Global Occurrence of Cyanotoxins in Drinking Water Systems: Recent Advances, Human Health Risks, Mitigation, and Future Directions
Abstract
:1. Introduction
2. Methods
Boolean Search and Retrieval Techniques
3. Cyanotoxins in DWSs
3.1. Diversity of Cyanotoxins
3.2. Global Occurrence of Cyanotoxins in Drinking Water Systems
3.2.1. Africa
3.2.2. Asia
3.2.3. Oceania/Australia
3.2.4. Europe
3.2.5. South/Latin America
3.2.6. North America and Antarctica
3.3. Factors Controlling Cyanotoxins in DWSs
3.3.1. Physical Factors Controlling Cyanotoxins in DWSs
Light and Temperature
3.3.2. Chemical Factors Controlling Cyanotoxins in DWSs
Nutrients
3.3.3. Effects of Microbes on Cyanotoxins in DWSs
3.3.4. Effect of Seasonality and Hydrodynamics on Cyanotoxins in DWSs
3.3.5. Effect of Climate Change on Cyanotoxins in DWSs
3.4. Cyanotoxin Detection Methods
3.4.1. Conventional Methods
3.4.2. Advanced/Emerging Methods
4. Human Health Risks
4.1. Exposure Pathways
4.1.1. Ingestion
4.1.2. Skin Contact (Dermal Route)
4.1.3. Intravenous (Hemodialysis)
4.1.4. Inhalation
4.2. Potential High-Risk Settings and Groups
4.3. Evidence of Human Health Risks
4.4. Behaviour and Fate of Cyanotoxins in the Human Body
4.4.1. Cyanotoxin Toxicokinetics: The ADME Concept
Absorption
Distribution
Metabolism/(Bio)Chemical Transformation
Excretion
4.4.2. Toxicity Effects and Mechanisms
Individual Effects of Cyanotoxins
Interactive Effects of Cyanotoxins and Other Toxicants
4.4.3. A Critique of the Evidence on Human Health Risks
5. Risk Mitigation Strategy
5.1. Hazard Identification
5.2. Risk Assessment
5.3. Preventive and Control Methods
5.4. Removal of Cyanotoxins
6. Future Perspectives
6.1. Harnessing Ethnomedicine and Ethnotoxicology of Cyanotoxins
6.2. Research Needs
- Understanding cyanotoxin occurrence, fate, and transport along the entire DWS chain—from the source to the point of use—including storage and conveyance infrastructure.
- Investigating potential interactive human health effects of cyanotoxins with other pollutants, particularly emerging contaminants.
- Further exploration of degradation pathways and their by-products, as well as the conditions of formation of these by-products and their toxicity compared to the parent compound.
- Developing and evaluating novel, low-cost cyanotoxin removal methods, such as biochar and metallic iron, which have demonstrated efficiency in removing various water contaminants.
- Exploring the potential methods to modify the cyanotoxins to regulate their toxic effects and make high-value products [374].
- Process modeling of cyanotoxin occurrence, dissemination, fate, and behavior in DWSs to identify human exposure hotspots, especially in low-income regions.
- Applying quantitative microbial risk assessment tools and disability-adjusted life years to profile cyanotoxin-related health risks in high-risk populations.
- Developing and validating simple, cost-effective cyanotoxin indicators based on easily measurable water quality parameters, including those informed by indigenous knowledge, for implementation in low-income settings.
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berdalet, E.; Kudela, R.; Urban, E.; Enevoldsen, H.; Banas, N.S. GlobalHAB: A New Program to Promote International Research, Observations, and Modeling of Harmful Algal Blooms in Aquatic Systems. Oceanography 2017, 30, 70–81. [Google Scholar] [CrossRef]
- Antoniou, M.G.; Cruz, A.A.d.l.; Dionysiou, D.D. Cyanotoxins: New Generation of Water Contaminants. J. Environ. Eng. 2005, 131, 1239–1243. [Google Scholar] [CrossRef]
- Loftin, K.A.; Graham, J.L.; Hilborn, E.D.; Lehmann, S.C.; Meyer, M.T.; Dietze, J.E.; Griffith, C.B. Cyanotoxins in inland lakes of the United States: Occurrence and potential recreational health risks in the EPA National Lakes Assessment 2007. Harmful Algae 2016, 56, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Bormans, M.; Amzil, Z.; Mineaud, E.; Brient, L.; Savar, V.; Robert, E.; Lance, E. Demonstrated transfer of cyanobacteria and cyanotoxins along a freshwater-marine continuum in France. Harmful Algae 2019, 87, 101639. [Google Scholar] [CrossRef]
- Zhang, Y.; Husk, B.R.; Duy, S.V.; Dinh, Q.T.; Sanchez, J.S.; Sauvé, S.; Whalen, J.K. Quantitative screening for cyanotoxins in soil and groundwater of agricultural watersheds in Quebec, Canada. Chemosphere 2021, 274, 129781. [Google Scholar] [CrossRef]
- Fastner, J.; Teikari, J.; Hoffmann, A.; Köhler, A.; Hoppe, S.; Dittmann, E.; Welker, M. Cyanotoxins associated with macrophytes in Berlin (Germany) water bodies—Occurrence and risk assessment. Sci. Total Environ. 2023, 858, 159433. [Google Scholar] [CrossRef] [PubMed]
- Huo, D.; Gan, N.; Geng, R.; Cao, Q.; Song, L.; Yu, G.; Li, R. Cyanobacterial blooms in China: Diversity, distribution, and cyanotoxins. Harmful Algae 2021, 109, 102106. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Mu, X.; Hu, X.; Ma, Y. Research Characteristics on Cyanotoxins in Inland Water: Insights from Bibliometrics. Water 2022, 14, 667. [Google Scholar] [CrossRef]
- Falfushynska, H.; Kasianchuk, N.; Siemens, E.; Henao, E.; Rzymski, P. A Review of Common Cyanotoxins and Their Effects on Fish. Toxics 2023, 11, 118. [Google Scholar] [CrossRef]
- Ndebele, M.R.; Magadza, C.H.D. The occurrence of microcystin-LR in Lake Chivero, Zimbabwe. Lakes Reserv. Res. Manag. 2006, 11, 57–62. [Google Scholar] [CrossRef]
- Mhlanga, L.; Day, J.; Cronberg, G.; Chimbari, M.; Siziba, N.; Annadotter, H. Cyanobacteria and cyanotoxins in the source water from Lake Chivero, Harare, Zimbabwe, and the presence of cyanotoxins in drinking water. Afr. J. Aquat. Sci. 2006, 31, 165–173. [Google Scholar] [CrossRef]
- Habtemariam, H.; Kifle, D.; Leta, S.; Beekman, W.; Lürling, M. Cyanotoxins in drinking water supply reservoir (Legedadi, Central Ethiopia): Implications for public health safety. SN Appl. Sci. 2021, 3, 328. [Google Scholar] [CrossRef]
- Willén, E.; Ahlgren, G.; Tilahun, G.; Spoof, L.; Neffling, M.-R.; Meriluoto, J. Cyanotoxin production in seven Ethiopian Rift Valley lakes. Inland Waters 2011, 1, 81–91. [Google Scholar] [CrossRef]
- Mwaura, F.; Koyo, A.O.; Zech, B. Cyanobacterial blooms and the presence of cyanotoxins in small high altitude tropical headwater reservoirs in Kenya. J. Water Health 2004, 2, 49–57. [Google Scholar] [CrossRef]
- Raffoul, M.H.; Enanga, E.M.; Senar, O.E.; Creed, I.F.; Trick, C.G. Assessing the potential health risk of cyanobacteria and cyanotoxins in Lake Naivasha, Kenya. Hydrobiologia 2020, 847, 1041–1056. [Google Scholar] [CrossRef]
- Muyodi, F.J.; Hecky, R.E.; Kitamirike, J.M.; Odong, R. Trends in health risks from water-related diseases and cyanotoxins in Ugandan portion of Lake Victoria basin. Lakes Reserv. Res. Manag. 2009, 14, 247–257. [Google Scholar] [CrossRef]
- Poste, A. Microcystin in Ugandan Lakes: Production Dynamics, Accumulation in Fish, and Risk Evaluation. Ph.D. Thesis, University of Waterloo: Waterloo, ON, Canada, 2010. [Google Scholar]
- Zhao, X.; Liu, Y.; Guo, Y.-M.; Xu, C.; Chen, L.; Codd, G.A.; Chen, J.; Wang, Y.; Wang, P.-Z.; Yang, L.-W.; et al. Meta-analysis reveals cyanotoxins risk across African inland waters. J. Hazard. Mater. 2023, 451, 131160. [Google Scholar] [CrossRef] [PubMed]
- Pantelić, D.; Svirčev, Z.; Simeunović, J.; Vidović, M.; Trajković, I. Cyanotoxins: Characteristics, production and degradation routes in drinking water treatment with reference to the situation in Serbia. Chemosphere 2013, 91, 421–441. [Google Scholar] [CrossRef] [PubMed]
- Fosso-Kankeu, E.; Jagals, P.; Preez, H.D. Exposure of rural households to toxic cyanobacteria in container-stored water. Water SA 2008, 34, 631–636. [Google Scholar] [CrossRef]
- Flores, C.; Caixach, J.; Barca, S.; Vieira-Lanero, R.; Cobo, F. Occurrence of Cyanotoxins in Mineral Water Sources and Hot Springs from NW Iberian Peninsula. Biol. Life Sci. Forum 2022, 14, 26. [Google Scholar] [CrossRef]
- Lovin, L.M.; Brooks, B.W. Global scanning of anatoxins in aquatic systems: Environment and health hazards, and research needs. Mar. Freshw. Res. 2019, 71, 689–700. [Google Scholar] [CrossRef]
- Sundaravadivelu, D.; Sanan, T.T.; Venkatapathy, R.; Mash, H.; Tettenhorst, D.; DAnglada, L.; Frey, S.; Tatters, A.O.; Lazorchak, J. Determination of Cyanotoxins and Prymnesins in Water, Fish Tissue, and Other Matrices: A Review. Toxins 2022, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Kajjumba, G.W.; Ejjada, M.; Masrura, S.U.; Marti, E.J.; Khan, E.; Jones-Lepp, T.L.; Abbas, T.; Kajjumba, G.W.; Ejjada, M.; et al. Recent Advancements in the Removal of Cyanotoxins from Water Using Conventional and Modified Adsorbents—A Contemporary Review. Water 2020, 12, 2756. [Google Scholar] [CrossRef]
- Wang, S.; Jiao, Y.; Rao, Z. Selective removal of common cyanotoxins: A review. Environ. Sci. Pollut. Res. 2021, 28, 28865–28875. [Google Scholar] [CrossRef]
- Agathokleous, E.; Peñuelas, J. Monitoring, Regulation, and Mitigation of Cyanotoxins in the Environment to Protect Human Health and Wildlife. Environ. Sci. Technol. 2022, 56, 14225–14227. [Google Scholar] [CrossRef]
- Gwenzi, W.; Marumure, J.; Makuvara, Z.; Simbanegavi, T.T.; Njomou-Ngounou, E.L.; Nya, E.L.; Kaetzl, K.; Noubactep, C.; Rzymski, P. The pit latrine paradox in low-income settings: A sanitation technology of choice or a pollution hotspot? Sci. Total Environ. 2023, 879, 163179. [Google Scholar] [CrossRef]
- Chia, M.A.; Ameh, I.; George, K.C.; Balogun, E.O.; Akinyemi, S.A.; Lorenzi, A.S. Genetic Diversity of Microcystin Producers (Cyanobacteria) and Microcystin Congeners in Aquatic Resources across Africa: A Review Paper. Toxics 2022, 10, 772. [Google Scholar] [CrossRef]
- Pestana, C.J.; Moura, D.S.; Capelo-Neto, J.; Edwards, C.; Dreisbach, D.; Spengler, B.; Lawton, L.A. Potentially Poisonous Plastic Particles: Microplastics as a Vector for Cyanobacterial Toxins Microcystin-LR and Microcystin-LF. Environ. Sci. Technol. 2021, 55, 15940. [Google Scholar] [CrossRef]
- Zaffiro, A.; Rosenblum, L.; Wendelken, S. Method 546: Determination of Total Microcystins and Nodularins in Drinking Water and Ambient Water by Adda Enzyme-Linked Immunosorbent Assay; United States Environmental Protection Agency: Columbus, OH, USA, 2016. [Google Scholar]
- Ballot, A.; Sandvik, M.; Rundberget, T.; Botha, C.J.; Miles, C.O. Diversity of cyanobacteria and cyanotoxins in Hartbeespoort Dam, South Africa. Mar. Freshw. Res. 2013, 65, 175–189. [Google Scholar] [CrossRef]
- Massey, I.Y.; Wu, P.; Wei, J.; Luo, J.; Ding, P.; Wei, H.; Yang, F. A Mini-Review on Detection Methods of Microcystins. Toxins 2020, 12, 641. [Google Scholar] [CrossRef]
- Wendelken, S. Method 545: Determination of Cylindrospermopsin and Anatoxin- in Drinking Water by Liquid Chromatography Electrospray Ionization Tandem Mass Spectrometry (LC/ESI-MS/MS); United States Envirnonmental Protection Agency: Columbus, OH, USA, 2015. [Google Scholar]
- Kaushik, R.; Balasubramanian, R. Methods and Approaches Used for Detection of Cyanotoxins in Environmental Samples: A Review. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1349–1383. [Google Scholar] [CrossRef]
- Sanseverino, I.; António, D.C.; Loos, R.; Lettieri, T. Cyanotoxins: Methods and Approaches for Their Analysis and Detection; JRC Technology Report; European Commission: Brussels, Belgium, 2017; pp. 1–70. [Google Scholar] [CrossRef]
- Mthembu, Z.; Govender, H.; Hendricks, N.; Omotola, E.O.; Kowtharapu, L.P.; Katari, N.K.; Gumbi, B. Detection of Microcystins in South African surface waters by high performance liquid chromatography in the light of Quality by Design statical tool. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.-a.; Zhao, Q.; Pu, C.; Qiu, Z.; Zhang, R.; Shu, W. A Cross-Sectional Investigation of Chronic Exposure to Microcystin in Relationship to Childhood Liver Damage in the Three Gorges Reservoir Region, China. Environ. Health Perspect. 2011, 119, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W.; Azevedo, S.M.; An, J.S.; Molica, R.J.; Jochimsen, E.M.; Lau, S.; Rinehart, K.L.; Shaw, G.R.; Eaglesham, G.K. Human fatalities from cyanobacteria: Chemical and biological evidence for cyanotoxins. Environ. Health Perspect. 2001, 109, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.C.; Gammie, A.J.; Hollinrake, K.; Codd, G.A. Pneumonia associated with contact with cyanobacteria. Br. Med. J. 1990, 300, 1440–1441. [Google Scholar] [CrossRef] [PubMed]
- Mrdjen, I.; Morse, M.A.; Ruch, R.J.; Knobloch, T.J.; Choudhary, S.; Weghorst, C.M.; Lee, J. Impact of Microcystin-LR on Liver Function Varies by Dose and Sex in Mice. Toxins 2018, 10, 435. [Google Scholar] [CrossRef]
- Labine, M.; Gong, Y.; Minuk, G.Y. Long-Term, Low-Dose Exposure to Microcystin-LR Does not Cause or Increase the Severity of Liver Disease in Rodents. Ann. Hepatol. 2017, 16, 959–965. [Google Scholar] [CrossRef]
