Spark Plasma Sintering of Pristine and Transition Metal-Doped Ti2AlC MAX Phases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sintering Temperature Optimisation
2.2. Optimisation of Excess Aluminium Content for Ti2AlC Synthesis
2.3. Synthesis of Doped MAX Phases
2.4. Sample Characterization
3. Results
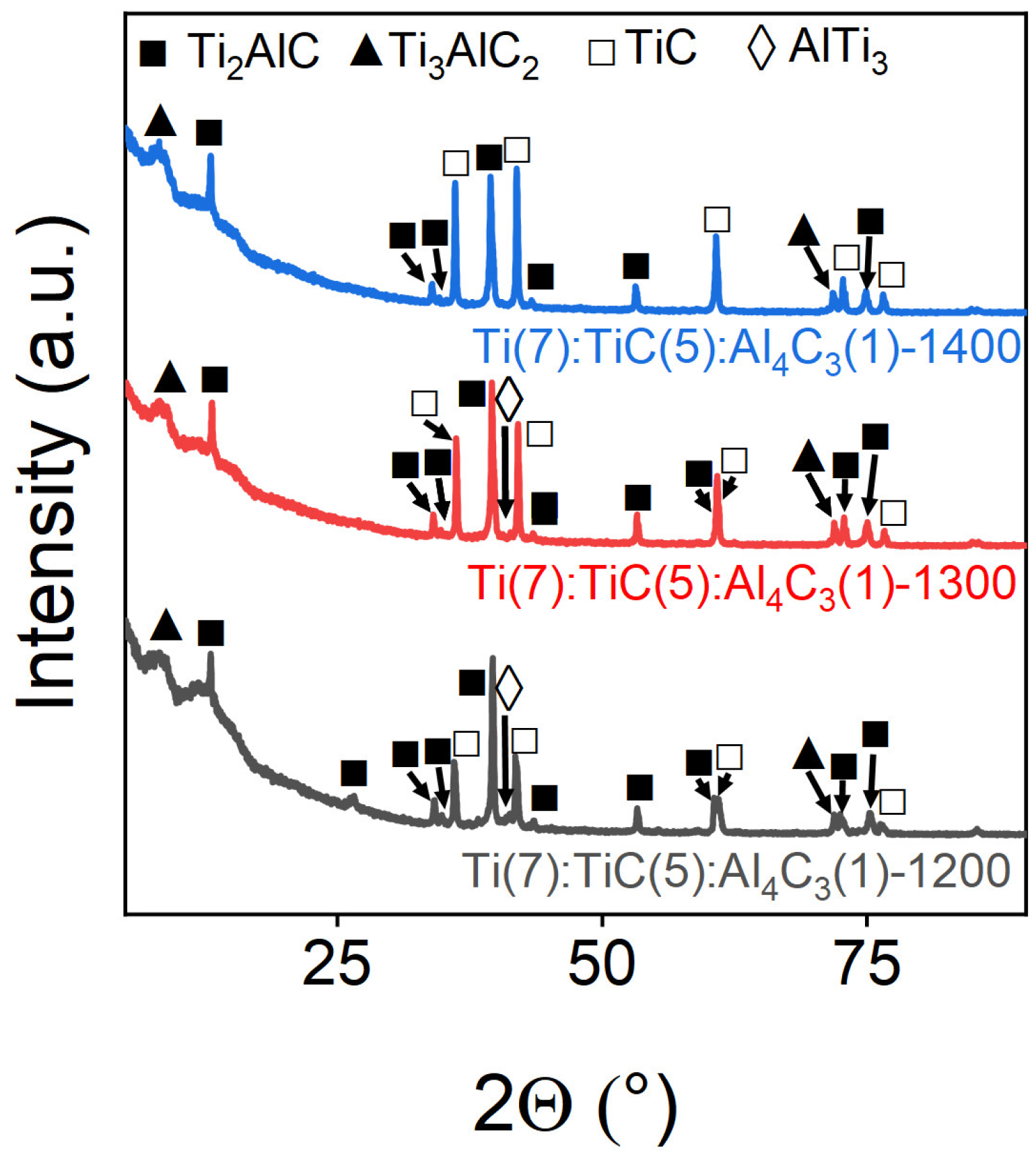
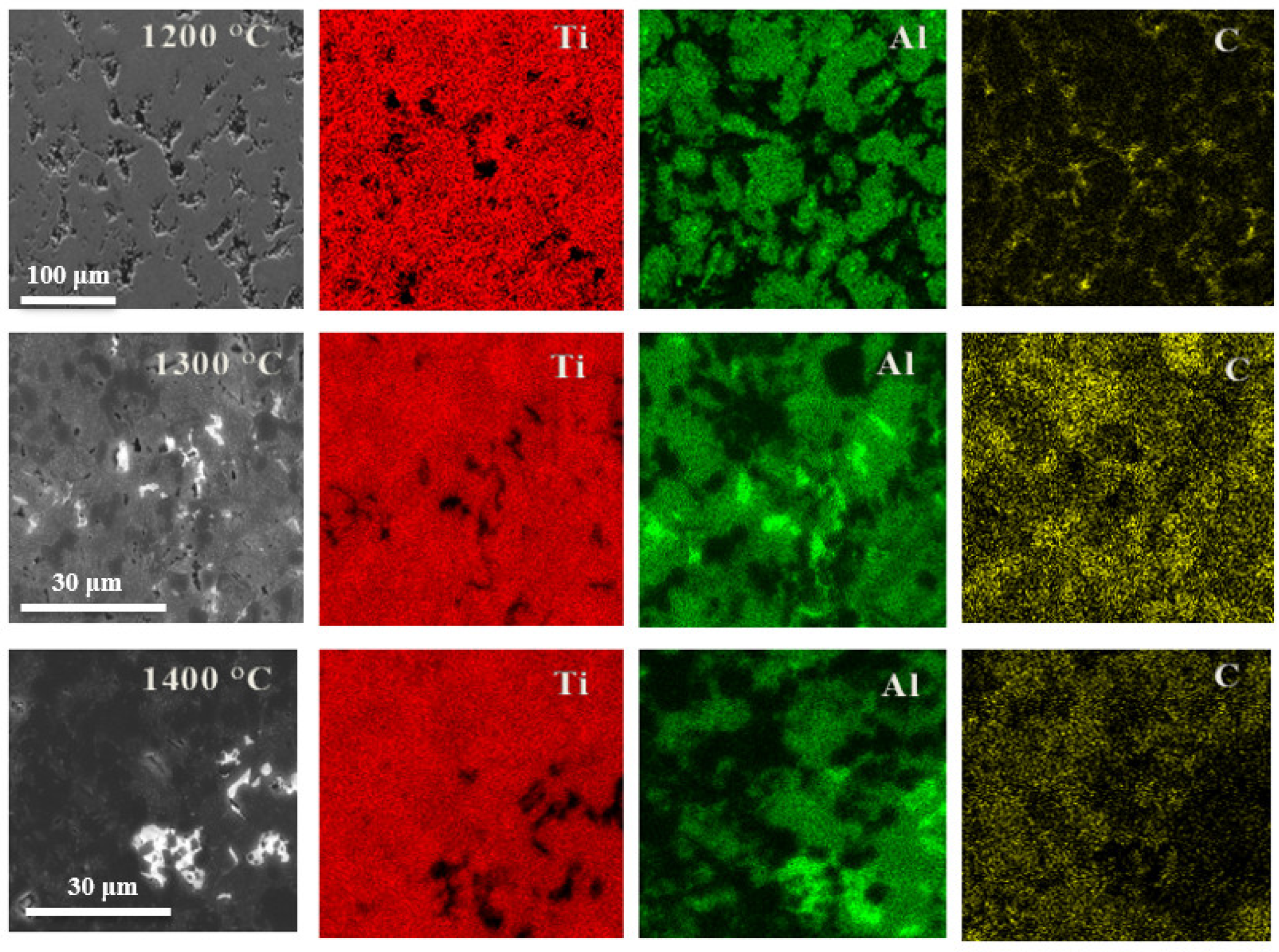
3.1. The Effect of Temperature on the Yield of the Target Phase
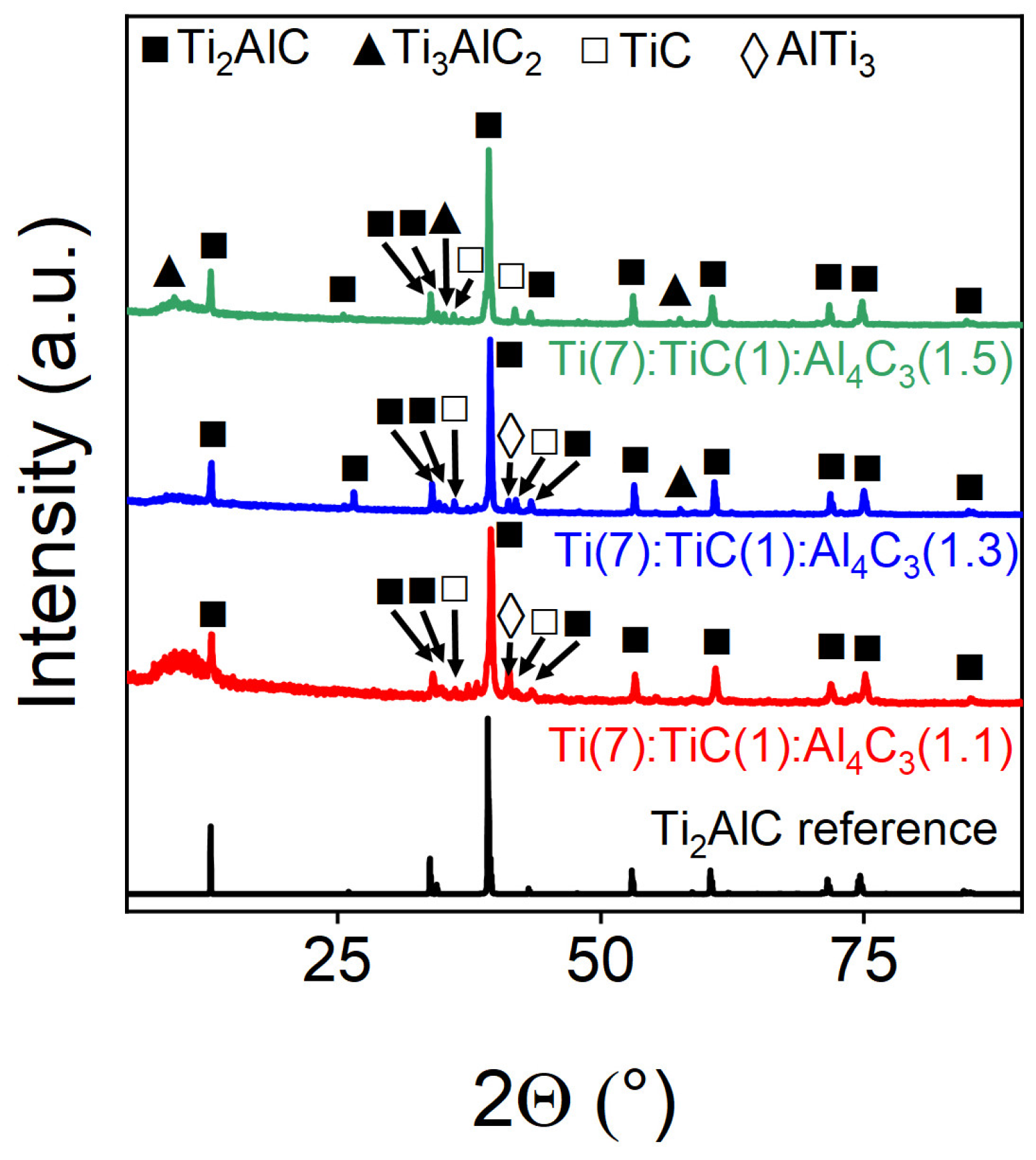
3.2. The Effect of the Precursor Ratio on the Yield of Ti2AlC
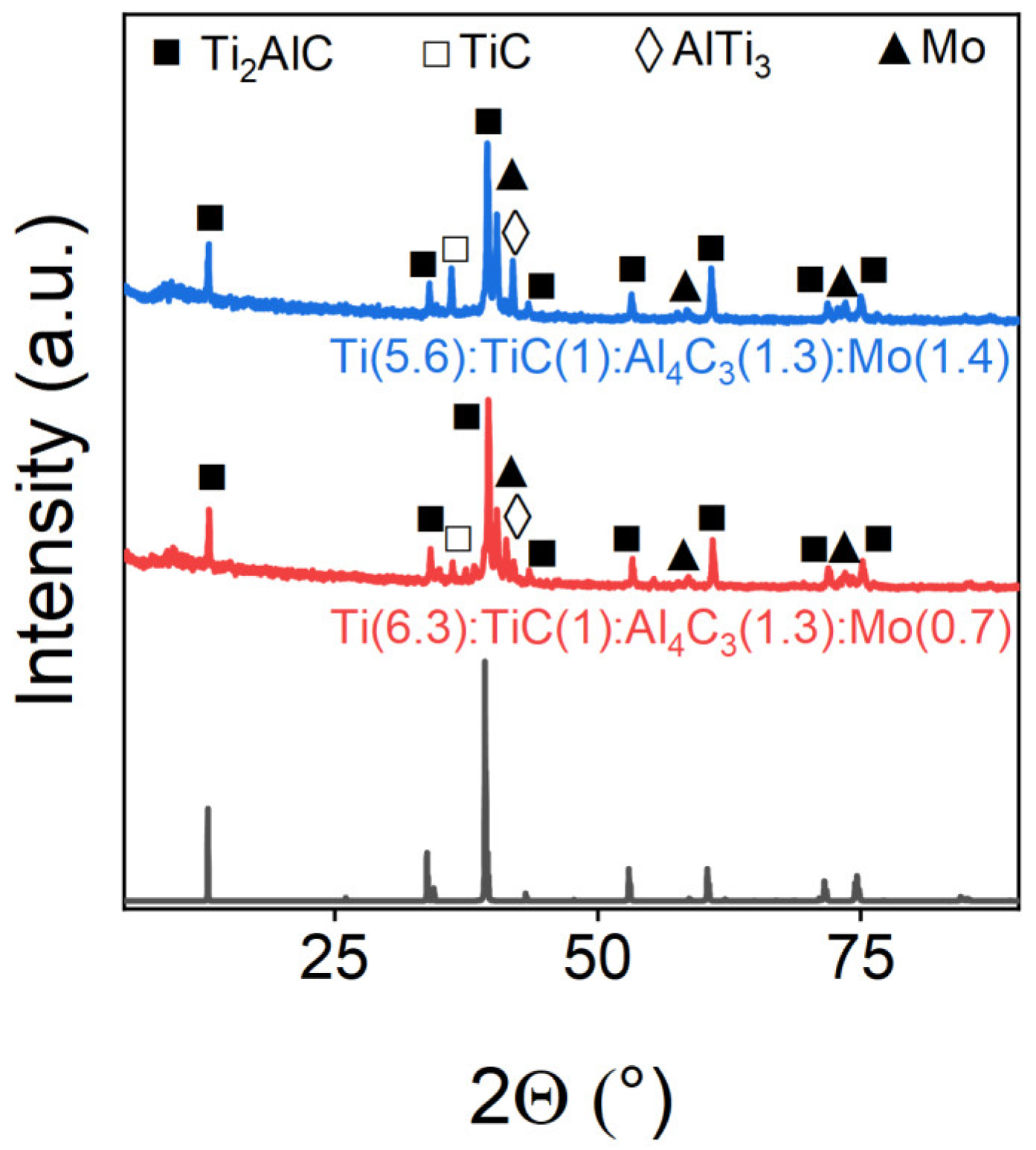
3.3. The Effect of Doping Additives on MAX-Phases
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Z.M. Progress in Research and Development on MAX Phases: A Family of Layered Ternary Compounds. Int. Mater. Rev. 2011, 56, 143–166. [Google Scholar] [CrossRef]
- Eklund, P.; Beckers, M.; Jansson, U.; Högberg, H.; Hultman, L. The M+1AX Phases: Materials Science and Thin-Film Processing. Thin Solid Films 2010, 518, 1851–1878. [Google Scholar] [CrossRef]
- Qureshi, M.W.; Ma, X.; Tang, G.; Miao, B.; Niu, J. Fabrication and Mechanical Properties of Cr2AlC MAX Phase Coatings on TiBw/Ti6Al4V Composite Prepared by HiPIMS. Materials 2021, 14, 826. [Google Scholar] [CrossRef] [PubMed]
