mechanical and thermal expansion properties at high temperature of a MCrAlY coated Ni-based superalloy. Surf. Coat. Technol. 2016, 307, 81–90. [Google Scholar]
- Sun, X.; Zhang, P.; Moverare, J.; Li, H.X.; Cui, L.Q.; Peng, R.L. Impeding the γ′ depletion during the interdiffusion between bond coatings and superalloys via introduction of tantalum in bond coatings. Mater. Des. 2023, 227, 111792. [Google Scholar] [CrossRef]
- Tan, X.P.; Hong, H.U.; Choi, B.G.; Kim, I.S.; Jo, C.Y.; Jin, T.; Hu, Z.Q. Characterization of topologically close-packed phases in secondary reaction zone in a coated CMSX-4 single crystal Ni-based superalloy. J. Mater. Sci. 2013, 48, 1085–1089. [Google Scholar] [CrossRef]
- Bai, Z.; Li, D.; Peng, H.; Wang, J.; Guo, H.; Gong, S. Suppressing the formation of SRZ in a Ni-based single crystal superalloy by RuNiAl diffusion barrier. Prog. Nat. Sci. 2012, 22, 146–152. [Google Scholar]
- Yin, B.; Ni, J.; Deng, P.; Li, Q.; Mao, J.; Zhang, L.; Deng, C. Microstructure evolution and interdiffusion of PtAl coated a third generation single crystal superalloy during thermal exposure. Vacuum 2022, 203, 111225. [Google Scholar] [CrossRef]
- Yang, L.; Chen, M.; Wang, J.; Qiao, Y.X.; Guo, P.Y.; Zhu, S.L.; Wang, F.H. Microstructure and composition evolution of a single-crystal superalloy caused by elements interdiffusion with an overlay NiCrAlY coating on oxidation. J. Mater. Sci. Technol. 2020, 45, 49–58. [Google Scholar]
- Bai, B.; Guo, H.; Peng, H.; Peng, L.Q.; Gong, S.K. Cyclic oxidation and interdiffusion behavior of a NiAlDy/RuNiAl coating on a Ni-based single crystal superalloy. Corros. Sci. 2011, 53, 2721–2727. [Google Scholar] [CrossRef]
- Zhong, J.; Liu, J.; Zhou, X.; Li, S.; Yu, M.; Xu, Z. Thermal cyclic oxidation and interdiffusion of NiCoCrAlYHf coating on a Ni-based single crystal superalloy. J. Alloy. Compd. 2016, 657, 616–625. [Google Scholar] [CrossRef]
- Geng, L.; Zhao, W.; Ru, Y.; Pei, Y.; Li, S.; Gong, S. Tailoring coating composition for the associated microstructural stability of a single-crystal superalloy: An experimental and simulation study. Corros. Sci. 2023, 211, 110916. [Google Scholar]
- Zhan, X.; Wang, D.; Ge, Z.; Zhang, Y.; Dong, J.; Lou, L.; Zhang, J. Microstructural evolution of NiCoCrAlY coated directionally solidified superalloy. Surf. Coat. Technol. 2022, 440, 128487. [Google Scholar] [CrossRef]
- Shi, L.; Xin, L.; Wang, X.; Wang, X.; Wei, H.; Zhu, S.; Wang, F. Influences of MCrAlY coatings on oxidation resistance of single crystal superalloy DD98M and their inter-diffusion behaviors. J. Alloy. Compd. 2015, 649, 515–530. [Google Scholar] [CrossRef]
- Liang, T.; Guo, H.; Peng, H.; Gong, S. Precipitation phases in the nickel-based superalloy DZ 125 with YSZ/CoCrAlY thermal barrier coating. J. Alloy. Compd. 2011, 509, 8542–8548. [Google Scholar] [CrossRef]
- Vorkötter, C.; Mack, D.E.; Guillon, O.; Vaßen, R. Superior cyclic life of thermal barrier coatings with advanced bond coats on single-crystal superalloys. Surf. Coat. Technol. 2019, 361, 150–158. [Google Scholar] [CrossRef]
- Naumenko, D.; Shemet, V.; Singheiser, L.; Quadakkers, W.J. Failure mechanisms of thermal barrier coatings on MCrAlY-type bondcoats associated with the formation of the thermally grown oxide. J. Mater. Sci. 2009, 44, 1687–1703. [Google Scholar]
- Walston, W.S.; Ross, E.W.; Pollock, T.M.; O’Hara, K.S.; Murphy, W.H. Nickel-Base Superalloy and Article with High Temperature Strength and Improved Stability. U.S. Patent 5,455,120, 3 October 1995. [Google Scholar]