- Koreivienė, J.; Anne, O.; Kasperovičienė, J.; Burškytė, V. Cyanotoxin management and human health risk mitigation in recreational waters. Environ. Monit. Assess. 2014, 186, 4443–4459. [Google Scholar] [CrossRef]
- Rzymski, P.; Poniedziałek, B.; Mankiewicz-Boczek, J.; Faassen, E.J.; Jurczak, T.; Gągała-Borowska, I.; Ballot, A.; Lürling, M.; Kokociński, M. Polyphasic toxicological screening of Cylindrospermopsis raciborskii and Aphanizomenon gracile isolated in Poland. Algal Res. 2017, 24, 72–80. [Google Scholar] [CrossRef]
- Fastner, J.; Heinze, R.; Humpage, A.R.; Mischke, U.; Eaglesham, G.K.; Chorus, I. Cylindrospermopsin occurrence in two German lakes and preliminary assessment of toxicity and toxin production of Cylindrospermopsis raciborskii (Cyanobacteria) isolates. Toxicon 2003, 42, 313–321. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, Y.; Xie, L.; Wang, L.; He, Y.; Wan, X.; Xue, Q. Long-term environmental exposure to microcystins increases the risk of nonalcoholic fatty liver disease in humans: A combined fisher-based investigation and murine model study. Environ. Int. 2020, 138, 105648. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xue, Q.; Su, X.; Xie, L.; Yan, Y.; Wang, L.; Steinman, A.D. First Identification of the Toxicity of Microcystins on Pancreatic Islet Function in Humans and the Involved Potential Biomarkers. Environ. Sci. Technol. 2016, 50, 3137–3144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xie, P.; Zhang, X.; Zhou, W.; Zhao, S.; Zhao, Y.; Cai, Y. Toxic effects of microcystin-LR on the HepG2 cell line under hypoxic and normoxic conditions. J. Appl. Toxicol. 2013, 33, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Sedan, D.; Laguens, M.; Copparoni, G.; Aranda, J.O.; Giannuzzi, L.; Marra, C.A.; Andrinolo, D. Hepatic and intestine alterations in mice after prolonged exposure to low oral doses of Microcystin-LR. Toxicon 2015, 104, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Hastie, C.J.; Borthwick, E.B.; Morrison, L.F.; Codd, G.A.; Cohen, P.T.W. Inhibition of several protein phosphatases by a non-covalently interacting microcystin and a novel cyanobacterial peptide, nostocyclin. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2005, 1726, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cui, J.; Yu, H.; Zong, W.; Xu, Y.; Cui, J.; Yu, H.; Zong, W. Insight into the Molecular Mechanism for the Discrepant Inhibition of Microcystins (MCLR, LA, LF, LW, LY) on Protein Phosphatase 2A. Toxins 2022, 14, 390. [Google Scholar] [CrossRef]
- Díez-Quijada, L.; Prieto, A.I.; Puerto, M.; Jos, Á.; Cameán, A.M. In Vitro Mutagenic and Genotoxic Assessment of a Mixture of the Cyanotoxins Microcystin-LR and Cylindrospermopsin. Toxins 2019, 11, 318. [Google Scholar] [CrossRef]
- Yang, F.; Wen, C.; Zheng, S.; Yang, S.; Chen, J.; Feng, X. Involvement of MAPK/ERK1/2 pathway in microcystin-induced microfilament reorganization in HL7702 hepatocytes. J. Toxicol. Environ. Health Part A 2018, 81, 1135–1141. [Google Scholar] [CrossRef]
- Torokne, A.; Palovics, A.; Bankine, M. Allergenic (sensitization, skin and eye irritation) effects of freshwater cyanobacteria—Experimental evidence. Environ. Toxicol. 2001, 16, 512–516. [Google Scholar] [CrossRef]
- Stewart, I.; Seawright, A.A.; Schluter, P.J.; Shaw, G.R. Primary irritant and delayed-contact hypersensitivity reactions to the freshwater cyanobacterium Cylindrospermopsis raciborskii and its associated toxin cylindrospermopsin. BMC Dermatol. 2006, 6, 5. [Google Scholar] [CrossRef]
- Hinojosa, M.G.; Gutiérrez-Praena, D.; López, S.; Prieto, A.I.; Moreno, F.J.; Jos, Á.; Cameán, A.M. Toxic effects of the cylindrospermopsin and chlorpyrifos combination on the differentiated SH-SY5Y human neuroblastoma cell line. Toxicon 2023, 227, 107091. [Google Scholar] [CrossRef] [PubMed]
- Mankiewicz-Boczek, J.; Palus, J.; Gągała, I.; Izydorczyk, K.; Jurczak, T.; Dziubałtowska, E.; Stępnik, M.; Arkusz, J.; Komorowska, M.; Skowron, A.; et al. Effects of microcystins-containing cyanobacteria from a temperate ecosystem on human lymphocytes culture and their potential for adverse human health effects. Harmful Algae 2011, 10, 356–365. [Google Scholar] [CrossRef]
- Pilotto, L.; Hobson, P.; Burch, M.D.; Ranmuthugala, G.; Attewell, R.; Weightman, W. Acute skin irritant effects of cyanobacteria (blue-green algae) in healthy volunteers. Aust. N. Z. J. Public Health 2004, 28, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, J.; Xiao, Y.; Zhang, Y.; Yu, Y.; Zheng, Z.; Liu, Y.; Li, Q. The Impact of Cyanobacteria Blooms on the Aquatic Environment and Human Health. Toxins 2022, 14, 658. [Google Scholar] [CrossRef]
- Vela, L.; Sevilla, E.; Gonzalez, C.; Bes, M.T.; Fillat, M.F.; Peleato, M.L. Exploring the interaction of microcystin-LR with proteins and DNA. Toxicol. Vitr. 2008, 22, 1714–1718. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhu, N.; Deng, S.; Du, C.; Tang, Y.; Tang, P.; Xu, S.; Liu, W.; Shen, M.; Xiao, X.; et al. Combination Effect of Microcystins and Arsenic Exposures on CKD: A Case-Control Study in China. Toxins 2023, 15, 144. [Google Scholar] [CrossRef] [PubMed]
- Rapala, J.; Lahti, K.; Räsänen, L.A.; Esala, A.-L.; Niemelä, S.I.; Sivonen, K. Endotoxins associated with cyanobacteria and their removal during drinking water treatment. Water Res. 2002, 36, 2627–2635. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, Z.A.; Deyab, M.A.; Abou-Dobara, M.I.; El-Raghi, W.M. Occurrence of toxic cyanobacteria and microcystin toxin in domestic water storage reservoirs, Egypt. J. Water Supply Res. Technol.-Aqua 2016, 65, 431–440. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, H.; Du, X.; Shi, Z.; Liu, X.; Wang, R.; Zhang, S.; Tian, Z.; Shi, L.; Guo, H.; et al. Advances in the toxicology research of microcystins based on Omics approaches. Environ. Int. 2021, 154, 106661. [Google Scholar] [CrossRef]
- Zamyadi, A.; Dorner, S.; Sauvé, S.; Ellis, D.; Bolduc, A.; Bastien, C.; Prévost, M. Species-dependence of cyanobacteria removal efficiency by different drinking water treatment processes. Water Res. 2013, 47, 2689–2700. [Google Scholar] [CrossRef]
- Xagoraraki, I. Fate of pharmaceuticals during water chlorination. In Proceedings of the Water Quality Technology Conference, Charlotte, NC, USA, 4–8 November 2007. [Google Scholar]
- Mohamed, Z.A. Breakthrough of Oscillatoria limnetica and microcystin toxins into drinking water treatment plants—Examples from the Nile River, Egypt. Water SA 2016, 42, 161–165. [Google Scholar] [CrossRef]
- Teixeira, M.R.; Rosa, M.J. Comparing dissolved air flotation and conventional sedimentation to remove cyanobacterial cells of Microcystis aeruginosa: Part II. The effect of water background organics. Sep. Purif. Technol. 2007, 53, 126–134. [Google Scholar] [CrossRef]
- Yuan, B.-L.; Qu, J.-H.; Fu, M.-L. Removal of cyanobacterial microcystin-LR by ferrate oxidation–coagulation. Toxicon 2002, 40, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Bu, L.; Deng, L.; Shi, Z.; Zhou, S. Removal of Microcystis aeruginosa by UV/chlorine process: Inactivation mechanism and microcystins degradation. Chem. Eng. J. 2018, 349, 408–415. [Google Scholar] [CrossRef]
- Lambert, T.W.; Holmes, C.F.B.; Hrudey, S.E. Adsorption of microcystin-LR by activated carbon and removal in full scale water treatment. Water Res. 1996, 30, 1411–1422. [Google Scholar] [CrossRef]
- Kumar, P.; Cledon, M.; Brar, S.K. A low-cost graphitized sand filter to deliver MC-LR-free potable water: Water treatment plants and household perspective. Sci. Total Environ. 2020, 747, 141135. [Google Scholar] [CrossRef]
- Mouchet, P.; Bonnelye, V. Solving algae problems: French expertise and worldwide applications. J. Water Supply Res. Technol. 1998, 47, 125–141. [Google Scholar] [CrossRef]
- Ho, L.; Meyn, T.; Keegan, A.; Hoefel, D.; Brookes, J.; Saint, C.P.; Newcombe, G. Bacterial degradation of microcystin toxins within a biologically active sand filter. Water Res. 2006, 40, 768–774. [Google Scholar] [CrossRef]
- Gijsbertsen-Abrahamse, A.J.; Schmidt, W.; Chorus, I.; Heijman, S.G.J. Removal of cyanotoxins by ultrafiltration and nanofiltration. J. Membr. Sci. 2006, 276, 252–259. [Google Scholar] [CrossRef]
- Drogui, P.; Daghrir, R.; Simard, M.-C.; Sauvageau, C.; Blais, J.F. Removal of microcystin-LR from spiked water using either activated carbon or anthracite as filter material. Environ. Technol. 2012, 33, 381–391. [Google Scholar] [CrossRef]
- Mashile, P.P.; Mpupa, A.; Nomngongo, P.N. Adsorptive removal of microcystin-LR from surface and wastewater using tyre-based powdered activated carbon: Kinetics and isotherms. Toxicon 2018, 145, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Kohler, E.; Villiger, J.; Posch, T.; Derlon, N.; Shabarova, T.; Morgenroth, E.; Pernthaler, J.; Blom, J.F. Biodegradation of Microcystins during Gravity-Driven Membrane (GDM) Ultrafiltration. PLoS ONE 2014, 9, e111794. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.W.K.; Panglisch, S.; House, J.; Drikas, M.; Burch, M.D.; Gimbel, R. A study of membrane filtration for the removal of cyanobacterial cells. Aqua 1997, 46, 324–334. [Google Scholar]
- Xiong, Q.; Liu, Z.H.; Zhang, Y.H.; Liang, H.F.; Yang, S.; Li, J.L. Algaecidal effect of a nanomaterial sheet. Huanjing Kexue 2006, 27, 715–719. [Google Scholar] [PubMed]
- Wang, H.; Ho, L.; Lewis, D.M.; Brookes, J.D.; Newcombe, G. Discriminating and assessing adsorption and biodegradation removal mechanisms during granular activated carbon filtration of microcystin toxins. Water Res. 2007, 41, 4262–4270. [Google Scholar] [CrossRef] [PubMed]
- Lopes, W.S.; Buriti, J.S.; Cebalos, B.S.O.; Sousa, J.T.; Leite, V.D.; Vieira, F.F. Removal of Microcystin-LR from Drinking Water Using a System Involving Oxidation and Adsorption. Water Air Soil Pollut. 2017, 228, 1–14. [Google Scholar] [CrossRef]
- Jian, Z.; Bai, Y.; Chang, Y.; Liang, J.; Qu, J. Removal of micropollutants and cyanobacteria from drinking water using KMnO4 pre-oxidation coupled with bioaugmentation. Chemosphere 2019, 215, 1–7. [Google Scholar] [CrossRef]
- Bai, M.; Zheng, Q.; Zheng, W.; Li, H.; Lin, S.; Huang, L.; Zhang, Z. •OH Inactivation of Cyanobacterial Blooms and Degradation of Toxins in Drinking Water Treatment System. Water Res. 2019, 154, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Bai, M.; Yang, X.; Zhong, Z.; Gao, M.; Tian, Y. OH pre-treatment of algae blooms and degradation of microcystin-LR in a drinking water system of 480 m3/day: Comparison with ClO2. Chem. Eng. J. 2019, 367, 189–197. [Google Scholar] [CrossRef]
- Hoeger, S.J.; Shaw, G.; Hitzfeld, B.C.; Dietrich, D.R. Occurrence and elimination of cyanobacterial toxins in two Australian drinking water treatment plants. Toxicon 2004, 43, 639–649. [Google Scholar] [CrossRef]
- Momani, F.A.A.; Jarrah, N. Treatment and kinetic study of cyanobacterial toxin by ozone. J. Environ. Sci. Health Part A 2010, 45, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Lawton, L.A.; Robertson, P.K.J.; Cornish, B.J.P.A.; Marr, I.L.; Jaspars, M. Processes influencing surface interaction and photocatalytic destruction of microcystins on titanium dioxide photocatalysts. J. Catal. 2003, 213, 109–113. [Google Scholar] [CrossRef]
- Yuan, B.; Li, Y.; Huang, X.; Liu, H.; Qu, J. Fe(VI)-assisted photocatalytic degradating of microcystin-LR using titanium dioxide. J. Photochem. Photobiol. A Chem. 2006, 178, 106–111. [Google Scholar] [CrossRef]
- Mohamed, Z.A.; Alamri, S.A. Biodegradation of cylindrospermopsin toxin by microcystin-degrading bacteria isolated from cyanobacterial blooms. Toxicon 2012, 60, 1390–1395. [Google Scholar] [CrossRef] [PubMed]
- Dziga, D.; Kokocinski, M.; Maksylewicz, A.; Czaja-Prokop, U.; Barylski, J. Cylindrospermopsin Biodegradation Abilities of Aeromonas sp. Isolated from Rusałka Lake. Toxins 2016, 8, 55. [Google Scholar] [CrossRef]
- Somdee, T.; Thunders, M.; Ruck, J.; Lys, I.; Allison, M.; Page, R. Degradation of [Dha7]MC-LR by a Microcystin Degrading Bacterium Isolated from Lake Rotoiti, New Zealand. Int. Sch. Res. Not. 2013, 2013, 596429. [Google Scholar] [CrossRef]
- Nhut, H.T.; Hung, N.T.Q.; Lap, B.Q.; Han, L.T.N.; Tri, T.Q.; Bang, N.H.K.; Hiep, N.T.; Ky, N.M. Use of Moringa oleifera seeds powder as bio-coagulants for the surface water treatment. Int. J. Environ. Sci. Technol. 2020, 18, 2173–2180. [Google Scholar] [CrossRef]
- Wan, J.; Chakraborty, T.; Xu, C.C.; Ray, M.B. Treatment train for tailings pond water using Opuntia ficus-indica as coagulant. Sep. Purif. Technol. 2019, 211, 448–455. [Google Scholar] [CrossRef]
- Bouaidi, W.E.; Essalhi, S.; Douma, M.; Tazart, Z.; Ounas, A.; Enaime, G.; Yaacoubi, A.; Loudiki, M. Evaluation of the potentiality of Vicia faba and Opuntia ficus indica as eco-friendly coagulants to mitigate Microcystis aeruginosa blooms. Desalination Water Treat. 2020, 196, 198–213. [Google Scholar] [CrossRef]
- Teixeira, M.R.; Camacho, F.P.; Sousa, V.S.; Bergamasco, R. Green technologies for cyanobacteria and natural organic matter water treatment using natural based products. J. Clean. Prod. 2017, 162, 484–490. [Google Scholar] [CrossRef]