- Alruqi, A.B. Engineering the Mechanics and Thermodynamics of Ti3AlC2, Hf3AlC2, Hf3GaC2, (ZrHf)3AlC2, and (ZrHf)4AlN3 MAX Phases via the Ab Initio Method. Crystals 2025, 15, 87. [Google Scholar] [CrossRef]
- Qureshi, M.W.; Ma, X.; Tang, G.; Paudel, R. Structural Stability, Electronic, Mechanical, Phonon, and Thermodynamic Properties of the M2GaC (M = Zr, Hf) MAX Phase: An Ab Initio Calculation. Materials 2020, 13, 5148. [Google Scholar] [CrossRef]
- Chiu, S.-C.; Huang, C.-W.; Li, Y.-Y. Synthesis of High-Purity Silicon Carbide Nanowires by a Catalyst-Free Arc-Discharge Method. J. Phys. Chem. C 2007, 111, 10294–10297. [Google Scholar] [CrossRef]
- Chen, X.; Bei, G. Toughening Mechanisms in Nanolayered MAX Phase Ceramics—A Review. Materials 2017, 10, 366. [Google Scholar] [CrossRef]
- Lyu, J.; Kashkarov, E.B.; Travitzky, N.; Syrtanov, M.S.; Lider, A.M. Sintering of MAX-Phase Materials by Spark Plasma and Other Methods. J. Mater. Sci. 2021, 56, 1980–2015. [Google Scholar] [CrossRef]