- Suzuki, A.S.; Rae, C.M.; Hobbs, R.; Murakami, H. Secondary reaction zone formations in Pt-Aluminised fourth generation Ni-base single crystal superalloys. Adv. Mater. Res. 2011, 278, 78–83. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, X.; Song, P.; Wang, Y.; Dong, J.S.; Lou, L.H. Anisotropy of interface characteristics between NiCoCrAlY coating and a hot corrosion resistant Ni-Based single crystal superalloy during thermal exposure at different temperatures. Appl. Surf. Sci. 2020, 532, 147405. [Google Scholar] [CrossRef]
- Yin, B.; Xie, G.; Lou, L.; Zhang, J. Effect of Ta on microstructural evolution of NiCrAlYSi coated Ni-base single crystal superalloys. J. Alloys Compd. 2020, 829, 154440. [Google Scholar] [CrossRef]
- Wang, R.; Gong, X.; Peng, H.; Ma, Y.; Guo, H. Interdiffusion behavior between NiAlHf coating and Ni-based single crystal superalloy with different crystal orientations. Appl. Surf. Sci. 2015, 326, 124–130. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, F.; Chen, M.; Wang, J.; Zhu, S.; Wang, F. An in-situ formed ceramic/alloy/ceramic sandwich barrier to resist elements interdiffusion between NiCrAlY coating and a Ni-based superalloy. J. Mater. Sci. Technol. 2021, 70, 1–11. [Google Scholar] [CrossRef]
- Shi, Z.X.; Liu, S.Z.; Wang, X.G.; Li, J.R. Hot-gas corrosion resistance of DD9 single crystal superalloy. In Materials Science Forum; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2016; Volume 849, pp. 463–467. [Google Scholar]
- Zhang, Z.; Wen, Z.; Yue, Z. Effects of tensile/compressive creeps on microstructure evolution of nickel-based single crystal superalloys. J. Alloy. Compd. 2021, 851, 156767. [Google Scholar] [CrossRef]
- GB 11373-89; The General Principle of Surface Preparation of Metallic Substrate for Thermal Spraying. Standards Press of China: Beijing, China, 1989.
- Liu, D.; Mu, R.; He, L.; Li, S.; Yang, W. Failure behaviour of EB-PVD YSZ thermal barrier coatings under simulated aero-engine operating conditions. Surf. Coat. Technol. 2023, 474, 130027. [Google Scholar] [CrossRef]
- Li, P.; Fan, X.; Lu, P.; Wang, H.; Jin, X.C. Effect of pre-treatment temperatures on the oxidation behaviors and surface roughening mechanisms of NiCoCrAlYHf coating. Corros. Sci. 2023, 224, 111548. [Google Scholar] [CrossRef]
- Xie, S.; Dai, M.; Lin, S.; Shi, Q.; Song, C.; Hou, H.; Qiu, W.; Wang, Y. Effect of bias voltage on the oxidation resistance of NiCoCrAlYTa coatings prepared by arc ion plating. Corros. Sci. 2019, 147, 330–341. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, K.; Jin, X.; Li, P.; Li, Z.; Li, J.; Hou, C.; Fan, X. Comparative study of temperature-dependent oxidation and interdiffusion behavior on NiCoCrAlYHf-coated nickel-based single-crystal superalloys. J. Mater. Sci. 2024, 59, 19977–19995. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Ding, K.; Deng, C.; Zhang, S.Y.; Zheng, R.G.; Yang, L.W.; Lin, X.P. Effects of TGO growth on the stress distribution and evolution of three-dimensional cylindrical thermal barrier coatings based on finite element simulations. Ceram. Int. 2022, 48, 7864–7875. [Google Scholar] [CrossRef]
- Xu, Q.L.; Liu, K.C.; Wang, K.Y.; Lou, L.Y.; Zhang, Y.; Li, C.J.; Li, C.X. TGO and Al diffusion behavior of CuAlxNiCrFe high-entropy alloys fabricated by high-speed laser cladding for TBC bond coats. Corros. Sci. 2021, 192, 109781. [Google Scholar] [CrossRef]
- Heuer, A.H.; Hovis, D.B.; Smialek, J.L.; Gleeson, B. Alumina scale formation: A new perspective. J. Am. Ceram. Soc. 2011, 94, s146–s153. [Google Scholar]
- Heuer, A.; Nakagawa, T.; Azar, M.; Hovis, D.; Smialek, J.; Gleeson, B.; Hine, N.; Guhl, H.; Lee, H.-S.; Tangney, P.; et al. On the growth of Al2O3 scales. Acta Mater. 2013, 61, 6670–6683. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, W.; Liu, L.; Yi, T.; Jiang, W.X.; Wang, J.; Zhang, Y.F.; Zhang, Z. In-situ SEM study on fatigue crack behavior of a nickel-based single crystal alloy at 950 °C and 1050 °C. Mater. Charact. 2023, 199, 112763. [Google Scholar] [CrossRef]