- Camacho, F.; Bongiovani, M.; Amorim, M.P.; Silva, M.; Coldebella, P.; Bergamasco, R. Coagulation/Flocculation/Flotation/Nanofiltration Processes Using Moringa Oleifera as Coagulant of Eutrophized River. Chem. Eng. Trans. 2015, 43, 1123–1128. [Google Scholar] [CrossRef]
- Corbel, S.; Mougin, C.; Bouaïcha, N. Cyanobacterial toxins: Modes of actions, fate in aquatic and soil ecosystems, phytotoxicity and bioaccumulation in agricultural crops. Chemosphere 2014, 96, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Codd, G.A.; Testai, E.; Funari, E.; Svirčev, Z. Cyanobacteria, Cyanotoxins, and Human Health. In Water Treatment for Purification from Cyanobacteria and Cyanotoxins, Hiskia, A.E., Triantis, T.M., Antoniou, M.G., Kaloudis, T., Dionysiou, D.D., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 37–68. [Google Scholar]
- Daly, R.I.; Ho, L.; Brookes, J.D. Effect of Chlorination on Microcystis aeruginosa Cell Integrity and Subsequent Microcystin Release and Degradation. Key Eng. Mater. 2007, 277–279, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Antunes, J.T.; Leão, P.N.; Vasconcelos, V.M. Cylindrospermopsis raciborskii: Review of the distribution, phylogeography, and ecophysiology of a global invasive species. Front. Microbiol. 2015, 6, 473. [Google Scholar] [CrossRef]
- Jiang, Y.; Ji, B.; Wong, R.N.S.; Wong, M.H. Statistical study on the effects of environmental factors on the growth and microcystins production of bloom-forming cyanobacterium—Microcystis aeruginosa. Harmful Algae 2008, 7, 127–136. [Google Scholar] [CrossRef]
- Fotiou, T.; Triantis, T.M.; Kaloudis, T.; O’Shea, K.E.; Dionysiou, D.D.; Hiskia, A. Assessment of the roles of reactive oxygen species in the UV and visible light photocatalytic degradation of cyanotoxins and water taste and odor compounds using C–TiO2. Water Res. 2016, 90, 52–61. [Google Scholar] [CrossRef]
- Yusof, T.N.; Rafatullah, M.; Mohamad, R.; Ismail, N.; Zainuddin, Z.; Lalung, J. Cyanobacteria Characteristics and Methods for Isolation and Accurate Identification of Cyanotoxins: A Review Article. Avicenna J. Environ. Health Eng. 2017, 4, 10051. [Google Scholar] [CrossRef]
- Spoof, L.; Catherine, A. Appendix 3 Tables of MCs and Nodularins. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis, 1st ed.; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017. [Google Scholar]
- Chorus, I.; Welker, M. Toxic Cyanobacteria in Water. A Guide to Their Public Health Consequences, Monitoring and Management, 2nd ed.; CRC Press: London, UK, 2021. [Google Scholar]
- Bouaïcha, N.; Miles, C.O.; Beach, D.G.; Labidi, Z.; Djabri, A.; Benayache, N.Y.; Nguyen-Quang, T. Structural Diversity, Characterization and Toxicology of Microcystins. Toxins 2019, 11, 714. [Google Scholar] [CrossRef]
- Wonnacott, S.; Jackman, S.; Swanson, K.L.; Rapoport, H.; Albuquerque, E.X. Nicotinic pharmacology of anatoxin analogs. II. Side chain structure-activity relationships at neuronal nicotinic ligand binding sites. J. Pharmacol. Exp. Ther. 1991, 259, 387–391. [Google Scholar] [CrossRef]
- Méjean, A.; Paci, G.; Gautier, V.; Ploux, O. Biosynthesis of anatoxin-a and analogues (anatoxins) in cyanobacteria. Toxicon 2014, 91, 15–22. [Google Scholar] [CrossRef]
- Bruno, M.; Ploux, O.; Metcalf, J.S.; Mejean, A.P.; Awlik-Skowronska, B.; Furey, A. Anatoxin-a, Homoanatoxin-a, and Natural Analogues. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Wiley Online Library: Hoboken, NJ, USA, 2016; pp. 138–147. [Google Scholar] [CrossRef]
- Robillot, C.; Llewellyn, L.E. Water analysis, algal and microbial toxins. In Encyclopedia of Analytical Science, 2nd ed.; Worsfold, P., Townshend, A.P., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2012; pp. 343–349. [Google Scholar]
- Poniedziałek, B.; Rzymski, P.; Kokociński, M. Cylindrospermopsin: Water-linked potential threat to human health in Europe. Environ. Toxicol. Pharmacol. 2012, 34, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.R.; Pinto, E.; Torres, M.A.; Dörr, F.; Mazur-Marzec, H.; Szubert, K.; Tartaglione, L.; Dell’Aversano, C.; Miles, C.O.; Beach, D.G.; et al. CyanoMetDB, a comprehensive public database of secondary metabolites from cyanobacteria. Water Res. 2021, 196, 117017. [Google Scholar] [CrossRef]
- Jones, R.J.; Pinto, E.; Torres, F.D.; Mazur-Marzec, H.; Szybert, K.; Tartaglione, L.; Cel’Aversano, C.; Miles, C.O.; Beach, D.G.; McCarron, P.; et al. Comprehensive database of secondary metabolites from cyanobacteria. bioRiv 2020. [Google Scholar] [CrossRef]
- Hrouzek, P.; Kapuścik, A.; Vacek, J.; Voráčová, K.; Paichlová, J.; Kosina, P.; Voloshko, L.; Ventura, S.; Kopecký, J. Cytotoxicity evaluation of large cyanobacterial strain set using selected human and murine in vitro cell models. Ecotoxicol. Environ. Saf. 2016, 124, 177–185. [Google Scholar] [CrossRef]
- Falfushynska, H.; Horyn, O.; Osypenko, I.; Rzymski, P.; Wejnerowski, Ł.; Dziuba, M.K.; Sokolova, I.M. Multibiomarker-based assessment of toxicity of central European strains of filamentous cyanobacteria Aphanizomenon gracile and Raphidiopsis raciborskii to zebrafish Danio rerio. Water Res. 2021, 194, 116923. [Google Scholar] [CrossRef] [PubMed]
- Hitzfeld, B.C.; Lampert, C.S.; Spaeth, N.; Mountfort, D.; Kaspar, H.; Dietrich, D.R. Toxin production in cyanobacterial mats from ponds on the McMurdo Ice Shelf, Antarctica. Toxicon 2000, 38, 1731–1748. [Google Scholar] [CrossRef] [PubMed]
- Kleinteich, J.; Hildebrand, F.; Wood, S.A.; Ciŕs, S.; Agha, R.; Quesada, A.; Pearce, D.A.; Convey, P.; Kpper, F.C.; Dietrich, D.R. Diversity of toxin and non-toxin containing cyanobacterial mats of meltwater ponds on the Antarctic Peninsula: A pyrosequencing approach. Antarct. Sci. 2014, 26, 521–532. [Google Scholar] [CrossRef]
- Jungblut, A.D.; Wilbraham, J.; Banack, S.A.; Metcalf, J.S.; Codd, G.A. Microcystins, BMAA and BMAA isomers in 100-year-old Antarctic cyanobacterial mats collected during Captain R.F. Scott’s Discovery Expedition. Eur. J. Phycol. 2018, 53, 115–121. [Google Scholar] [CrossRef]
- Usman, A.S.; Merican, F.; Zaki, S.; Broady, P.; Convey, P.; Muangmai, N. Microcystin production by oscillatorialean cyanobacteria isolated from cryopreserved Antarctic mats. Harmful Algae 2022, 120, 102336. [Google Scholar] [CrossRef]
- Addico, G.N.D.; Hardege, J.D.; Kohoutek, J.; deGraft-Johnson, K.A.A.; Babica, P. Cyanobacteria and microcystin contamination in untreated and treated drinking water in Ghana. Adv. Oceanogr. Limnol. 2017, 8, 92–106. [Google Scholar] [CrossRef]
- Ballot, A.; Krienitz, L.; Kotut, K.; Wiegand, C.; Pflugmacher, S. Cyanobacteria and cyanobacterial toxins in the alkaline crater lakes Sonachi and Simbi, Kenya. Harmful Algae 2005, 4, 139–150. [Google Scholar] [CrossRef]
- Magonono, M.; Oberholster, P.J.; Shonhai, A.; Makumire, S.; Gumbo, J.R. The Presence of Toxic and Non-Toxic Cyanobacteria in the Sediments of the Limpopo River Basin: Implications for Human Health. Toxins 2018, 10, 269. [Google Scholar] [CrossRef]
- Mchau, G.J.; Machunda, R.; Kimanya, M.; Makule, E.; Gong, Y.Y.; Mpolya, E.; Meneely, J.P.; Elliott, C.T.; Greer, B. First Report of the Co-occurrence of Cylindrospermopsin, Nodularin and Microcystins in the Freshwaters of Lake Victoria, Tanzania. Expo. Health 2020, 13, 185–194. [Google Scholar] [CrossRef]
- Otoigo, L. Identification and quantification of Cyanotoxins in Lake and household water around Lake Victoria, Kenya. AfricArXiv Preprints 2020. [Google Scholar] [CrossRef]
- Olokotum, M.; Humbert, J.-F.; Quiblier, C.; Okello, W.; Semyalo, R.; Troussellier, M.; Marie, B.; Baumann, K.; Kurmayer, R.; Bernard, C. Characterization of Potential Threats from Cyanobacterial Toxins in Lake Victoria Embayments and during Water Treatment. Toxins 2022, 14, 664. [Google Scholar] [CrossRef]
- Mohamed, Z.A.; Alamri, S.; Hashem, M. The link between microcystin levels in groundwater and surface Nile water, and assessing their potential risk to human health. J. Contam. Hydrol. 2022, 244, 103921. [Google Scholar] [CrossRef] [PubMed]
- Addico, G.; Hardege, J.; Komarek, J.; Babica, P.; Graft-Johnson, K.D. Cyanobacteria species identified in the Weija and Kpong reservoirs, Ghana, and their implications for drinking water quality with respect to microcystin. Afr. J. Mar. Sci. 2006, 28, 451–456. [Google Scholar] [CrossRef]
- Zewde, T.; Kifle, D.; Johansen, J.; Demissie, T.; Hansen, J.; Tadesse, Z. Cyanobacterial abundance and microcystins in water, seston and fish tissues in Lake Hora-Arsedi (Ethiopia). Afr. J. Aquat. Sci. 2020, 45, 475–485. [Google Scholar] [CrossRef]
- Mohamed, Z.A.; Deyab, M.A.; Abou-Dobara, M.I.; El-Sayed, A.K.; El-Raghi, W.M. Occurrence of cyanobacteria and microcystin toxins in raw and treated waters of the Nile River, Egypt: Implication for water treatment and human health. Environ. Sci. Pollut. Res. 2015, 22, 11716–11727. [Google Scholar] [CrossRef] [PubMed]
- Mokoena, M.M.; Mukhola, M.S.; Mokoena, M.M.; Mukhola, M.S. Current Effects of Cyanobacteria Toxin in Water Sources and Containers in the Hartbeespoort Dam Area, South Africa. Int. J. Environ. Res. Public Health 2019, 16, 4468. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Kong, F.; Zhang, M. Groundwater contamination by microcystin from toxic cyanobacteria blooms in Lake Chaohu, China. Environ. Monit. Assess. 2016, 188, 280. [Google Scholar] [CrossRef]
- Tamele, I.J.; Vasconcelos, V. Microcystin Incidence in the Drinking Water of Mozambique: Challenges for Public Health Protection. Toxins 2020, 12, 368. [Google Scholar] [CrossRef]
- Kulabhusan, P.K.; Campbell, K. Recent trends in the detection of freshwater cyanotoxins with a critical note on their occurrence in Asia. Trends Environ. Anal. Chem. 2021, 32, e00150. [Google Scholar] [CrossRef]
- Bashir, F.; Bashir, A.; Rajput, V.D.; Bouaïcha, N.; Fazili, K.M.; Adhikari, S.; Negi, Y.; Minkina, T.; Almalki, W.H.; Ganai, B.A. Microcystis sp. AE03 strain in Dal Lake harbors cylindrospermopsin and microcystin synthetase gene cluster. Front. Sustain. Food Syst. 2022, 6, 1036111. [Google Scholar] [CrossRef]
- Anahas, A.M.P.; Gayathri, M.; Muralitharan, G. Isolation and Characterization of Microcystin-Producing Microcystis ae. In Microbiological Research in Agroecosystem Management; Velu, R., Ed.; Springer: Pune, India, 2013; pp. 235–248. [Google Scholar]
- Batool, U.; Tromas, N.; Simon, D.F.; Sauvé, S.; Shapiro, B.J.; Ahmed, M. Snapshot of cyanobacterial toxins in Pakistani freshwater bodies. Environ. Sci. Pollut. Res. 2024, 31, 24648–24661. [Google Scholar] [CrossRef]
- Ishikawa, K.; Kumagai, M.; Vincent, W.F.; Tsujimura, S.; Nakahara, H. Transport and accumulation of bloom-forming cyanobacteria in a large, mid-latitude lake: The gyre-Microcystis hypothesis. Limnology 2002, 3, 87–96. [Google Scholar] [CrossRef]
- Watanabe, M.F.; Tsujimura, S.; Oishi, S.; Niki, T.; Namikoshi, M. Isolation and identification of homoanatoxin-a from a toxic strain of the cyanobacterium Raphidiopsis mediterranea Skuja isolated from Lake Biwa, Japan. Phycologia 2003, 42, 364–369. [Google Scholar] [CrossRef]
- Gurbuz, F.; Metcalf, J.S.; Karahan, A.G.; Codd, G.A. Analysis of dissolved microcystins in surface water samples from Kovada Lake, Turkey. Sci. Total Environ. 2009, 407, 4038–4046. [Google Scholar] [CrossRef] [PubMed]
- Swe, T.; Miles, C.O.; Cerasino, L.; Mjelde, M.; Kleiven, S.; Ballot, A. Microcystis, Raphidiopsis raciborskii and Dolichospermum smithii, toxin producing and non-toxigenic cyanobacteria in Yezin Dam, Myanmar. Limnologica 2021, 90, 125901. [Google Scholar] [CrossRef]
- Welker, M.; Chorus, I.; Fastner, J.; Khan, S.; Haque, M.M.; Islam, S.; Khan, N.H. Microcystins (cyanobacterial toxins) in surface waters of rural Bangladesh: Pilot study. J. Water Health 2005, 3, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Joung, S.-H.; Oh, H.-M.; Ko, S.-R.; Ahn, C.-Y. Correlations between environmental factors and toxic and non-toxic Microcystis dynamics during bloom in Daechung Reservoir, Korea. Harmful Algae 2011, 10, 188–193. [Google Scholar] [CrossRef]
- Somdee, T.; Kaewkhiaw, K.; Somdee, A. Detection of toxic cyanobacteria and quantifi cation of microcystins in four recreational water reservoirs in Khon Kaen, Thailand. Asia-Pac. J. Sci. Technol. 2017, 18, 1–8. [Google Scholar]
- Trung, B.; Dao, T.-S.; Faassen, E.; Lürling, M.; Trung, B.; Dao, T.-S.; Faassen, E.; Lürling, M. Cyanobacterial Blooms and Microcystins in Southern Vietnam. Toxins 2018, 10, 471. [Google Scholar] [CrossRef] [PubMed]
- Te, S.H.; Gin, K.Y.-H. The dynamics of cyanobacteria and microcystin production in a tropical reservoir of Singapore. Harmful Algae 2011, 10, 319–329. [Google Scholar] [CrossRef]
- Tavakoli, Y.; Mohammadipanah, F.; Te, S.H.; You, L.; Gin, K.Y.-H. Biodiversity, phylogeny and toxin production profile of cyanobacterial strains isolated from lake Latyan in Iran. Harmful Algae 2021, 106, 102054. [Google Scholar] [CrossRef] [PubMed]