- Meng, F.; Liang, B.; Wang, M. Investigation of Formation Mechanism of Ti3SiC2 by Self-Propagating High-Temperature Synthesis. Int. J. Refract. Met. Hard Mater. 2013, 41, 152–161. [Google Scholar] [CrossRef]
- Bartoletti, A.; Mercadelli, E.; Gondolini, A.; Sanson, A. Exploring the Potential of Cold Sintering for Proton-Conducting Ceramics: A Review. Materials 2024, 17, 5116. [Google Scholar] [CrossRef]
- Rybakov, K.I.; Olevsky, E.A.; Krikun, E.V. Microwave Sintering: Fundamentals and Modeling. J. Am. Ceram. Soc. 2013, 96, 1003–1020. [Google Scholar] [CrossRef]
- Kulkarni, S.R.; Wu, A.V.D.K.-H. Synthesis of Ti2AlC by Spark Plasma Sintering of TiAl–Carbon Nanotube Powder Mixture. J. Alloys Compd. 2010, 490, 155–159. [Google Scholar] [CrossRef]
- Syuy, A.; Shtarev, D.; Lembikov, A.; Gurin, M.; Kevorkyants, R.; Tselikov, G.; Arsenin, A.; Volkov, V. Effective Method for the Determination of the Unit Cell Parameters of New MXenes. Materials 2022, 15, 8798. [Google Scholar] [CrossRef]
- Du, C.-F.; Xue, Y.; Zeng, Q.; Wang, J.; Zhao, X.; Wang, Z.; Wang, C.; Yu, H.; Liu, W. Mo-Doped Cr-Ti-Mo Ternary o-MAX with Ultra-Low Wear at Elevated Temperatures. J. Eur. Ceram. Soc. 2022, 42, 7403–7413. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, W.; Li, Y.; Zhang, J.; Zhang, Z.; Zhang, Q.; Liu, H.; Han, G.; Zhang, W. Preparation, Mechanical Properties, and Corrosion Resistance Behavior of (Zr, Mo, Cr)-Doped Ti3AlC2 Ceramics. Ceram. Int. 2024, 50, 20694–20705. [Google Scholar] [CrossRef]
- Zhu, S.; Li, Y.; Liu, D.; Huang, Q.; Kuang, Y. Excellent CoOxHy/C Oxygen Evolution Catalysts Evolved from the Rapid In Situ Electrochemical Reconstruction of Cobalt Transition Metals Doped into the V2 SnC MAX Phase at A Layers. ACS Appl. Energy Mater. 2023, 6, 1116–1125. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Du, S.; Zhao, X.; Zhang, S.; Wang, R.; Lu, X.; Guan, C.; Zhao, Z. Trends in Structural, Electronic and Elastic Properties of Ti2AC (A = Al, Zn, Cu, Ni) Based on Late Transition Metals Substitution. Phys. B Condens. Matter 2023, 662, 414936. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Zhang, C.; Li, J.; Wang, X.; Zhou, Y. Nb Doping in Ti3AlC2: Effects on Phase Stability, High-Temperature Compressive Properties, and Oxidation Resistance. J. Eur. Ceram. Soc. 2017, 37, 3641–3645. [Google Scholar] [CrossRef]