- Pei, H.; Wang, J.; Li, Z.; Yao, X.; Wen, Z.; Yue, Z. Oxidation behavior of recast layer of air-film hole machined by EDM technology of Ni-based single crystal blade and its effect on creep strength. Surf. Coat. Technol. 2021, 419, 127285. [Google Scholar] [CrossRef]
- Gao, F.; Nishikawa, H.; Takemoto, T. Additive effect of kirkendall void formation in Sn-3.5Ag solder joints on common substrates. J. Electron. Mater. 2008, 37, 45–50. [Google Scholar] [CrossRef]
- Cho, M.G.; Kang, S.K.; Shih, D.Y.; Lee, H.M. Effects of minor additions of Zn on interfacial reactions of Sn-Ag-Cu and Sn-Cu solders with various Cu substrates during thermal aging. J. Electron. Mater. 2007, 36, 1501–1509. [Google Scholar] [CrossRef]
- Tsai, J.Y.; Hu, Y.C.; Tsai, C.M.; Kao, C.R. A study on the reaction between Cu and Sn3.5Ag solder doped with small amounts of Ni. J. Electron. Mater. 2003, 32, 1203–1208. [Google Scholar] [CrossRef]
- Liang, T.; Guo, H.; Peng, H.; Gong, S. Microstructural evolution of CoCrAlY bond coat on Ni-based superalloy DZ 125 at 1050 °C. Surf. Coat. Technol. 2011, 205, 4374–4379. [Google Scholar] [CrossRef]
- Tian, S.; Zhang, Y.; He, A.; Liu, J.H.; Zeng, S.W.; Jiang, H.T. Interdiffusion mechanism at the interface between TiAl alloy and NiCoCrAlY bond coating. Surf. Coat. Technol. 2022, 444, 128687. [Google Scholar] [CrossRef]
- Sun, J.; Liu, J.; Chen, C.; Li, J.; Wang, X.; Sun, X. Effect of Ru on γ/γ′ microstructural evolutions and precipitation of TCP phases during thermal exposure at 1100 °C in a single crystal superalloy. Mater. Charact. 2022, 186, 111794. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, J.; Zhang, Y.; Zhang, W.Y.; Ma, S.Y.; Mao, S.C.; Du, Y.Q.; Wang, Z.H.; Qin, J.Y.; Wang, Q. Effects of the orientation relationships between TCP phases and matrix on the morphologies of TCP phases in Ni-based single crystal superalloys. Mater. Charact. 2022, 183, 111609. [Google Scholar] [CrossRef]

| Ni | Co | Cr | Ta | Al | W | Re | Hf | Y | C | Nb | Mo | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DD9 | Bal | 7 | 3.5 | 7.5 | 5.6 | 6.5 | 4.5 | 0.1 | 0.001 | 0.008 | 0.5 | 2 |
| DD6 | Bal | 9 | 4 | 7 | 5.7 | 8 | 2.2 | 1 | / | / | 1 | 2 |
| BC | Bal | 12.5 | 21 | / | 10 | / | / | 0.4 | 0.3 | / | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Xin, Z.; Sun, F.; Jin, X.; Zhang, C. Microstructure Evolution and Damage Mechanism of DD9 Single Crystal Superalloy-Thermal Barrier Coating System Under High Temperature Oxidation: A Comparative Study with DD6. Materials 2025, 18, 4332. https://doi.org/10.3390/ma18184332
Li P, Xin Z, Sun F, Jin X, Zhang C. Microstructure Evolution and Damage Mechanism of DD9 Single Crystal Superalloy-Thermal Barrier Coating System Under High Temperature Oxidation: A Comparative Study with DD6. Materials. 2025; 18(18):4332. https://doi.org/10.3390/ma18184332
Chicago/Turabian StyleLi, Pan, Zhenyu Xin, Fan Sun, Xiaochao Jin, and Chao Zhang. 2025. "Microstructure Evolution and Damage Mechanism of DD9 Single Crystal Superalloy-Thermal Barrier Coating System Under High Temperature Oxidation: A Comparative Study with DD6" Materials 18, no. 18: 4332. https://doi.org/10.3390/ma18184332
APA StyleLi, P., Xin, Z., Sun, F., Jin, X., & Zhang, C. (2025). Microstructure Evolution and Damage Mechanism of DD9 Single Crystal Superalloy-Thermal Barrier Coating System Under High Temperature Oxidation: A Comparative Study with DD6. Materials, 18(18), 4332. https://doi.org/10.3390/ma18184332