- Belykh, O.I.; Sorokovikova, E.G.; Tomberg, I.V.; Fedorova, G.A.; Kuzmin, A.V.; Krasnopeev, A.Y.; Suslova, M.Y.; Potapov, S.A.; Belykh, T.I.; Norovsuren, J.; et al. Water Quality, Toxicity and Diversity of Planktonic and Benthic Cyanobacteria in Pristine Ancient Lake Khubsugul (Hövsgöl), Mongolia. Toxins 2023, 15, 213. [Google Scholar] [CrossRef]
- Mohamed, Z.A.; Shehri, A.M.A. Microcystins in groundwater wells and their accumulation in vegetable plants irrigated with contaminated waters in Saudi Arabia. J. Hazard. Mater. 2009, 172, 310–315. [Google Scholar] [CrossRef]
- Chatziefthimiou, A.D.; Metcalf, J.S.; Glover, W.B.; Banack, S.A.; Dargham, S.R.; Richer, R.A. Cyanobacteria and cyanotoxins are present in drinking water impoundments and groundwater wells in desert environments. Toxicon 2016, 114, 75–84. [Google Scholar] [CrossRef]
- Al-Tebrineh, J.; Merrick, C.; Ryan, D.; Humpage, A.; Bowling, L.; Neilan, B.A. Community Composition, Toxigenicity, and Environmental Conditions during a Cyanobacterial Bloom Occurring along 1,100 Kilometers of the Murray River. Appl. Environ. Microbiol. 2012, 78, 263–272. [Google Scholar] [CrossRef]
- Griffiths, D.J.; Saker, M.L. The Palm Island mystery disease 20 years on: A review of research on the cyanotoxin cylindrospermopsin. Environ. Toxicol. 2003, 18, 78–93. [Google Scholar] [CrossRef]
- Ryan, E.F.; Hamilton, D.P.; Barnes, G.E. Recent occurrence of Cylindrospermopsis raciborskii in Waikato lakes of New Zealand. N. Z. J. Mar. Freshw. Res. 2003, 37, 829–836. [Google Scholar] [CrossRef]
- Wood, S.A.; Hamilton, D.P.; Paul, W.J.; Safi, K.A.; Williamson, W.M. New Zealand Guidelines for Cyanobacteria in Recreational Fresh Waters: Interim Guidelines; Ministry for the Environment and Ministry of Health: Wellington, New Zealand, 2009. [Google Scholar]
- Wood, S.A. Bloom Forming and Toxic Cyanobacteria in New Zealand Species Diversity and Distribution, Cyanotoxin Production and Accumulation of Microcystins in Selected Freshwater Organisms. Ph.D. Thesis, Victoria University of Wellington, Wellington, New Zealand, 2004. [Google Scholar] [CrossRef]
- Wood, S.A.; Stirling, D.J. First identification of the cylindrospermopsin-producing cyanobacterium Cylindrospermopsis raciborskii in New Zealand. N. Z. J. Mar. Freshw. Res. 2003, 37, 821–828. [Google Scholar] [CrossRef]
- Svirčev, Z.; Krstič, S.; Miladinov-Mikov, M.; Baltič, V.; Vidovič, M. Freshwater Cyanobacterial Blooms and Primary Liver Cancer Epidemiological Studies in Serbia. J. Environ. Sci. Health Part C 2009, 27, 36–55. [Google Scholar] [CrossRef] [PubMed]
- Cirés, S.; Wörmer, L.; Carrasco, D.; Quesada, A. Sedimentation Patterns of Toxin-Producing Microcystis Morphospecies in Freshwater Reservoirs. Toxins 2013, 5, 939–957. [Google Scholar] [CrossRef] [PubMed]
- Stefanelli, M.; Scardala, S.; Cabras, P.A.; Orrù, A.; Vichi, S.; Testai, E.; Funari, E.; Manganelli, M. Cyanobacterial dynamics and toxins concentrations in Lake Alto Flumendosa, Sardinia, Italy. Adv. Oceanogr. Limnol. 2017, 8, 71–86. [Google Scholar] [CrossRef]
- Kabziński, A.K.M.; Juszczak, R.; Miękoś, E.; Tarczynska, E.; Sivonen, K.; Rapala, J. The First Report about the Presence of Cyanobacterial Toxins in Polish Lakes. Pol. J. Environ. Stud. 2000, 9, 171–178. [Google Scholar]
- Jurczak, T.; Tarczynska, M.; Karlsson, K.; Meriluoto, J. Characterization and diversity of cyanobacterial hepatotoxins (Microcystins) in blooms from polish freshwaters identified by liquid chromatography-electrospray ionisation mass spectrometry. Chromatographia 2004, 59, 571–578. [Google Scholar] [CrossRef]
- Grabowska, M.; Pawlik-Skowrońska, B. Replacement of chroococcales and nostocales by oscillatoriales caused a significant increase in microcystin concentrations in a dam reservoir. Oceanol. Hydrobiol. Stud. 2009, 37, 23–33. [Google Scholar] [CrossRef]
- Jančula, D.; Straková, L.; Sadílek, J.; Maršálek, B.; Babica, P. Survey of cyanobacterial toxins in Czech water reservoirs—The first observation of neurotoxic saxitoxins. Environ. Sci. Pollut. Res. 2014, 21, 8006–8015. [Google Scholar] [CrossRef]
- Kobos, J.; Błaszczyk, A.; Hohlfeld, N.; Toruńska-Sitarz, A.; Krakowiak, A.; Hebel, A.; Sutryk, K.; Grabowska, M.; Toporowska, M.; Kokociński, M.; et al. Cyanobacteria and cyanotoxins in Polish freshwater bodies. Oceanol. Hydrobiol. Stud. 2014, 42, 358–378. [Google Scholar] [CrossRef]
- Czyżewska, W.; Piontek, M.; Łuszczyńska, K. The Occurrence of Potential Harmful Cyanobacteria and Cyanotoxins in the Obrzyca River (Poland), a Source of Drinking Water. Toxins 2020, 12, 284. [Google Scholar] [CrossRef] [PubMed]
- Mooney, K.M.; Hamilton, J.T.G.; Floyd, S.D.; Foy, R.H.; Elliott, C.T. Initial studies on the occurrence of cyanobacteria and microcystins in Irish lakes. Environ. Toxicol. 2011, 26, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Pekar, H.; Westerberg, E.; Bruno, O.; Lääne, A.; Persson, K.M.; Sundström, L.F.; Thim, A.-M. Fast, rugged and sensitive ultra high pressure liquid chromatography tandem mass spectrometry method for analysis of cyanotoxins in raw water and drinking water—First findings of anatoxins, cylindrospermopsins and microcystin variants in Swedish source waters and infiltration ponds. J. Chromatogr. A 2016, 1429, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Sorokovikova, E.; Tikhonova, I.; Naidanova, Y.; Belykh, O. Identification of microcystin producing cyanobacteria in the plankton of Lake Baikal and Irkutsk Reservoir. Limnol. Freshw. Biol. 2024, 8, 1101–1108. [Google Scholar] [CrossRef]
- Sysolyatina, M.A.; Sidelev, S.I.; Kutyavina , T.I. Identification of toxin-producing cyanobacteria in water reservoirs of Kirov region and assessment of their relationship with water toxicity to hydrobionts. Limnol. Freshw. Biol. 2024, 8, 1109–1114. [Google Scholar] [CrossRef]
- Chernova, E.; Sidelev, S.; Russkikh, I.; Korneva, L.; Solovyova, V.; Mineeva, N.; Stepanova, I.; Zhakovskaya, Z. Spatial distribution of cyanotoxins and ratios of microcystin to biomass indicators in the reservoirs of the Volga, Kama and Don Rivers, the European part of Russia. Limnologica 2020, 84, 125819. [Google Scholar] [CrossRef]
- Belykh, O.I.; Gladkikh, S.S.; Sorokovikova, E.G.; Tikhonova, I.V.; Potapov, S.A.; Fedorova, G.A. Microcystin-Producing Cyanobacteria in Water Reservoirs of Russia, Belarus and Ukraine. Chem. Sustain. Dev. 2013, 21, 347–361. [Google Scholar]
- Rzymski, P.; Horyn, O.; Budzyńska, A.; Jurczak, T.; Kokociński, M.; Niedzielski, P.; Klimaszyk, P.; Falfushynska, H. A report of Cylindrospermopsis raciborskii and other cyanobacteria in the water reservoirs of power plants in Ukraine. Environ. Sci. Pollut. Res. 2018, 25, 15245–15252. [Google Scholar] [CrossRef]
- Lorenzi, A.S.; Cordeiro-Araújo, M.K.; Chia, M.A.; Bittencourt-Oliveira, M.d.C. Cyanotoxin contamination of semiarid drinking water supply reservoirs. Environ. Earth Sci. 2018, 77, 595. [Google Scholar] [CrossRef]
- Oliveira, E.D.C.; Castelo-Branco, R.; Silva, L.; Silva, N.; Azevedo, J.; Vasconcelos, V.; Faustino, S.; Cunha, A. First Detection of Microcystin-LR in the Amazon River at the Drinking Water Treatment Plant of the Municipality of Macapá, Brazil. Toxins 2019, 11, 669. [Google Scholar] [CrossRef]
- Ruiz, M.; Galanti, L.; Ruibal, A.L.; Rodriguez, M.I.; Wunderlin, D.A.; Amé, M.V. First Report of Microcystins and Anatoxin-a Co-occurrence in San Roque Reservoir (Córdoba, Argentina). Water Air Soil Pollut. 2013, 224, 1–17. [Google Scholar] [CrossRef]
- Forastier, M.; Zalocar, Y.; Andrinolo, D.; Domitrovic, H.A. Occurrence and toxicity of Microcystis aeruginosa (Cyanobacteria) in the Paraná River, downstream of the Yacyretá dam (Argentina). Rev. Biol. Tropical 2016, 64, 203–211. [Google Scholar] [CrossRef]
- Caly, L.F.; Rodríguez, D.C.; Peñuela, G.A. Monitoring of cyanobacteria and cyanotoxins in a Colombian tropical reservoir. Environ. Sci. Pollut. Res. 2022, 29, 52775–52787. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.E.; Gutiérrez-Hoyos, N.; Benedetti, J.S.G.; Vives, M.J.; Sivasubramanian, V. Clarification of cyanotoxins in El Guajaro Reservoir, Colombia using a microalgae-based consortium MPMC. J. Chem. Technol. Biotechnol. 2022, 97, 1468–1481. [Google Scholar] [CrossRef]
- Ballesteros, I.; Cruz, S.D.L.; Rojas, M.; Salazar, G.; Martínez-Fresneda, M.; Castillejo, P. Screening of cyanotoxin producing genes in Ecuadorian freshwater systems. Acta Limnol. Bras. 2022, 34, e24. [Google Scholar] [CrossRef]
- Cuellar-Martinez, T.; Ruiz-Fernández, A.C.; Alonso-Hernández, C.; Amaya-Monterrosa, O.; Quintanilla, R.; Carrillo-Ovalle, H.L.; Arbeláez, M.N.; Díaz-Asencio, L.; Méndez, S.M.; Vargas, M.; et al. Addressing the Problem of Harmful Algal Blooms in Latin America and the Caribbean- A Regional Network for Early Warning and Response. Front. Mar. Sci. 2018, 5, 409. [Google Scholar] [CrossRef]
- López-Cortés, D.J.; Núñez Vázquez, E.J.; Dorantes-Aranda, J.J.; Band-Schmidt, C.J.; Hernández-Sandoval, F.E.; Bustillos-Guzmán, J.J.; Leyva-Valencia, I.; Fernández-Herrera, L.J. The State of Knowledge of Harmful Algal Blooms of Margalefidinium polykrikoides (a.k.a. Cochlodinium polykrikoides) in Latin America. Front. Mar. Sci. 2019, 6, 463. [Google Scholar] [CrossRef]
- Sunesen, I.; Méndez, S.M.; Mancera-Pineda, J.E.; Bottein, M.-Y.D.; Enevoldsen, H. The Latin America and Caribbean HAB status report based on OBIS and HAEDAT maps and databases. Harmful Algae 2021, 102, 101920. [Google Scholar] [CrossRef]
- Garita-Alvarado, C.A.; Bojorge-García, M.G.; Cantoral-Uriza, E.A. Cyanotoxins bioaccumulation in freshwater ecosystems in Latin America: A review. Hidrobiológica 2023, 33, 353–365. [Google Scholar] [CrossRef]
- Aguilera, A.; Almanza, V.; Haakonsson, S.; Palacio, H.; Rodas, G.A.B.; Barros, M.U.G.; Capelo-Neto, J.; Urrutia, R.; Aubriot, L.; Bonilla, S. Cyanobacterial bloom monitoring and assessment in Latin America. Harmful Algae 2023, 125, 102429. [Google Scholar] [CrossRef]
- Zamyadi, A.; McQuaid, N.; Prévost, M.; Dorner, S. Monitoring of potentially toxic cyanobacteria using an online multi-probe in drinking water sources. J. Environ. Monit. 2012, 14, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.R.; Beversdorf, L.J.; Weirich, C.A.; Bartlett, S.L. Cyanobacterial Toxins of the Laurentian Great Lakes, Their Toxicological Effects, and Numerical Limits in Drinking Water. Mar. Drugs 2017, 15, 160. [Google Scholar] [CrossRef]
- US. EPA. The Great Lakes: Lake Erie; United States Environmental Protection Agency: Washington, DC, USA, 2023.
- Steffen, M.M.; Davis, T.W.; McKay, R.M.L.; Bullerjahn, G.S.; Krausfeldt, L.E.; Stough, J.M.A.; Neitzey, M.L.; Gilbert, N.E.; Boyer, G.L.; Johengen, T.H.; et al. Ecophysiological Examination of the Lake Erie Microcystis Bloom in 2014: Linkages between Biology and the Water Supply Shutdown of Toledo, OH. Environ. Sci. Technol. 2017, 51, 6745–6755. [Google Scholar] [CrossRef]
- Mercado Borrayo, B.M. Estudio sobre la remoción de cianobacterias y sus metabolitos en la Planta Potabilizadora “Los Berros” Sistema Cutzamala. Ph.D. Thesis, Universidad Nacional Autónoma de México: Mexico City, Mexico, 2007. [Google Scholar]
- Kim, H.R.; Kim, C.K.; Ahn, T.S.; Yoo, S.A.; Lee, D.H. Effects of Temperature and Light on Microcystin Synthetase Gene Transcription in Microcystis Aeruginosa. Key Eng. Mater. 2005, 277–279, 606–611. [Google Scholar] [CrossRef]
- Sevilla, E.; Martin-Luna, B.; Bes, M.T.; Fillat, M.F.; Peleato, M.L. An active photosynthetic electron transfer chain required for mcyD transcription and microcystin synthesis in Microcystis aeruginosa PCC7806. Ecotoxicology 2011, 21, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.; Harel, M.; Kaplan-Levy, R.N.; Hadas, O.; Sukenik, A.; Dittmann, E. Frontiers | The Languages Spoken in the Water Body (or the Biological Role of Cyanobacterial Toxins). Front. Microbiol. 2012, 3, 138. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Wang, P.; Jia, P.; Zhang, Y.; Chu, X.; Wang, Y. A review on factors affecting microcystins production by algae in aquatic environments. World J. Microbiol. Biotechnol. 2016, 32, 51. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, B.; Majedi, S.M.; Pavagadhi, S.; Te, S.H.; Boo, C.Y.; Gin, K.Y.-H.; Swarup, S. Effects of Light and Temperature on the Metabolic Profiling of Two Habitat-Dependent Bloom-Forming Cyanobacteria. Metabolites 2022, 12, 406. [Google Scholar] [CrossRef]
- Hobson, P.; Fallowfield, H.J. Effect of irradiance, temperature and salinity on growth and toxin production by Nodularia spumigena. Hydrobiologia 2003, 493, 7–15. [Google Scholar] [CrossRef]
- Bui, T.; Dao, T.-S.; Vo, T.-G.; Lürling, M. Warming Affects Growth Rates and Microcystin Production in Tropical Bloom-Forming Microcystis Strains. Toxins 2018, 10, 123. [Google Scholar] [CrossRef]