- Siebert, J.P.; Mallett, S.; Juelsholt, M.; Pazniak, H.; Wiedwald, U.; Page, K.; Birkel, C.S. Structure Determination and Magnetic Properties of the Mn-Doped MAX Phase Cr2 GaC. Mater. Chem. Front. 2021, 5, 6082–6091. [Google Scholar] [CrossRef]
- Mandegari, M.; Nasouri, K.; Ghasemi-Mobarakeh, L. Synthesis of Low-Cost Ti3AlC2-Ti2AlC Dual MAX Phase with High-Electrical Conductivity Using Economical Raw Materials and Novel Compositions. Mater. Today Commun. 2023, 36, 106868. [Google Scholar] [CrossRef]
- Gilardi, E.; Fabbri, E.; Bi, L.; Rupp, J.L.M.; Lippert, T.; Pergolesi, D.; Traversa, E. Effect of Dopant–Host Ionic Radii Mismatch on Acceptor-Doped Barium Zirconate Microstructure and Proton Conductivity. J. Phys. Chem. C 2017, 121, 9739–9747. [Google Scholar] [CrossRef]
- Distl, B.; Stein, F. Ti-Al-Based Alloys with Mo: High-Temperature Phase Equilibria and Microstructures in the Ternary System. Philos. Mag. 2024, 104, 28–54. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, Z.; Tang, Y.; Li, S.; Bai, S. Enhancing Phase Stability in Low-Activation TiVTa-Based Alloys Through CALPHAD-Guided Design. JOM 2025, 77, 1524–1535. [Google Scholar] [CrossRef]



| Sample | Atomic Content, at. % | Ti:Al:C Ratio (Normalized to Aluminium) | ||