- Stark, G.F.; Martin, R.M.; Smith, L.E.; Wei, B.; Hellweger, F.L.; Bullerjahn, G.S.; McKay, R.M.L.; Boyer, G.L.; Wilhelm, S.W. Microcystin aids in cold temperature acclimation: Differences between a toxic Microcystis wildtype and non-toxic mutant. Harmful Algae 2023, 129, 102531. [Google Scholar] [CrossRef] [PubMed]
- Wejnerowski, Ł.; Dulić, T.; Akter, S.; Font-Nájera, A.; Rybak, M.; Kamiński, O.; Czerepska, A.; Dziuba, M.K.; Jurczak, T.; Meriluoto, J.; et al. Community Structure and Toxicity Potential of Cyanobacteria during Summer and Winter in a Temperate-Zone Lake Susceptible to Phytoplankton Blooms. Toxins 2024, 16, 357. [Google Scholar] [CrossRef] [PubMed]
- Wejnerowski, Ł.; Rzymski, P.; Kokociński, M.; Meriluoto, J. The structure and toxicity of winter cyanobacterial bloom in a eutrophic lake of the temperate zone. Ecotoxicology 2018, 27, 752–760. [Google Scholar] [CrossRef]
- Wang, Q.; Niu, Y.; Xie, P.; Chen, J.; Ma, Z.; Tao, M.; Qi, M.; Wu, L.; Guo, L. Factors Affecting Temporal and Spatial Variations of Microcystins in Gonghu Bay of Lake Taihu, with Potential Risk of Microcystin Contamination to Human Health. Sci. World J. 2010, 10, 1795–1809. [Google Scholar] [CrossRef] [PubMed]
- Downing, S.; Banack, S.A.; Metcalf, J.S.; Cox, P.A.; Downing, T.G. Nitrogen starvation of cyanobacteria results in the production of β-N-methylamino-L-alanine. Toxicon 2011, 58, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, J.S.M.; Giani, A. Microcystin Production and Regulation under Nutrient Stress Conditions in Toxic Microcystis Strains. Appl. Environ. Microbiol. 2014, 80, 5836–5843. [Google Scholar] [CrossRef] [PubMed]
- Brookes, J.D.; Carey, C.C. Resilience to Blooms. Science 2011, 334, 46–47. [Google Scholar] [CrossRef] [PubMed]
- MacKeigan, P.W.; Zastepa, A.; Taranu, Z.E.; Westrick, J.A.; Liang, A.; Pick, F.R.; Beisner, B.E.; Gregory-Eaves, I. Microcystin concentrations and congener composition in relation to environmental variables across 440 north-temperate and boreal lakes. Sci. Total Environ. 2023, 884, 163811. [Google Scholar] [CrossRef] [PubMed]
- Jablonska, M.; Cerasino, L.; Boscaini, A.; Capelli, C.; Greco, C.; Klemenčič, A.K.; Mischke, U.; Salmaso, N.; Kurmayer, R. Distribution of toxigenic cyanobacteria in Alpine lakes and rivers as revealed by molecular screening. Water Res. 2024, 258, 121783. [Google Scholar] [CrossRef]
- Li, X.; Tikhonova, I.V.; Potapov, S.A.; Krasnopeev, A.Y.; Zhuchenko, N.A.; Niao, X.; Wang, L.; Sorokovikova, E.G.; Wang, W.; Belykh, O.I. World’s largest oligotrophic Lake Baikal: Concerns about cyanobacterial blooms and potential microcystin producers along the littoral zone. Harmful Algae 2025, 144, 102841. [Google Scholar] [CrossRef]
- Reinl, K.L.; Sterner, R.W.; Lafrancois, B.M.; Brovold, S. Fluvial seeding of cyanobacterial blooms in oligotrophic Lake Superior. Harmful Algae 2020, 100, 101941. [Google Scholar] [CrossRef]
- Cizmas, L.; Sharma, V.K.; Gray, C.M.; McDonald, T.J. Pharmaceuticals and personal care products in waters: Occurrence, toxicity, and risk. Environ. Chem. Lett. 2015, 13, 381–394. [Google Scholar] [CrossRef]
- Tijani, J.O.; Fatoba, O.O.; Petrik, L.F. A Review of Pharmaceuticals and Endocrine-Disrupting Compounds: Sources, Effects, Removal, and Detections. Water Air Soil Pollut. 2013, 224, 1770. [Google Scholar] [CrossRef]
- Edwards, C.; Lawton, L.A. Chapter 4 Bioremediation of Cyanotoxins. Adv. Appl. Microbiol. 2009, 67, 109–129. [Google Scholar] [CrossRef] [PubMed]
- Dziga, D.; Nybom, S.; Gagala, I.; Wasylewski, M. Biological Treatment for the Destruction of Cyanotoxins. In Water Treatment for Purification from Cyanobacteria and Cyanotoxins; Hiskia, A.E., Triantis, T.M., Antoniou, M.G., Kaloudis, T., Dionysiou, D.D., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 117–153. [Google Scholar]
- Ho, L.; Hoefel, D.; Saint, C.P.; Newcombe, G. Isolation and identification of a novel microcystin-degrading bacterium from a biological sand filter. Water Res. 2007, 41, 4685–4695. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, Z.A.; Hashem, M.; Alamri, S.; Campos, A.; Vasconcelos, V. Fungal biodegradation and removal of cyanobacteria and microcystins: Potential applications and research needs. Environ. Sci. Pollut. Res. 2021, 28, 37041–37050. [Google Scholar] [CrossRef] [PubMed]
- Boopathi, T.; Ki, J.-S. Impact of Environmental Factors on the Regulation of Cyanotoxin Production. Toxins 2014, 6, 1951–1978. [Google Scholar] [CrossRef]
- Dalu, T.; Wasserman, R.J. Cyanobacteria dynamics in a small tropical reservoir: Understanding spatio-temporal variability and influence of environmental variables. Sci. Total Environ. 2018, 643, 835–841. [Google Scholar] [CrossRef]
- Martínez-Jerónimo, F.; Antuna-González, P.d.C.; Hernández-Zamora, M.; Martínez-Jerónimo, L.; Munoz, G.; Simon, D.F.; Sauvé, S. Year-long monitoring of phytoplankton community, toxigenic cyanobacteria, and total microcystins in a eutrophic tropical dam supplying the Mexico megacity. Front. Environ. Sci. 2022, 10, 984365. [Google Scholar] [CrossRef]
- Peng, L.; Tang, Q.; Gu, J.; Lei, L.; Chen, W.; Song, L. Seasonal variation of microcystins and their accumulation in fish in two large shallow lakes of China. Ecotoxicology 2020, 29, 790–800. [Google Scholar] [CrossRef]
- Filatova, D.; Picardo, M.; Núñez, O.; Farré, M. Analysis, levels and seasonal variation of cyanotoxins in freshwater ecosystems. Trends Environ. Anal. Chem. 2020, 26, e00091. [Google Scholar] [CrossRef]
- Melo, D.S.; Gontijo, E.S.J.; Frascareli, D.; Simonetti, V.C.; Machado, L.S.; Barth, J.A.C.; Moschini-Carlos, V.; Pompêo, M.L.; Rosa, A.H.; Friese, K. Self-Organizing Maps for Evaluation of Biogeochemical Processes and Temporal Variations in Water Quality of Subtropical Reservoirs. Water Resour. Res. 2019, 55, 10268–10281. [Google Scholar] [CrossRef]
- Visser, P.M.; Verspagen, J.M.H.; Sandrini, G.; Stal, L.J.; Matthijs, H.C.P.; Davis, T.W.; Paerl, H.W.; Huisman, J. How rising CO2 and global warming may stimulate harmful cyanobacterial blooms. Harmful Algae 2016, 54, 145–159. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Machado, L.; Dörr, F.; Dörr, F.A.; Frascareli, D.; Melo, D.S.; Gontijo, E.S.J.; Friese, K.; Pinto, E.; Rosa, A.H.; Pompêo, M.M.; et al. Permanent occurrence of Raphidiopsis raciborskii and cyanotoxins in a subtropical reservoir polluted by domestic effluents (Itupararanga reservoir, São Paulo, Brazil). Environ. Sci. Pollut. Res. 2021, 29, 18653–18664. [Google Scholar] [CrossRef] [PubMed]
- Vione, D.; Rosario-Ortiz, F.L. Foreseen Effects of Climate-Impacted Scenarios on the Photochemical Fate of Selected Cyanotoxins in Surface Freshwaters. Environ. Sci. Technol. 2021, 55, 10928–10934. [Google Scholar] [CrossRef] [PubMed]
- Manning, S.R.; Nobles, D.R. Impact of global warming on water toxicity: Cyanotoxins. Curr. Opin. Food Sci. 2017, 18, 14–20. [Google Scholar] [CrossRef]
- Zepernick, B.N.; Wilhelm, S.W.; Bullerjahn, G.S.; Paerl, H.W. Climate change and the aquatic continuum: A cyanobacterial comeback story. Environ. Microbiol. Rep. 2023, 15, 3–12. [Google Scholar] [CrossRef]
- Mantzouki, E.; Lürling, M.; Fastner, J.; Domis, L.D.S.; Wilk-Woźniak, E.; Koreivienė, J.; Seelen, L.; Teurlincx, S.; Verstijnen, Y.; Krztoń, W.; et al. Temperature Effects Explain Continental Scale Distribution of Cyanobacterial Toxins. Toxins 2018, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Van de Waal, D.B.; Gsell, A.S.; Harris, T.; Paerl, H.W.; de Senerpont Domis, L.N.; Huisman, J. Hot summers raise public awareness of toxic cyanobacterial blooms. Water Res. 2024, 249, 120817. [Google Scholar] [CrossRef]
- Paerl, H.W.; Barnard, M.A. Mitigating the global expansion of harmful cyanobacterial blooms: Moving targets in a human- and climatically-altered world. Harmful Algae 2020, 96, 101845. [Google Scholar] [CrossRef]
- Free, G.; Bresciani, M.; Pinardi, M.; Peters, S.; Laanen, M.; Padula, R.; Cingolani, A.; Charavgis, F.; Giardino, C. Shorter blooms expected with longer warm periods under climate change: An example from a shallow meso-eutrophic Mediterranean lake. Hydrobiologia 2022, 849, 3963–3978. [Google Scholar] [CrossRef]
- Chorus, I.; Fastner, J.; Welker, M. Cyanobacteria and Cyanotoxins in a Changing Environment: Concepts, Controversies, Challenges. Water 2021, 13, 2463. [Google Scholar] [CrossRef]
- Amórtegui, J.C.E.; Pekar, H.; Retrato, M.D.C.; Persson, M.; Karlson, B.; Bergquist, J.; Zuberovic-Muratovic, A.; España Amórtegui, J.C.; Pekar, H.; Retrato, M.D.C.; et al. LC-MS/MS Analysis of Cyanotoxins in Bivalve Mollusks—Method Development, Validation and First Evidence of Occurrence of Nodularin in Mussels (Mytilus edulis) and Oysters (Magallana gigas) from the West Coast of Sweden. Toxins 2023, 15, 329. [Google Scholar] [CrossRef] [PubMed]
- Almuhtaram, H.; Kibuye, F.A.; Ajjampur, S.; Glover, C.M.; Hofmann, R.; Gaget, V.; Owen, C.; Wert, E.C.; Zamyadi, A. State of knowledge on early warning tools for cyanobacteria detection. Ecol. Indic. 2021, 133, 108442. [Google Scholar] [CrossRef]
- Oberholster, P.J.; Botha, A.-M.; Cloete, T.E. Toxic cyanobacterial blooms in a shallow, artificially mixed urban lake in Colorado, USA. Lakes Reserv. Res. Manag. 2006, 11, 111–123. [Google Scholar] [CrossRef]
- Vogiazi, V.; Cruz, A.d.l.; Mishra, S.; Shanov, V.; Heineman, W.R.; Dionysiou, D.D. A Comprehensive Review: Development of Electrochemical Biosensors for Detection of Cyanotoxins in Freshwater. ACS Sens. 2019, 4, 1151–1173. [Google Scholar] [CrossRef] [PubMed]
- Massey, I.Y.; Yang, F.; Ding, Z.; Yang, S.; Guo, J.; Tezi, C.; Al-Osman, M.; Kamegni, R.B.; Zeng, W. Exposure routes and health effects of microcystins on animals and humans: A mini-review. Toxicon 2018, 151, 156–162. [Google Scholar] [CrossRef]
- Haande, S.; Ballot, A.; Rohrlack, T.; Fastner, J.; Wiedner, C.; Edvardsen, B. Diversity of Microcystis aeruginosa isolates (Chroococcales, Cyanobacteria) from East-African water bodies. Arch. Microbiol. 2007, 188, 15–25. [Google Scholar] [CrossRef]
- Bertani, P.; Lu, W. Cyanobacterial toxin biosensors for environmental monitoring and protection. Med. Nov. Technol. Devices 2021, 10, 100059. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, C.; Sun, Y.; Liu, Z.; Xu, F.; Zhang, Y.; Wen, Z.; Li, Z. Interaction between DNA and Microcystin-LR Studied by Spectra Analysis and Atomic Force Microscopy. Biomacromolecules 2011, 12, 797–803. [Google Scholar] [CrossRef]
- Bilibana, M.P.; Williams, A.R.; Rassie, C.; Sunday, C.E.; Makelane, H.; Wilson, L.; Ntshongontshi, N.; Jijana, A.N.; Masikini, M.; Baker, P.G.L.; et al. Electrochemical Aptatoxisensor Responses on Nanocomposites Containing Electro-Deposited Silver Nanoparticles on Poly(Propyleneimine) Dendrimer for the Detection of Microcystin-LR in Freshwater. Sensors 2016, 16, 1901. [Google Scholar] [CrossRef] [PubMed]
- McLellan, N.L.; Manderville, R.A. Toxic mechanisms of microcystins in mammals. Toxicol. Res. 2017, 6, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Plaas, H.E.; Paerl, H.W. Toxic Cyanobacteria: A Growing Threat to Water and Air Quality. Environ. Sci. Technol. 2020, 55, 44–64. [Google Scholar] [CrossRef] [PubMed]
- Svirčev, Z.; Chen, L.; Sántha, K.; Drobac Backović, D.; Šušak, S.; Vulin, A.; Palanački Malešević, T.; Codd, G.A.; Meriluoto, J.; Svirčev, Z.; et al. A review and assessment of cyanobacterial toxins as cardiovascular health hazards. Arch. Toxicol. 2022, 96, 2829–2863. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Gupta, S.K. Occurrence and Exposure to Trihalomethanes in Drinking Water: A Systematic Review and Meta-analysis. Expo. Health 2022, 14, 915–939. [Google Scholar] [CrossRef]
- Mutoti, M.I.; Edokpayi, J.N.; Mutileni, N.; Durowoju, O.S.; Munyai, F.L. Cyanotoxins in groundwater; occurrence, potential sources, health impacts and knowledge gap for public health. Toxicon 2023, 226, 107077. [Google Scholar] [CrossRef]
- Drobac, D.; Tokodi, N.; Simeunović, J.; Baltić, V.; Stanić, D.; Svirčev, Z. Human Exposure to Cyanotoxins and their Effects on Health. Arch. Ind. Hyg. Toxicol. 2013, 64, 305–315. [Google Scholar] [CrossRef]
- Annadotter, H.; Cronberg, G.; Lawton, L.; Hansson, H.-B.; Göthe, J.; Skulberg, O.M.; Cronberg, G.; Lawton, L.; Hansson, H.B.; Skulberg, O. An extensive outbreak of gastroenteritis associated with the toxic cyanobacterium (Oscillatoriales, Cyanophyceae) in Scania, South Sweden. In Cyanotoxins—Occurrence, Causes, Consequences; Chorus, I., Ed.; Springer: Berlin, Germany, 2001; pp. 200–208. [Google Scholar]
- Svirčev, Z.; Drobac, D.; Tokodi, N.; Mijović, B.; Codd, G.A.; Meriluoto, J. Toxicology of microcystins with reference to cases of human intoxications and epidemiological investigations of exposures to cyanobacteria and cyanotoxins. Arch. Toxicol. 2017, 91, 621–650. [Google Scholar] [CrossRef]
- Ohtani, I.; Moore, R.E.; Runnegar, M.T.C. Cylindrospermopsin: A potent hepatotoxin from the blue-green alga Cylindrospermopsis raciborskii. J. Am. Chem. Soc. 2002, 114, 7941–7942. [Google Scholar] [CrossRef]
- Hoeger, S.J.; Hitzfeld, B.C.; Dietrich, D.R. Occurrence and elimination of cyanobacterial toxins in drinking water treatment plants. Toxicol. Appl. Pharmacol. 2005, 203, 231–242. [Google Scholar] [CrossRef]
- Nielsen, M.C.; Jiang, S.C. Can cyanotoxins penetrate human skin during water recreation to cause negative health effects? Harmful Algae 2020, 98, 101872. [Google Scholar] [CrossRef] [PubMed]
- Osborne, N.J.; Shaw, G.R.; Webb, P.M. Health effects of recreational exposure to Moreton Bay, Australia waters during a Lyngbya majuscula bloom. Environ. Int. 2007, 33, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Niamien-Ebrottie, J.E.; Bhattacharyya, S.; Deep, P.R.; Nayak, B. Cyanobacteria and cyanotoxins in the World: Review. Int. J. Appl. Res. Vet. Med. 2015, 1, 563–569. [Google Scholar]
- Azevedo, S.M.F.O.; Carmichael, W.W.; Jochimsen, E.M.; Rinehart, K.L.; Lau, S.; Shaw, G.R.; Eaglesham, G.K. Human intoxication by microcystins during renal dialysis treatment in Caruaru—Brazil. Toxicology 2002, 181–182, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Pouria, S.; Andrade, A.d.; Barbosa, J.; Cavalcanti, R.; Barreto, V.; Ward, C.; Preiser, W.; Poon, G.K.; Neild, G.; Codd, G. Fatal microcystin intoxication in haemodialysis unit in Caruaru, Brazil. Lancet 1998, 352, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Facciponte, D.N.; Bough, M.W.; Seidler, D.; Carroll, J.L.; Ashare, A.; Andrew, A.S.; Tsongalis, G.J.; Vaickus, L.J.; Henegan, P.L.; Butt, T.H.; et al. Identifying aerosolized cyanobacteria in the human respiratory tract: A proposed mechanism for cyanotoxin-associated diseases. Sci. Total Environ. 2018, 645, 1003–1013. [Google Scholar] [CrossRef]
- Olson, N.E.; Cooke, M.E.; Shi, J.H.; Birbeck, J.A.; Westrick, J.A.; Ault, A.P. Harmful Algal Bloom Toxins in Aerosol Generated from Inland Lake Water. Environ. Sci. Technol. 2020, 54, 4769–4780. [Google Scholar] [CrossRef]
- Wiśniewska, K.; Śliwińska-Wilczewska, S.; Savoie, M.; Lewandowska, A.U. Quantitative and qualitative variability of airborne cyanobacteria and microalgae and their toxins in the coastal zone of the Baltic Sea. Sci. Total Environ. 2022, 826, 154152. [Google Scholar] [CrossRef] [PubMed]
- Labohá, P.; Sychrová, E.; Brózman, O.; Sovadinová, I.; Bláhová, L.; Prokeš, R.; Ondráček, J.; Babica, P. Cyanobacteria, cyanotoxins and lipopolysaccharides in aerosols from inland freshwater bodies and their effects on human bronchial cells. Environ. Toxicol. Pharmacol. 2023, 98, 104073. [Google Scholar] [CrossRef]
- Murby, A.L.; Haney, J.F. Field and laboratory methods to monitor lake aerosols for cyanobacteria and microcystins. Aerobiologia 2015, 32, 395–403. [Google Scholar] [CrossRef]
- Backer, L.C.; Landsberg, J.H.; Miller, M.; Keel, K.; Taylor, T.K. Canine Cyanotoxin Poisonings in the United States (1920s–2012): Review of Suspected and Confirmed Cases from Three Data Sources. Toxins 2013, 5, 1597–1628. [Google Scholar] [CrossRef] [PubMed]
- Picanço, M.R.; Soares, R.M.; Cagido, V.R.; Azevedo, S.M.F.O.; Rocco, P.R.M.; Zin, W.A. Toxicity of a cyanobacterial extract containing microcystins to mouse lungs. Braz. J. Med. Biol. Res. 2004, 37, 1225–1229. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.M.; Cagido, V.R.; Ferraro, R.B.; Meyer-Fernandes, J.R.; Rocco, P.R.M.; Zin, W.A.; Azevedo, S.M.F.O. Effects of microcystin-LR on mouse lungs. Toxicon 2007, 50, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W.; Boyer, G.L. Health impacts from cyanobacteria harmful algae blooms: Implications for the North American Great Lakes. Harmful Algae 2016, 54, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Poniedziałek, B.; Karczewski, J. Gastroenteritis and liver carcinogenesis induced by cyanobacterial toxins. Gastroenterol. Pol. 2011, 18, 159–162. [Google Scholar]
- Zilberg, B. Gastroenteritis in Salisbury European children—A five-year study. Cent. Afr. J. Med. 1996, 12, 164–168. [Google Scholar]
- Wu, J.-X.; Huang, H.; Yang, L.; Zhang, X.-F.; Zhang, S.-S.; Liu, H.-H.; Wang, Y.-Q.; Yuan, L.; Cheng, X.-M.; Zhuang, D.-G.; et al. Gastrointestinal toxicity induced by microcystins. World J. Clin. Cases 2018, 6, 344–354. [Google Scholar] [CrossRef]
- Backer, L.C.; McNeel, S.V.; Barber, T.; Kirkpatrick, B.; Williams, C.; Irvin, M.; Zhou, Y.; Johnson, T.B.; Nierenberg, K.; Aubel, M.; et al. Recreational exposure to microcystins during algal blooms in two California lakes. Toxicon 2010, 55, 909–921. [Google Scholar] [CrossRef]
- Stewart, I.; Webb, P.M.; Schluter, P.J.; Fleming, L.E.; Burns, J.W.; Gantar, M.; Backer, L.C.; Shaw, G.R. Epidemiology of recreational exposure to freshwater cyanobacteria—An international prospective cohort study. BMC Public Health 2006, 6, 93. [Google Scholar] [CrossRef]
- Zheng, C.; Zeng, H.; Lin, H.; Wang, J.; Feng, X.; Qiu, Z.; Chen, J.a.; Luo, J.; Luo, Y.; Huang, Y.; et al. Serum microcystin levels positively linked with risk of hepatocellular carcinoma: A case-control study in southwest China. Hepatology 2017, 66, 1519–1528. [Google Scholar] [CrossRef]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Ingested Nitrate and Nitrite, and Cyanobacterial Peptide toxins; International Agency for Research on Cancer: Lyon, France, 2010. [Google Scholar]
- Gu, S.; Jiang, M.; Zhang, B. Microcystin-LR in Primary Liver Cancers: An Overview. Toxins 2022, 14, 715. [Google Scholar] [CrossRef] [PubMed]
- Malta, J.F.; Nardocci, A.C.; Razzolini, M.T.P.; Diniz, V.; Cunha, D.G.F. Exposure to microcystin-LR in tropical reservoirs for water supply poses high risks for children and adults. Environ. Monit. Assess. 2022, 194, 253. [Google Scholar] [CrossRef] [PubMed]
- Zanchett, G.; Oliveira-Filho, E.C. Cyanobacteria and Cyanotoxins: From Impacts on Aquatic Ecosystems and Human Health to Anticarcinogenic Effects. Toxins 2013, 5, 1896–1917. [Google Scholar] [CrossRef] [PubMed]
- Mulvenna, V.; Dale, K.; Priestly, B.; Mueller, U.; Humpage, A.; Shaw, G.; Allinson, G.; Falconer, I. Health Risk Assessment for Cyanobacterial Toxins in Seafood. Int. J. Environ. Res. Public Health 2012, 9, 807–820. [Google Scholar] [CrossRef]
- Arman, T.; Clarke, J.D. Microcystin Toxicokinetics, Molecular Toxicology, and Pathophysiology in Preclinical Rodent Models and Humans. Toxins 2021, 13, 537. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Codd, G.A. Co-Occurrence of Cyanobacteria and Cyanotoxins with Other Environmental Health Hazards: Impacts and Implications. Toxins 2020, 12, 629. [Google Scholar] [CrossRef]
- Greer, B.; Maul, R.; Campbell, K.; Elliott, C.T. Detection of freshwater cyanotoxins and measurement of masked microcystins in tilapia from Southeast Asian aquaculture farms. Anal. Bioanal. Chem. 2017, 409, 4057–4069. [Google Scholar] [CrossRef]
- Merwe, D.v.d.; Sebbag, L.; Nietfeld, J.C.; Aubel, M.T.; Foss, A.; Carney, E. Investigation of a Microcystis aeruginosa cyanobacterial freshwater harmful algal bloom associated with acute microcystin toxicosis in a dog. J. Vet. Diagn. Investig. 2012, 24, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Gorham, T.; Root, E.D.; Jia, Y.; Shum, C.K.; Lee, J. Relationship between cyanobacterial bloom impacted drinking water sources and hepatocellular carcinoma incidence rates. Harmful Algae 2020, 95, 101801. [Google Scholar] [CrossRef]
- Dietrich, D.R.; Fischer, A.; Michel, C.; Hoeger, S. Toxin mixture in cyanobacterial blooms—A critical comparison o. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Hudnell, K.H., Ed.; Springer: New York, NY, USA, 2008; pp. 885–912. [Google Scholar]
- Henri, J.; Huguet, A.; Delmas, J.-M.; Besson, A.; Sanders, P.; Fessard, V. Low in vitro permeability of the cyanotoxin microcystin-LR across a Caco-2 monolayer: With identification of the limiting factors using modelling. Toxicon 2014, 91, 5–14. [Google Scholar] [CrossRef]
- Henri, J.; Lanceleur, R.; Delmas, J.-M.; Fessard, V.; Huguet, A. Permeability of the Cyanotoxin Microcystin-RR across a Caco-2 Cells Monolayer. Toxins 2021, 13, 178. [Google Scholar] [CrossRef] [PubMed]
- Nishiwaki, R.; Ohta, T.; Sueoka, E.; Suganuma, M.; Harada, K.-I.; Watanabe, M.F.; Fujiki, H. Two significant aspects of microcystin-LR: Specific binding and liver specificity. Cancer Lett. 1994, 83, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Kemp, A.; John, J. Microcystins associated with Microcystis dominated blooms in the Southwest wetlands, Western Australia. Environ. Toxicol. 2006, 21, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.L.; Loftin, K.A.; Meyer, M.T.; Ziegler, A.C. Cyanotoxin Mixtures and Taste-and-Odor Compounds in Cyanobacterial Blooms from the Midwestern United States. Environ. Sci. Technol. 2010, 44, 7361–7368. [Google Scholar] [CrossRef]
- Li, Z.; Yu, J.; Yang, M.; Zhang, J.; Burch, M.D.; Han, W. Cyanobacterial population and harmful metabolites dynamics during a bloom in Yanghe Reservoir, North China. Harmful Algae 2010, 9, 481–488. [Google Scholar] [CrossRef]
- Sabart, M.; Pobel, D.; Briand, E.; Combourieu, B.; Salençon, M.J.; Humbert, J.F.; Latour, D. Spatiotemporal Variations in Microcystin Concentrations and in the Proportions of Microcystin-Producing Cells in Several Microcystis aeruginosa Populations. Appl. Environ. Microbiol. 2010, 76, 4750–4759. [Google Scholar] [CrossRef]
- Srivastava, A.; Choi, G.-G.; Ahn, C.-Y.; Oh, H.-M.; Ravi, A.K.; Asthana, R.K. Dynamics of microcystin production and quantification of potentially toxigenic Microcystis sp. using real-time PCR. Water Res. 2012, 46, 817–827. [Google Scholar] [CrossRef]
- Kotak, B.G.; Zurawell, R.W. Cyanobacterial toxins in Canadian freshwaters: A review. Lake Reserv. Manag. 2007, 23, 109–122. [Google Scholar] [CrossRef]
- Watson, S.B.; Zastepa, A.; Boyer, G.L.; Matthews, E. Algal bloom response and risk management: On-site response tools. Toxicon 2017, 129, 144–152. [Google Scholar] [CrossRef]
- Boyer, G.L. The occurrence of cyanobacterial toxins in New York lakes: Lessons from the MERHAB-Lower Great Lakes program. Lake Reserv. Manag. 2007, 23, 153–160. [Google Scholar] [CrossRef]
- Jacoby, J.M.; Kann, J. The occurrence and response to toxic cyanobacteria in the Pacific Northwest, North America. Lake and Reservoir Management 2007, 23, 123–143. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, P.; Chen, J.; Liang, G. Distribution of microcystins in various organs (heart, liver, intestine, gonad, brain, kidney and lung) of Wistar rat via intravenous injection. Toxicon 2008, 52, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.A.; Pace, J.G.; Matson, C.F.; Miura, G.A.; Lawrence, W.B. Tissue distribution, excretion and hepatic biotransformation of microcystin-LR in mice. J. Pharmacol. Exp. Ther. 1991, 256, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Falconer, I.; Buckley, T.; Runnegar, M. Biological half-life, organ distribution and excretion of 125-I-labelled toxic peptide from the blue-green alga Microcystis aeruginosa. Aust. J. Biol. Sci. 1986, 39, 17–21. [Google Scholar] [CrossRef]
- Clarke, J.D.; Dzierlenga, A.; Arman, T.; Toth, E.; Li, H.; Lynch, K.D.; Tian, D.-D.; Goedken, M.; Paine, M.F.; Cherrington, N. Nonalcoholic fatty liver disease alters microcystin-LR toxicokinetics and acute toxicity. Toxicon 2019, 162, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, L.; Chen, J.; Xie, P.; Li, S.; He, J.; Li, W.; Fan, H.; Yu, D.; Zeng, C. Quantitatively evaluating detoxification of the hepatotoxic microcystin-LR through the glutathione (GSH) pathway in SD rats. Environ. Sci. Pollut. Res. 2015, 22, 19273–19284. [Google Scholar] [CrossRef]
- Fischer, W.J.; Altheimer, S.; Cattori, V.; Meier, P.J.; Dietrich, D.R.; Hagenbuch, B. Organic anion transporting polypeptides expressed in liver and brain mediate uptake of microcystin. Toxicol. Appl. Pharmacol. 2005, 203, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Shang, X.; Qin, X.; Lu, J.; Liu, M.; Wang, X. Characterization of organic anion transporting polypeptide 1b2 knockout rats generated by CRISPR/Cas9: A novel model for drug transport and hyperbilirubinemia disease. Acta Pharm. Sin. B 2020, 10, 850–860. [Google Scholar] [CrossRef]
- Kaur, G.; Fahrner, R.; Wittmann, V.; Stieger, B.; Dietrich, D.R. Human MRP2 exports MC-LR but not the glutathione conjugate. Chem.-Biol. Interact. 2019, 311, 108761. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, G.; Wu, L.; Tuo, X.; Wang, W.; Chen, J.; Xie, P. Why mammals more susceptible to the hepatotoxic microcystins than fish: Evidences from plasma and albumin protein binding through equilibrium dialysis. Ecotoxicology 2013, 22, 1012–1019. [Google Scholar] [CrossRef]
- Feurstein, D.; Holst, K.; Fischer, A.; Dietrich, D.R. Oatp-associated uptake and toxicity of microcystins in primary murine whole brain cells. Toxicol. Appl. Pharmacol. 2009, 234, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.R.; Wilhelm, S.W.; Boyer, G.L. The Fate of Microcystins in the Environment and Challenges for Monitoring. Toxins 2014, 6, 3354–3387. [Google Scholar] [CrossRef]