|---|---|---|---|---|
| Ti | Al | C | ||
| Ti(7):TiC(5):Al4C3(1)-1200 | 46.13 ± 0.09 | 11.71 ± 0.03 | 42.16 ± 0.10 | 3.94:1:3.6 |
| Ti(7):TiC(5):Al4C3(1)-1300 | 48.09 ± 0.09 | 14.72 ± 0.03 | 37.20 ± 0.10 | 3.27:1:2.53 |
| Ti(7):TiC(5):Al4C3(1)-1400 | 47.53 ± 0.09 | 12.70 ± 0.03 | 39.77 ± 0.10 | 3.74:1:3.13 |
| Sample | Ti2AlC, wt.% | TiC, wt.% | AlTi3, wt.% | Ti3AlC2, wt.% |
|---|---|---|---|---|
| Ti(7):TiC(1):Al4C3(1.1) | 86.18 | 2.48 | 11.34 | - |
| Ti(7):TiC(1): Al4C3(1.3) | 90.32 | 4.17 | 5.51 | - |
| Ti(7):TiC(1):Al4C3 (1.5) | 70.22 | 1.62 | - | 28.16 |
| Sample | Ti2AlC, wt.% | TiC, wt.% | AlTi3, wt.% | Mo, wt.% |
|---|---|---|---|---|
| Ti(6.3):TiC(1):Al4C3(1.3):Mo(0.7) | 79.6 | 3.4 | 8.7 | 8.3 |
| Ti(5.6):TiC(1):Al4C3(1.3):Mo(1.4) | 71.11 | 14.43 | - | 14.46 |
| Sample | Ti2AlC, wt.% | TiC, wt.% | Ta, wt.% | Ti3AlC2, wt.% |
|---|---|---|---|---|
| Ti(6.3):TiC(1):Al4C3(1.3):Ta(0.7) | 83.36 | 13.48 | 3.15 | - |
| Ti(5.6):TiC(1):Al4C3(1.3):Ta(1.4) | 89.06 | 8.56 | 2.39 | - |
| Ti(4.9):TiC(1):Al4C3(1.3):Ta(2.1) | 93.89 | 7.03 | 4.05 | - |
| Ti(3.5):TiC(1):Al4C3(1.3):Ta(2.5) | 45.62 | 2.77 | 0.61 | 51.00 |
| Sample | Ti2AlC, wt.% | TiC, wt.% | AlTi3, wt.% |