- Kondo, F.; Ikai, Y.; Oka, H.; Ishikawa, N.; Watanabe, M.F.; Watanabe, M.; Harada, K.-I.; Suzuki, M. Separation and identification of microcystins in cyanobacteria by frit-fast atom bombardment liquid chromatography/mass spectrometry. Toxicon 1992, 30, 227–237. [Google Scholar] [CrossRef]
- Pflugmacher, S.; Wiegand, C.; Oberemm, A.; Beattie, K.A.; Krause, E.; Codd, G.A.; Steinberg, C.E.W. Identification of an enzymatically formed glutathione conjugate of the cyanobacterial hepatotoxin microcystin-LR: The first step of detoxication. Biochim. Biophys. Acta (BBA) Gen. Subj. 1998, 1425, 527–533. [Google Scholar] [CrossRef]
- Buratti, F.M.; Scardala, S.; Funari, E.; Testai, E. Human Glutathione Transferases Catalyzing the Conjugation of the Hepatoxin Microcystin-LR. Chem. Res. Toxicol. 2011, 24, 926–933. [Google Scholar] [CrossRef]
- He, J.; Chen, J.; Wu, L.; Li, G.; Xie, P. Metabolic Response to Oral Microcystin-LR Exposure in the Rat by NMR-Based Metabonomic Study. J. Proteome Res. 2012, 11, 5934–5946. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, J.; Xie, P.; Guo, X.; Fan, H.; Yu, D.; Zeng, C.; Chen, L. The role of glutathione detoxification pathway in MCLR-induced hepatotoxicity in SD rats. Environ. Toxicol. 2015, 30, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.S.; Beattie, K.A.; Pflugmacher, S.; Codd, G.A. Immuno-crossreactivity and toxicity assessment of conjugation products of the cyanobacterial toxin, microcystin-LR. FEMS Microbiol. Lett. 2000, 189, 155–158. [Google Scholar] [CrossRef]
- Arman, T.; Lynch, K.D.; Goedken, M.; Clarke, J.D. Sub-chronic microcystin-LR renal toxicity in rats fed a high fat/high cholesterol diet. Chemosphere 2021, 269, 128773. [Google Scholar] [CrossRef] [PubMed]
- Pace, J.G.; Robinson, N.A.; Miura, G.A.; Matson, C.F.; Geisbert, T.W.; White, J.D. Toxicity and kinetics of [3H]microcystin-LR in isolated perfused rat livers. Toxicol. Appl. Pharmacol. 1991, 107, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Botha, N.; Venter, M.v.d.; Downing, T.G.; Shephard, E.G.; Gehringer, M.M. The effect of intraperitoneally administered microcystin-LR on the gastrointestinal tract of Balb/c mice. Toxicon 2004, 43, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, J.; Zhang, X.; Xie, P. A review of reproductive toxicity of microcystins. J. Hazard. Mater. 2016, 301, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef] [PubMed]
- Ahari, H.; Nowruzi, B.; Anvar, A.A.; Porzani, S.J. The Toxicity Testing of Cyanobacterial Toxins In vivo and In vitro by Mouse Bioassay: A Review. Mini-Rev. Med. Chem. 2022, 22, 1131–1151. [Google Scholar] [CrossRef]
- Aranda, Y.N.; Bhatt, P.; Ates, N.; Engel, B.A.; Simsek, H. Cyanophage-cyanobacterial interactions for sustainable aquatic environment. Environ. Res. 2023, 229, 115728. [Google Scholar] [CrossRef]
- Gupta, N.; Pant, S.C.; Vijayaraghavan, R.; Rao, P.V.L. Comparative toxicity evaluation of cyanobacterial cyclic peptide toxin microcystin variants (LR, RR, YR) in mice. Toxicology 2003, 188, 285–296. [Google Scholar] [CrossRef]
- Žegura, B.; Štraser, A.; Filipič, M. Genotoxicity and potential carcinogenicity of cyanobacterial toxins—A review. Mutat. Res./Rev. Mutat. Res. 2011, 727, 16–41. [Google Scholar] [CrossRef] [PubMed]
- Poniedziałek, B.; Rzymski, P.; Karczewski, J. The role of the enzymatic antioxidant system in cylindrospermopsin-induced toxicity in human lymphocytes. Toxicol. Vitr. 2015, 29, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Mello, F.D.; Braidy, N.; Marçal, H.; Guillemin, G.; Nabavi, S.M.; Neilan, B.A. Mechanisms and Effects Posed by Neurotoxic Products of Cyanobacteria/Microbial Eukaryotes/Dinoflagellates in Algae Blooms: A Review. Neurotox. Res. 2017, 33, 153–167. [Google Scholar] [CrossRef]
- Colas, S.; Marie, B.; Lance, E.; Quiblier, C.; Tricoire-Leignel, H.; Mattei, C. Anatoxin-a: Overview on a harmful cyanobacterial neurotoxin from the environmental scale to the molecular target. Environ. Res. 2021, 193, 110590. [Google Scholar] [CrossRef]
- Smith, J.L.; Boyer, G.L.; Zimba, P.V. A review of cyanobacterial odorous and bioactive metabolites: Impacts and management alternatives in aquaculture. Aquaculture 2008, 280, 5–20. [Google Scholar] [CrossRef]
- Du, X.; Liu, H.; Yuan, L.; Wang, Y.; Ma, Y.; Wang, R.; Chen, X.; Losiewicz, M.D.; Guo, H.; Zhang, H. The Diversity of Cyanobacterial Toxins on Structural Characterization, Distribution and Identification: A Systematic Review. Toxins 2019, 11, 530. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.; Wang, X.; Du, Y.; Zhang, S.; Zhang, Y.; Teng, Y. Molecular Mechanism for the Regulation of Microcystin Toxicity to Protein Phosphatase 1 by Glutathione Conjugation Pathway. BioMed Res. Int. 2017, 2017, 9676504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.; Wang, X.; Liu, S.; Zhou, L.; Liu, X. Evaluation of Microplastics and Microcystin-LR Effect for Asian Clams (Corbicula fluminea) by a Metabolomics Approach. Mar. Biotechnol. 2023, 25, 763–777. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Wang, B.; Chen, T.; Wang, X.; Huang, P.; Xu, L.; Guo, Z. Microcystin-LR promotes proliferation by activating Akt/S6K1 pathway and disordering apoptosis and cell cycle associated proteins phosphorylation in HL7702 cells. Toxicol. Lett. 2016, 240, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, B.; Huang, P.; Wang, H.; Xu, K.; Wang, X.; Xu, L.; Guo, Z. Microcystin-LR promotes cell proliferation in the mice liver by activating Akt and p38/ERK/JNK cascades. Chemosphere 2016, 163, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Toivola, D.M.; Eriksson, J.E.; Brautigan, D.L. Identification of protein phosphatase 2A as the primary target for microcystin-LR in rat liver homogenates. FEBS Lett. 1994, 344, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Runnegar, M.T.; Kong, S.; Berndt, N. Protein phosphatase inhibition and in vivo hepatotoxicity of microcystins. Am. J. Physiol.-Gastrointest. Liver Physiol. 1993, 265, G224–G230. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Lee, T.-H.; Lee, S.-J.; Huang, H.-B.; Huang, R.; Chou, H.-N. Comparison of protein phosphatase inhibition activities and mouse toxicities of microcystins. Toxicon 2006, 47, 742–746. [Google Scholar] [CrossRef]
- Ito, E.; Takai, A.; Kondo, F.; Masui, H.; Imanishi, S.; Harada, K.-i. Comparison of protein phosphatase inhibitory activity and apparent toxicity of microcystins and related compounds. Toxicon 2002, 40, 1017–1025. [Google Scholar] [CrossRef]
- Botha, N.; Gehringer, M.M.; Downing, T.G.; Venter, M.v.d.; Shephard, E.G. The role of microcystin-LR in the induction of apoptosis and oxidative stress in CaCo2 cells. Toxicon 2004, 43, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.-X.; Nam Ong, C. Role of oxidative stress and mitochondrial changes in cyanobacteria-induced apoptosis and hepatotoxicity. FEMS Microbiol. Lett. 2003, 220, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lad, A.; Hunyadi, J.; Connolly, J.; Breidenbach, J.D.; Khalaf, F.K.; Dube, P.; Zhang, S.; Kleinhenz, A.L.; Baliu-Rodriguez, D.; Isailovic, D.; et al. Antioxidant Therapy Significantly Attenuates Hepatotoxicity following Low Dose Exposure to Microcystin-LR in a Murine Model of Diet-Induced Non-Alcoholic Fatty Liver Disease. Antioxidants 2022, 11, 1625. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ying, F.; Chen, Y.; Han, X. Microcystin (-LR) affects hormones level of male mice by damaging hypothalamic-pituitary system. Toxicon 2012, 59, 205–214. [Google Scholar] [CrossRef]
- Ding, W.-X.; Shen, H.-M.; Ong, C.-N. Calpain Activation after Mitochondrial Permeability Transition in Microcystin-Induced Cell Death in Rat Hepatocytes. Biochem. Biophys. Res. Commun. 2002, 291, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Menezes, C.; Alverca, E.; Dias, E.; Sam-Bento, F.; Pereira, P. Involvement of endoplasmic reticulum and autophagy in microcystin-LR toxicity in Vero-E6 and HepG2 cell lines. Toxicol. Vitr. 2013, 27, 138–148. [Google Scholar] [CrossRef]
- Moreno, I.; Pichardo, S.; Jos, A.; Gómez-Amores, L.; Mate, A.; Vazquez, C.M.; Cameán, A.M. Antioxidant enzyme activity and lipid peroxidation in liver and kidney of rats exposed to microcystin-LR administered intraperitoneally. Toxicon 2005, 45, 395–402. [Google Scholar] [CrossRef]
- Wei, Y.; Weng, D.; Li, F.; Zou, X.; Young, D.O.; Ji, J.; Shen, P. Involvement of JNK regulation in oxidative stress-mediated murine liver injury by microcystin-LR. Apoptosis 2008, 13, 1031–1042. [Google Scholar] [CrossRef]
- Weng, D.; Lu, Y.; Wei, Y.; Liu, Y.; Shen, P. The role of ROS in microcystin-LR-induced hepatocyte apoptosis and liver injury in mice. Toxicology 2007, 232, 15–23. [Google Scholar] [CrossRef]
- Falconer, I.R.; Dornbusch, M.; Moran, G.; Yeung, S.K. Effect of the cyanobacterial (blue-green algal) toxins from Microcystis aeruginosa on isolated enterocytes from the chicken small intestine. Toxicon 1992, 30, 790–793. [Google Scholar] [CrossRef]
- Ding, W.-X.; Shen, H.-M.; Ong, C.-N. Pivotal Role of Mitochondrial Ca2+ in Microcystin-Induced Mitochondrial Permeability Transition in Rat Hepatocytes. Biochem. Biophys. Res. Commun. 2001, 285, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.P.; Ryan, T.P.; Searfoss, G.H.; Davis, M.A.; Hooser, S.B. Chronic Microcystin Exposure Induces Hepatocyte Proliferation with Increased Expression of Mitotic and Cyclin-associated Genes in P53-deficient Mice. Toxicol. Pathol. 2008, 36, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Casas-Rodriguez, A.; Cameán, A.M.; Jos, A. Potential Endocrine Disruption of Cyanobacterial Toxins, Microcystins and Cylindrospermopsin: A Review. Toxins 2022, 14, 882. [Google Scholar] [CrossRef] [PubMed]
- Hataley, E.K.; Shahmohamadloo, R.S.; Almirall, X.O.; Harrison, A.L.; Rochman, C.M.; Zou, S.; Orihel, D.M. Experimental Evidence from the Field that Naturally Weathered Microplastics Accumulate Cyanobacterial Toxins in Eutrophic Lakes. Environ. Toxicol. Chem. 2022, 41, 3017–3028. [Google Scholar] [CrossRef] [PubMed]
- Moura, D.S.; Pestana, C.J.; Moffat, C.F.; Hui, J.; Irvine, J.T.S.; Edwards, C.; Lawton, L.A. Adsorption of cyanotoxins on polypropylene and polyethylene terephthalate: Microplastics as vector of eight microcystin analogues. Environ. Pollut. 2022, 303, 119135. [Google Scholar] [CrossRef]
- Feng, S.; Deng, S.; Tang, Y.; Liu, Y.; Yang, Y.; Xu, S.; Tang, P.; Lu, Y.; Duan, Y.; Wei, J.; et al. Microcystin-LR Combined with Cadmium Exposures and the Risk of Chronic Kidney Disease: A Case–Control Study in Central China. Environ. Sci. Technol. 2022, 56, 15818–15827. [Google Scholar] [CrossRef]
- Lacagnina, S. The Developmental Origins of Health and Disease (DOHaD). Am. J. Lifestyle Med. 2019, 14, 47–50. [Google Scholar] [CrossRef]
- Marumure, J.; Simbanegavi, T.T.; Makuvara, Z.; Karidzagundi, R.; Alufasi, R.; Goredema, M.; Gufe, C.; Chaukura, N.; Halabowski, D.; Gwenzi, W. Emerging organic contaminants in drinking water systems: Human intake, emerging health risks, and future research directions. Chemosphere 2024, 356, 141699. [Google Scholar] [CrossRef]
- Lin, X.; Xu, J.; Keller, A.A.; He, L.; Gu, Y.; Zheng, W.; Sun, D.; Lu, Z.; Huang, J.; Huang, X.; et al. Occurrence and risk assessment of emerging contaminants in a water reclamation and ecological reuse project. Sci. Total Environ. 2020, 744, 140977. [Google Scholar] [CrossRef]
- Jensen, J.; Mesman, M.; Bierkens, J.; Loibner, A.; Rutgers, M.; Bogolte, T.; Celis, R.; Dirven-van Breemen, E.M.; Erlacher, E.; Ehlers, C.; et al. Ecological Risk Assessment of Contaminated Land-DECISION Support for Site Specific Investigations; Rijksinstituut voor Volksgezondheid en Milieu RIVM: Bilthoven, The Netherlands, 2006; pp. 1–136. [Google Scholar]
- Gwenzi, W.; Muhoyi, E.; Mukura, T.J. Health risk assessment and mitigation of emerging contaminants: A call for an integrated approach. In Emerging Contaminants in the Terrestrial-Aquatic-Atmosphere Continuum: Occurrence, Health Risks, and Mitigation, Gwenzi, W., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 325–342. [Google Scholar]
- Damikouka, I.; Katsiri, A.; Tzia, C. Application of HACCP principles in drinking water treatment. Desalination 2007, 210, 138–145. [Google Scholar] [CrossRef]