|---|---|---|---|
| Ti(6.3):TiC(1):Al4C3(1.3):HfC(0.7) | 82.24 | 17.76 | - |
| Ti(5.6):TiC(1):Al4C3(1.3):HfC(1.4) | 90.51 | - | 9.49 |
| Sample | Weight Content of the Phase, % | ||
|---|---|---|---|
| Ti2AlC | TiC | W2C | |
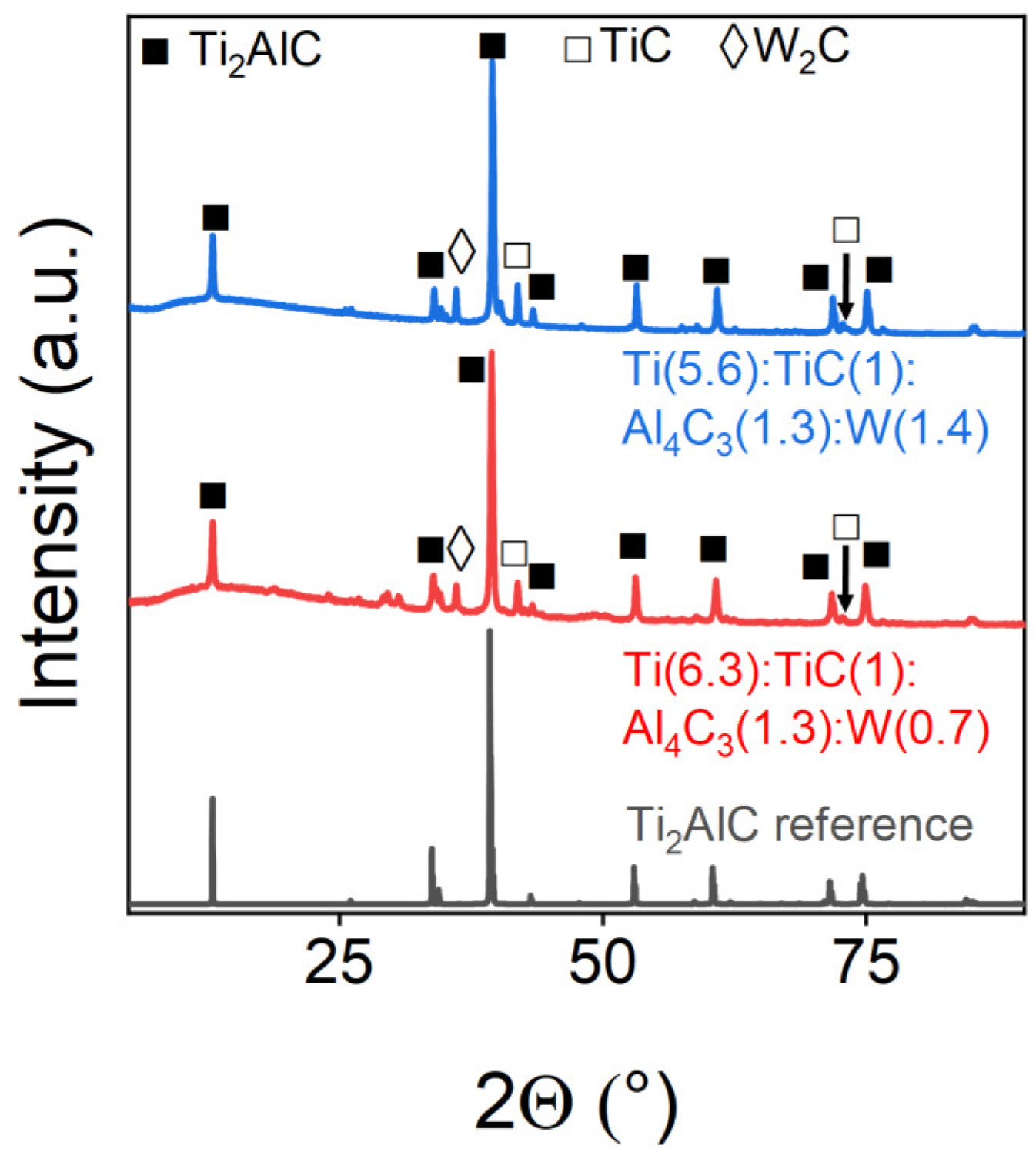
| Ti(6.3):TiC(1):Al4C3(1.3):W(0.7) | 93.81 | 6.0 | 0.18 |
| Ti(5.6):TiC(1):Al4C3(1.3):W(1.4) | 91.61 | 7.9 | 0.49 |
| Sample | Ti2AlC, wt.% | TiC, wt.% | Y, wt.% |
|---|---|---|---|
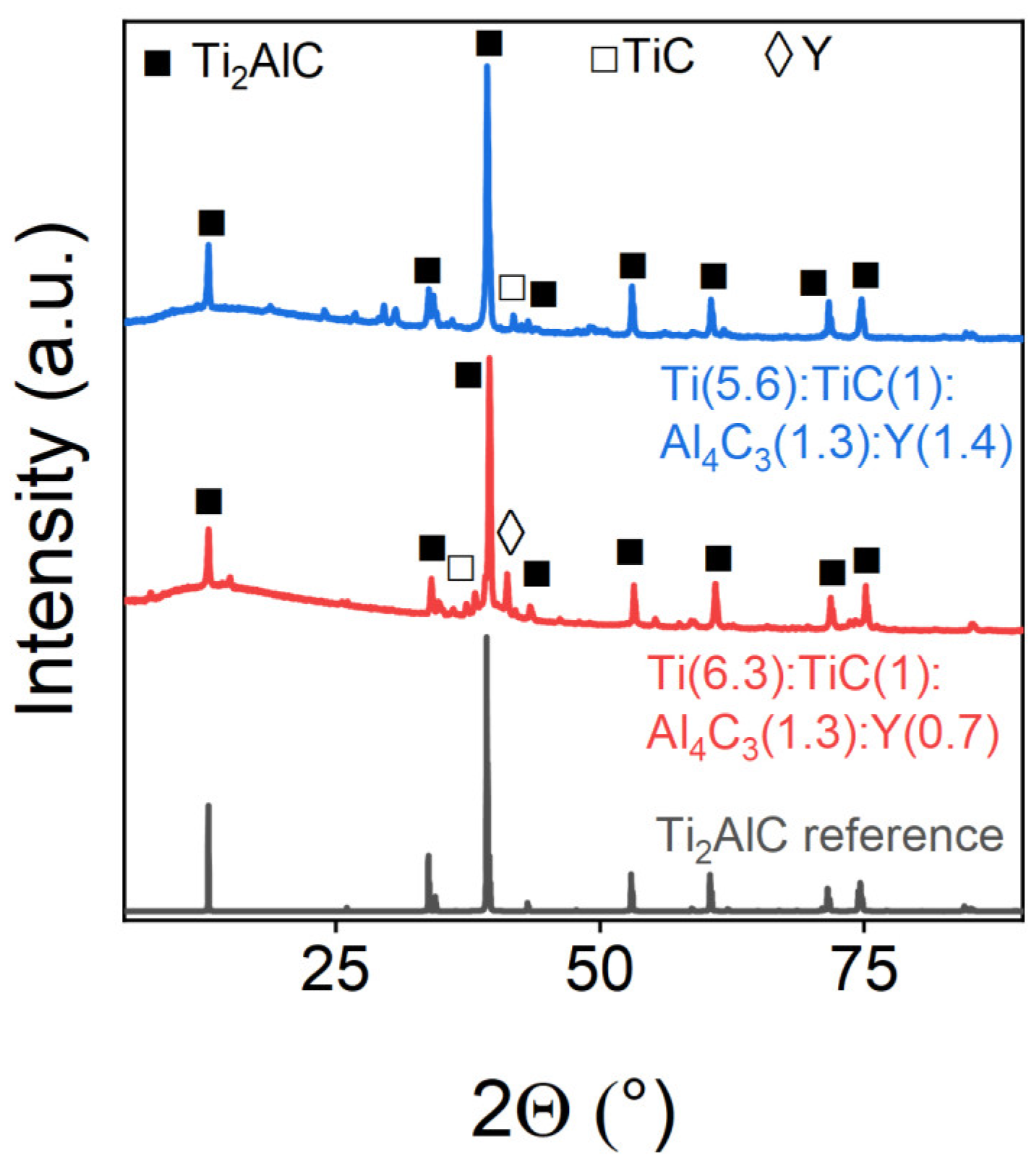
| Ti(6.3):TiC(1):Al4C3(1.3):Y(0.7) | 88.91 | 4.36 | 6.74 |
| Ti(5.6):TiC(1):Al4C3(1.3):Y(1.4) | 97.79 | 2.21 | - |
| Sample | MnO, wt.% | TiC, wt.% |
|---|---|---|
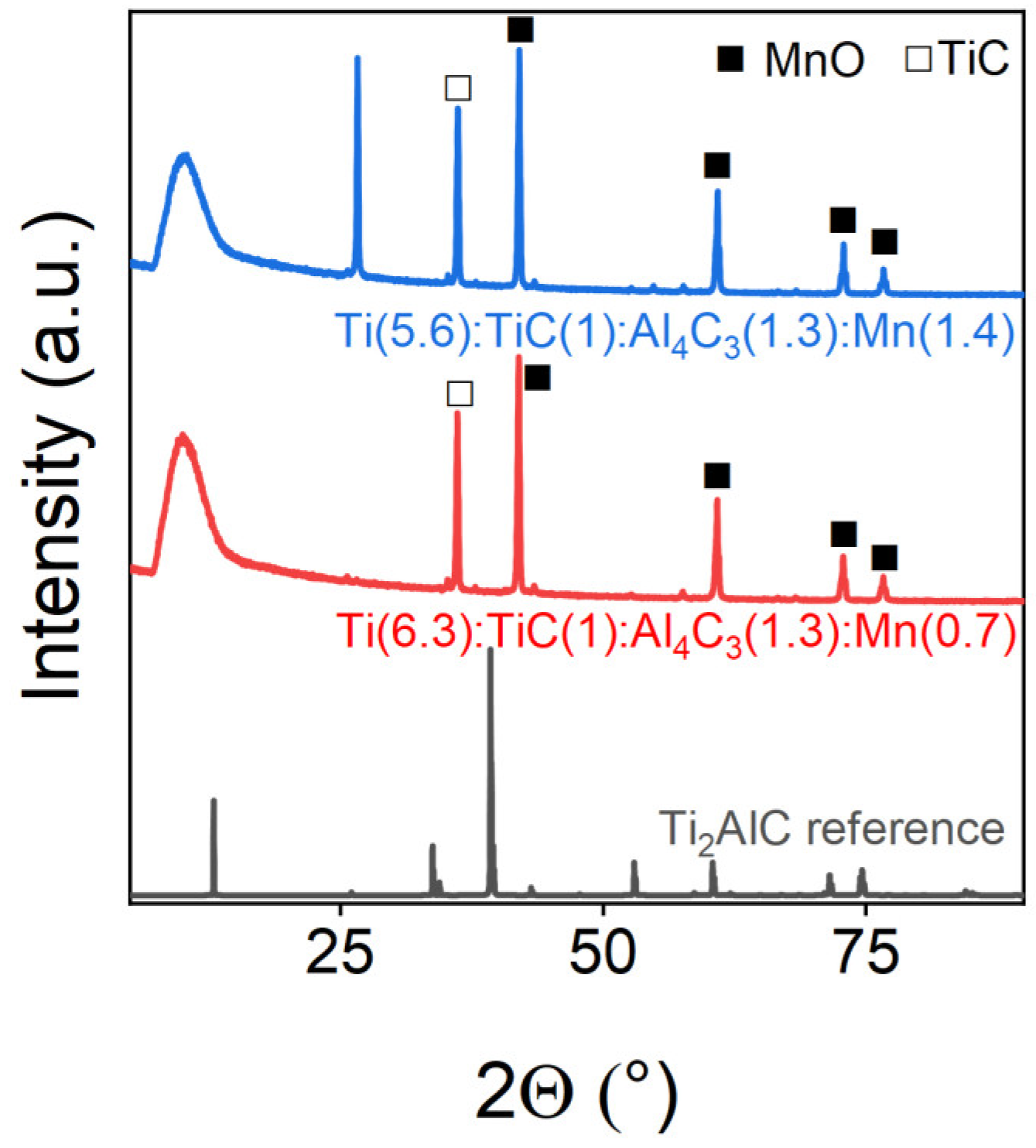
| Ti(6.3):TiC(1):Al4C3(1.3):Mn(0.7) | 75.7 | 24.3 |
| Ti(5.6):TiC(1):Al4C3(1.3):Mn(1.4) | 61.5 | 38.5 |
| Sample | Maximum Yeld of the Target Phase, % |
|---|---|
| Ti(7):TiC(1):Al4C3 (1.5) | 70.22 |
| Ti(7):TiC(1):Al4C3(1.1) | 86.18 |
| Ti(7):TiC(1):Al4C3(1.3) | 90.32 |
| Ti(7):TiC(1):Al4C3(1.3) doped by: | Maximum fraction of doping metal, mol.% |
| Mo | 0 |
| Ta | 30 |
| Hf | 20 |
| W | 20 |
| Y | 20 |
| Mn | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurin, M.S.; Shtarev, D.S.; Zavidovskiy, I.A.; Kolodeznikov, E.S.; Vyshnevyy, A.A.; Arsenin, A.V.; Bolshakov, A.D.; Syuy, A.V. Spark Plasma Sintering of Pristine and Transition Metal-Doped Ti2AlC MAX Phases. Materials 2025, 18, 1957. https://doi.org/10.3390/ma18091957
Gurin MS, Shtarev DS, Zavidovskiy IA, Kolodeznikov ES, Vyshnevyy AA, Arsenin AV, Bolshakov AD, Syuy AV. Spark Plasma Sintering of Pristine and Transition Metal-Doped Ti2AlC MAX Phases. Materials. 2025; 18(9):1957. https://doi.org/10.3390/ma18091957
Chicago/Turabian StyleGurin, Mikhail S., Dmitry S. Shtarev, Ilya A. Zavidovskiy, Erkhan S. Kolodeznikov, Andrey A. Vyshnevyy, Aleksey V. Arsenin, Alexey D. Bolshakov, and Alexander V. Syuy. 2025. "Spark Plasma Sintering of Pristine and Transition Metal-Doped Ti2AlC MAX Phases" Materials 18, no. 9: 1957. https://doi.org/10.3390/ma18091957
APA StyleGurin, M. S., Shtarev, D. S., Zavidovskiy, I. A., Kolodeznikov, E. S., Vyshnevyy, A. A., Arsenin, A. V., Bolshakov, A. D., & Syuy, A. V. (2025). Spark Plasma Sintering of Pristine and Transition Metal-Doped Ti2AlC MAX Phases. Materials, 18(9), 1957. https://doi.org/10.3390/ma18091957