- Tsitsifli, S.; Tsoukalas, D.S. Water Safety Plans and HACCP implementation in water utilities around the world: Benefits, drawbacks and critical success factors. Environ. Sci. Pollut. Res. 2019, 28, 18837–18849. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Liu, R.; Liu, H.; Qu, J. Mn(VII)–Fe(II) pre-treatment for Microcystis aeruginosa removal by Al coagulation: Simultaneous enhanced cyanobacterium removal and residual coagulant control. Water Res. 2014, 65, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E.; Onstad, G.D.; Kull, T.P.J.; Metcalf, J.S.; Acero, J.L.; Gunten, U.v. Oxidative elimination of cyanotoxins: Comparison of ozone, chlorine, chlorine dioxide and permanganate. Water Res. 2007, 41, 3381–3393. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qu, R.; Pan, X.; Wang, Z. Oxidative degradation of triclosan by potassium permanganate: Kinetics, degradation products, reaction mechanism, and toxicity evaluation. Water Res. 2016, 103, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Banker, R.; Carmeli, S.; Werman, M.; Teltsch, B.; Porat, R.; Sukenik, A. Uracil Moiety is Required for Toxicity of the Cyanobacterial Hepatotoxin Cylindrospermopsin. J. Toxicol. Environ. Health Part A 2001, 62, 281–288. [Google Scholar] [CrossRef]
- Falconer, I.R.; Humpage, A.R.; Falconer, I.R.; Humpage, A.R. Health Risk Assessment of Cyanobacterial (Blue-green Algal) Toxins in Drinking Water. Int. J. Environ. Res. Public Health 2005, 2, 43–50. [Google Scholar] [CrossRef]
- Ho, L.; Tanis-Plant, P.; Kayal, N.; Slyman, N.; Newcombe, G. Optimising water treatment practices for the removal of Anabaena circinalis and its associated metabolites, geosmin and saxitoxins. J. Water Health 2009, 7, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Laszakovits, J.R.; MacKay, A.A. Removal of cyanotoxins by potassium permanganate: Incorporating competition from natural water constituents. Water Res. 2019, 155, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Bláha, L. Advanced oxidation processes for the removal of cyanobacterial toxins from drinking water. Environ. Sci. Eur. 2020, 32, 94. [Google Scholar] [CrossRef]
- Reitzel, K.; Hansen, J.; Andersen, F.Ø.; Hansen, K.S.; Jensen, H.S. Lake Restoration by Dosing Aluminum Relative to Mobile Phosphorus in the Sediment. Environ. Sci. Technol. 2005, 39, 4134–4140. [Google Scholar] [CrossRef]
- Kibuye, F.A.; Zamyadi, A.; Wert, E.C. A critical review on operation and performance of source water control strategies for cyanobacterial blooms: Part I-chemical control methods. Harmful Algae 2021, 109, 102099. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Hong, L.; Luo, J.; You, Y.; Zhang, J.; Hua, P.; Du, B.; Zhan, J.; Ning, R.; Bao, M. Photocatalytic inactivation of harmful algae and degradation of cyanotoxins microcystin-LR using GO-based Z-scheme nanocatalysts under visible light. Chem. Eng. J. 2020, 392, 123767. [Google Scholar] [CrossRef]
- Fan, J.; Ho, L.; Hobson, P.; Daly, R.; Brookes, J.; Fan, J.; Ho, L.; Hobson, P.; Daly, R.; Brookes, J. Application of Various Oxidants for Cyanobacteria Control and Cyanotoxin Removal in Wastewater Treatment. J. Environ. Eng. 2014, 140, 04014022. [Google Scholar] [CrossRef]
- Newcombe, G.; Ho, L.; Neto, J.C. Controlling cyanotoxin occurrence. In Toxic Cyanobacteria in Water; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Deas, M.L.; Tanaka, S.K.; Limanto, E.; Miao, E. Pilot Testing of. Environmentally-Safe Algaecide on Copco reservoir Water—2011 Study Results; California for PacifiCorp: Portland, OR, USA, 2012. [Google Scholar]
- Deas, M.L.; Vaughn, J.C.; Tanaka, S.K. Algaecide Pilot Study: Copco Reservoir; California for PacifiCorp: Portland, OR, USA, 2009. [Google Scholar]
- Qian, H.; Hu, B.; Yu, S.; Pan, X.; Wu, T.; Fu, Z. The Effects of Hydrogen Peroxide on the Circadian Rhythms of Microcystis aeruginosa. PLoS ONE 2012, 7, e33347. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Kong, H.; Gao, Y.; Wu, M.; Kong, F. Low concentrations of polycyclic aromatic hydrocarbons promote the growth of Microcystis aeruginosa. J. Hazard. Mater. 2012, 237–238, 371–375. [Google Scholar] [CrossRef]
- Sandrini, G.; Piel, T.; Xu, T.; White, E.; Qin, H.; Slot, P.C.; Huisman, J.; Visser, P.M. Sensitivity to hydrogen peroxide of the bloom-forming cyanobacterium Microcystis PCC 7806 depends on nutrient availability. Harmful Algae 2020, 99, 101916. [Google Scholar] [CrossRef] [PubMed]
- Krippner, R.; Staples, J. Suspected Allergy to Artemether—Lumefantrine Treatment of Malaria. J. Travel Med. 2003, 10, 303–305. [Google Scholar] [CrossRef]
- Mahapatra, A.D.; Bhowmik, P.; Banerjee, A.; Das, A.; Ojha, D.; Chattopadhyay, D. Ethnomedicinal Wisdom: An Approach for Antiviral Drug Development. In New Look to Phytomedicine, Khan, M.S.A., Ahmad, I., Chattopadhyay, D., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 35–61. [Google Scholar]
- Kim, J.K.; Kim, K.H.; Shin, Y.C.; Jang, B.-H.; Ko, S.-G. Utilization of traditional medicine in primary health care in low- and middle-income countries: A systematic review. Health Policy Plan. 2020, 35, 1070–1083. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, X.; Wu, Z.; Sun, T.; Tong, Y. Recent Advances in Cyanotoxin Synthesis and Applications: A Comprehensive Review. Microorganisms 2023, 11, 2636. [Google Scholar] [CrossRef]
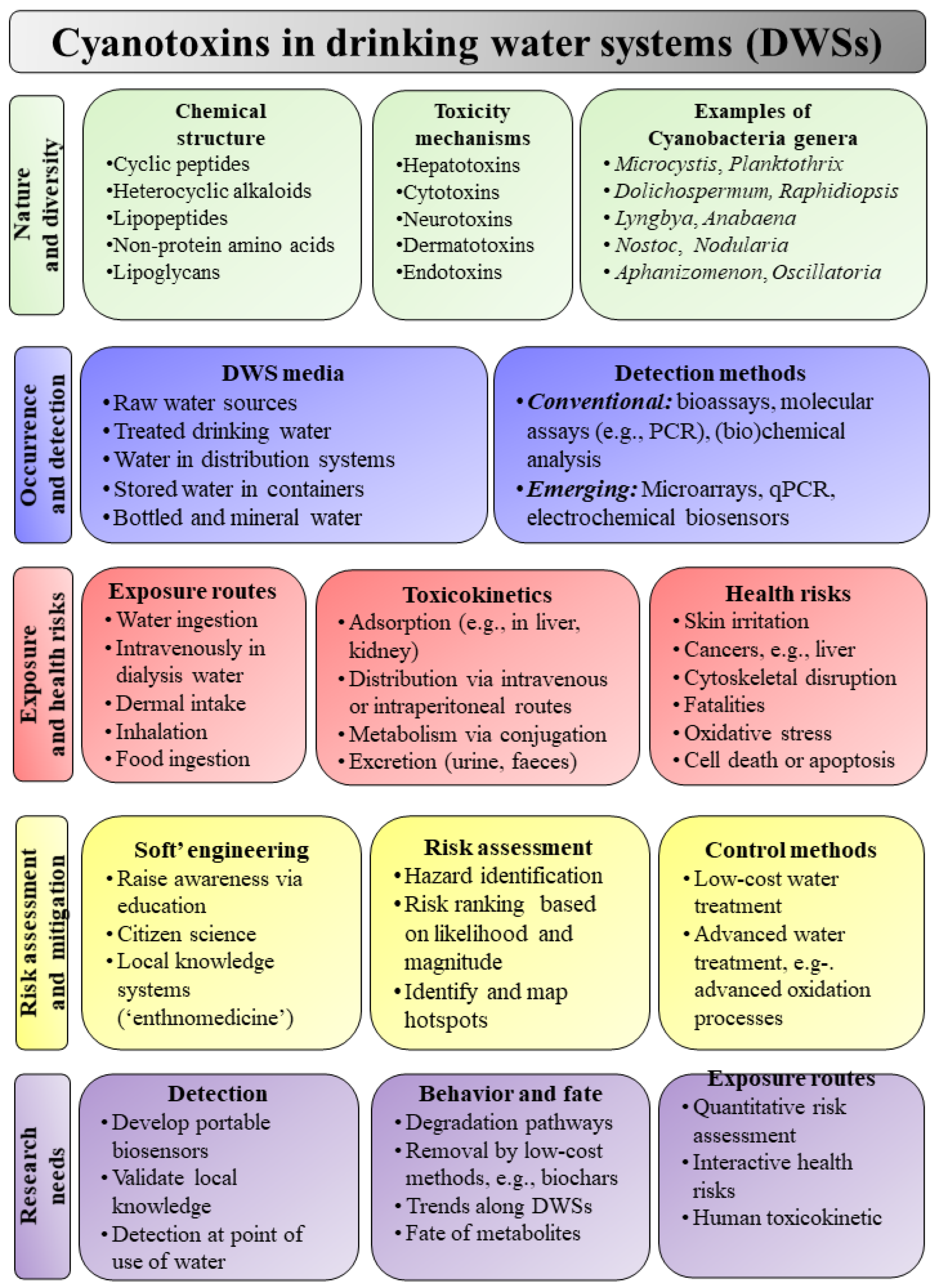
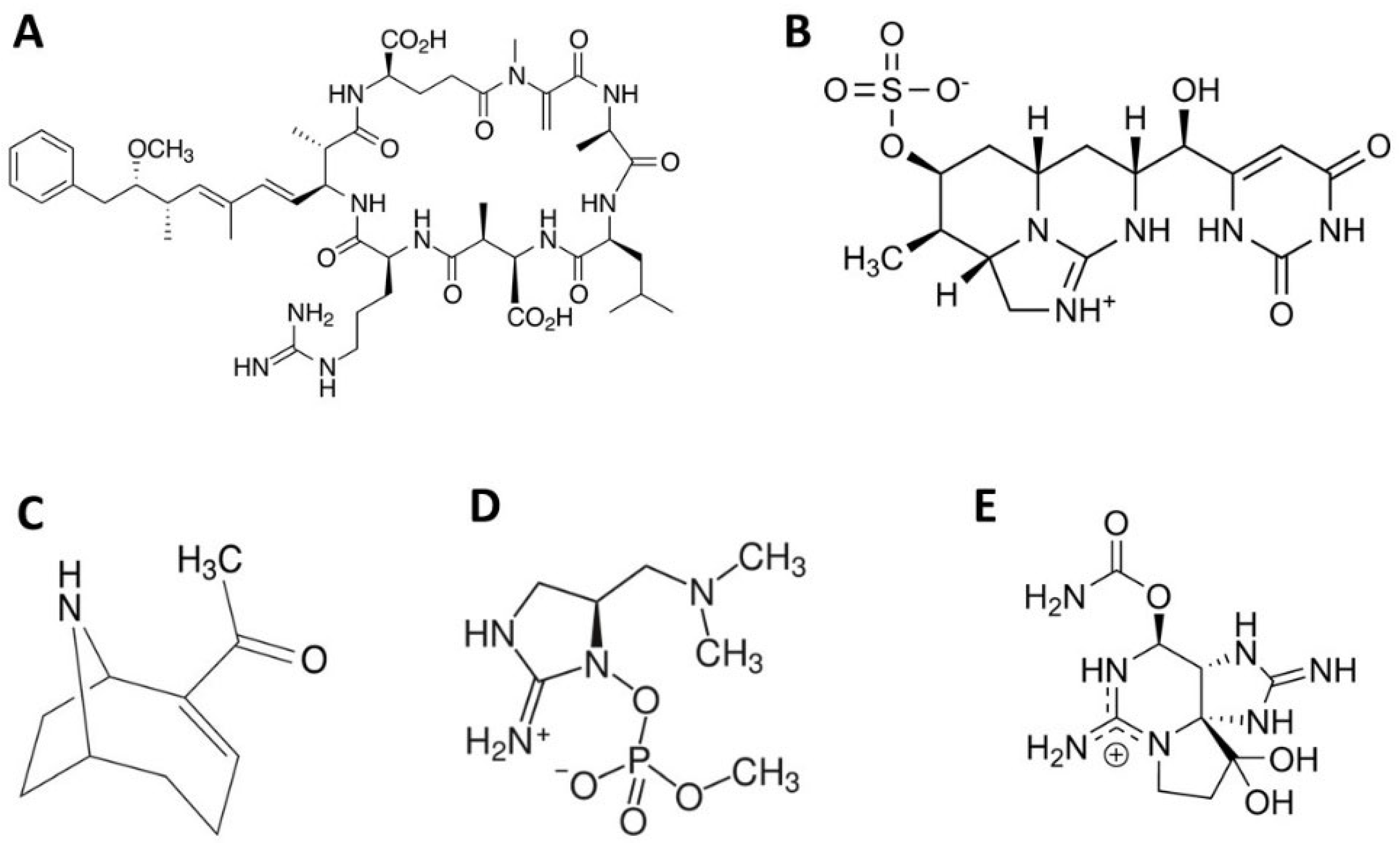
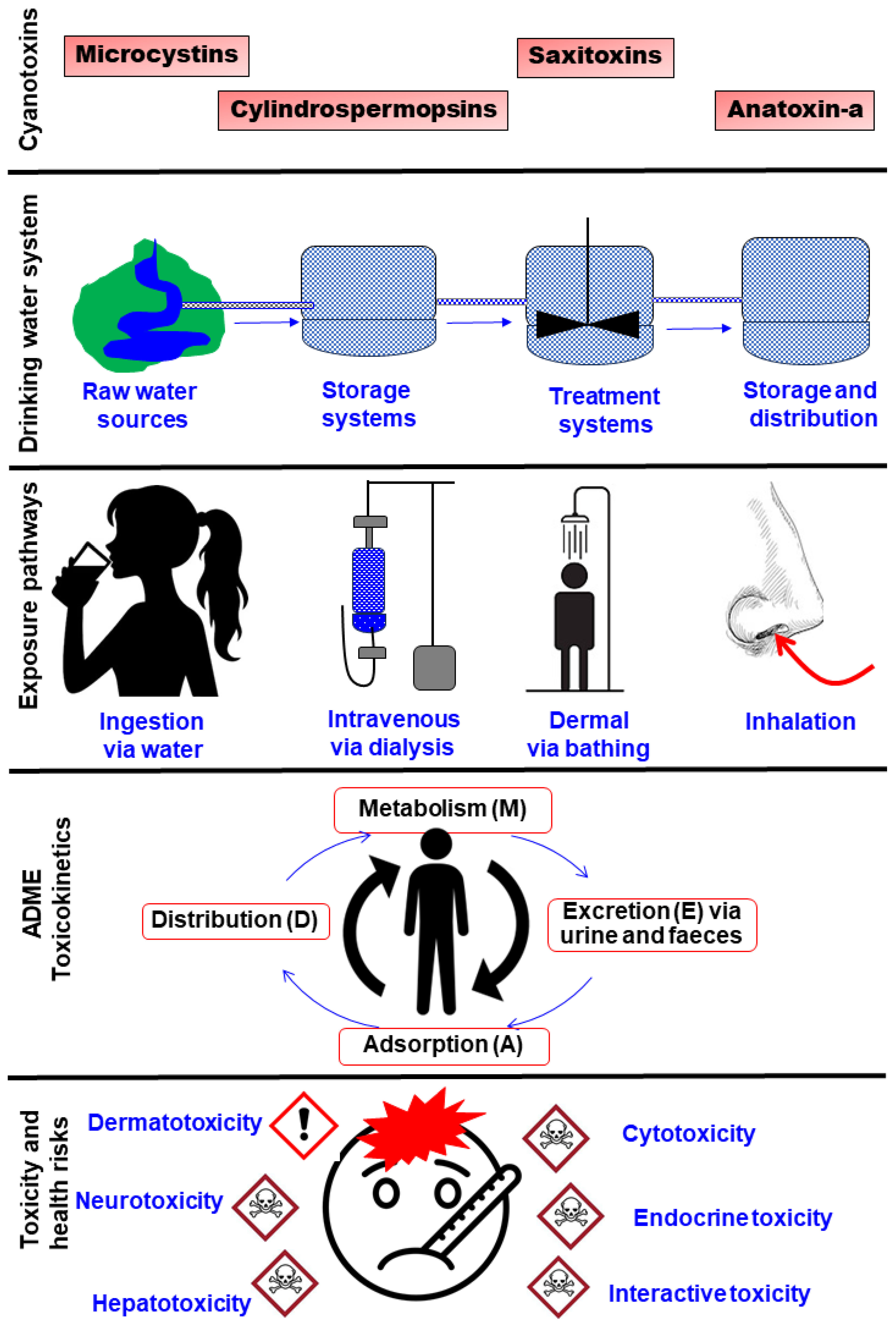
| Method | Key Capabilities | Merits | Demerits | Reference |
|---|---|---|---|---|
| Conventional PCR | Qualitative presence/absence test | Highly qualitative | Low sensitivity | [28] |
| Electrochemical biosensors | Analysis of low concentration of MCs | High recovery rates and low detection limits can be integrated into smartphones | Affected by temperature, pH, and ion concentration | [29] |
| ELISA | Detects 100 MC congeners | Applicable for field screening | Non-congener specific, low selectivity, cross-reactivity | [30] |
| GC/FID or MS | Uses gas chromatography | Inexpensive, sensitive | Affected by the co-occurrence of cyanobacterial species, requires equipment setup, and requires expertise | [30] |
| HPCE | Separates based on charge and molecular mass | Fast, easily applicable for the separation of cyanotoxin variants | Highly expensive | [31] |
| HPLC- (-MS, MS/MS, UV, PDA) | Can be coupled with other techniques for reliable, reproducible results | High congener resolution | Low selectivity, problematic quantitation, and high sample matrix interference | [32] |
| LC/ESI-MS/MS | Precisely and accurately identifies specific MC congeners | Low matrix interference | Limited standards | [33] |
| LC/TOF/MS | Precisely and accurately identifies specific MC congeners | Minimizes matrix interference | Limited standards for congeners | [33] |
| Microarrays | High-throughput gene expression studies, applicable in monitoring and tracking | Efficient, quick, and simultaneous analysis | High cost | [34] |
| PPIA | Specialized colourimetry for MC detection | Low instrument requirements | Cannot distinguish MC variants, low sensitivity | [23] |
| qPCR | Detects expression of toxin-encoding genes | Improved sensitivity | The presence of toxin-encoding genes does not translate to transcription, translation, and expression | [35] |
| Whole organism assay, e.g., mouse bioassay | May employ microbes, invertebrates, vertebrates, cell cultures, plants, and plant extracts | Can be calibrated against specific variants, highly qualitative | Low sensitivity, low specificity (no toxin identity), and not suitable for routine detection | [36] |
| Cyanotoxin | Exposure route | Toxicity | Mode of Action | Reference |
|---|---|---|---|---|
| Microcystin (MC) | Reservoir water | Toxin covalently binds and inhibits catalytic subunits on protein PP1 and PP2A, causing hyper-phosphorylation of cellular microtubules, hence loss of cellular structure. | MC-LR daily intake of 2.03 µg/L was associated with liver damage in children. Causes cellular apoptosis. | [37] |
| MC | Water used for dialysis treatments | MCs disrupted the liver plate pattern. MCs are inhibitors of eukaryotic protein serine/threonine phosphatases 1 and 2A. | Liver cell damage | [38] |
| MC | Contact and swallowing MC-contaminated reservoir water | Pulmonary intravascular formation of protein deposits and removal of platelets from circulation. | Liver damage, pneumonia, and pulmonary thrombosis. Death. | [39] |
| Microcystin-LR (MC-LR) | Injection | Intrahepatic hemorrhaging. MC-LR covalently binds to protein phosphatase 1/2A. | MC-LR dose-dependent hepatocellular hypertrophy, degradation and necrosis. Hepatic inflammation. Fibrosis in the livers of rats. | [40] |
| MC-LR plus thioacetamide (TAA) (MC-LR/TAA) | Drinking contaminated water | Inhibits serine/threonine phosphatase activity, enhances proliferative activity, or activates HSCs to transform to a myofibroblast phenotype. | Liver inflammation. | [41] |
| Saxitoxin | Contact with water during recreational activities | Blocking sodium channels in nerve axons. Inhibits the transfer of excitatory signals. | Causes paralysis of the respiratory muscles and death by respiratory failure. | [42] |
| Cylindrospermopsin (CYN) | Contact and drinking CYN-contaminated water | Causes oxidative stress in human neutrophils, eventually leading to lipid peroxidation and decreased cell survival. | Apoptosis in human T-lymphocytes. | [43] |
| CYN | Intraperitoneal administration | Causes oxidative stress in human neutrophils, eventually leading to lipid peroxidation and decreased cell survival. | Human hepatoblastoma and human colon adenocarcinoma cells. | [44] |
| MC | Drinking contaminated lake water and food | MC-LR leads to hepatic steatosis with molecular alterations in circadian rhythm regulation, lipid metabolic processes, and the cell cycle pathway. | Liver damage and lipid metabolism dysfunction. Long-term exposure leads to non-alcoholic fatty liver disease (NAFLD) | [45] |
| MC | Injection | Damages diabetes genes and other proteins, e.g., Ppp3ca, Ide, Marcks, Pgk1, and Ndufs4. | Increased risk of diabetes in humans. Reduction in blood insulin levels. | [46] |
| MC-LR | Human hepatocellular carcinoma cell line (HepG2) was exposed to solutions of MC-LR | Under normoxic conditions, MC-LR stimulates the proliferation of the HepG2 cell line, promoting liver tumor and liver cancer cell growth. Under hypoxic conditions, MC-LR induced apoptosis in the HepG2 cell line by inducing prolonged oxidative stress. | Liver cancer | [47] |
| MC-LR | Oral intake of MC-LR-contaminated water | MC-LR causes alterations in TBARS, superoxide dismutase (SOD) activity, and glutathione content in the liver and intestine of mice. | Exposure to 50 mg MC-LR/kg every 48 h generates significant damage to the liver and intestine. | [48] |
| Cyanotoxin | Receptor Organ, Tissue or Process | Toxicity | Reference |
|---|---|---|---|
| Hepatotoxicity | |||
| Microcystin-LR (MC-LR) and dehydrobutyrine (Dhb)-containing MC variant [Asp3, ADMAdda5, Dhb7] MC-HtyR isolated from Nostoc spp. | In vivo phosphoprotein phosphatase (PPP) family (PP1, PP2A, PPP4, and PPP5) |
| [49] |
| Microcystinss (MCLR, LA, LF, LW, LY) with changed Z4 residues | Protein phosphatase 2A |
| [50] |
| MC-LR (MC-LR) and Cylindrospermopsin (CYN) | Salmonella typhimurium, L5178Y Tk+/− cells, and Caco-2 cells |
| [51] |
| MC-LR | Microfilament depolarization and expression of microRNA-451a (miR-451a) in HL7702 liver cells |
| [52] |
| Neurotoxicity | |||
| CYN | The skin of albino Californian rabbits |
| [53] |
| CYN | Abdominal skin of Balb/c mice exposed to 100 µg of CYN mL−1 via topical application |
| [54] |
| Cytotoxicity | |||
| Interactive cytotoxic effects of CYN and a pesticide (chlorpyrifos) | Differentiated SH-SY5Y human neuronal (neuroblastoma) cells |
| [55] |
| Variants of cyanobacterial extracts (crude MC-containing, purified MC-containing, and non-MC-containing extracts) | Cultured human lymphocytes |
| [56] |
| Dermatotoxicity | |||
| Extracts of M. aeruginosa (non-toxic strain), A. circinalis, N. spumigena M. aeruginosa (toxic strain), A. incerta, and C. raciborskii | Skin of human volunteers |
| [57] |
| Miscellaneous toxicity effects | |||
| MC-leucine arginine (MC-LR) | DNA |
| [58] |
| MC-LR | Interaction of MC-LR with proteins and DNA |
| [59] |
| Combination of serum MCs, plasma As, and Cd | Human kidneys |
| [60] |
| Cyanotoxin | Receptor Organ, Tissue, or Process | Effect | Reference |
|---|---|---|---|
| Conventional methods | |||
| North America | Coagulation and sand filtration | Reduced intracellular cyanotoxins by 59–97%, with most MC removal occuring during coagulation, filtration, and settling. | [61] |
| Egypt | Sedimentation/ sand filtration and coagulation (flocculation) | Sedimentation, coagulation, and filtration removed 91–98.9% of MCs, but flocculation may release MCs. Chlorination partially degraded toxins, sometimes exceeding WHO limits. | [62] |
| Egypt | Coagulation, sedimentation, and flocculation | Removed various toxin-producing Cyanobacteria, but O. limnetica cells were only partially removed. | [62] |
| China | Coagulation (flocculation) with flocculants and coagulants, including ferric chloride and aluminum salts | Removal of cyanotoxins by 90% from drinking water. | [63] |
| Canada | Clarification, coagulation, and filtration | Cyanotoxin-producing Aphanizomenon cells were poorly removed by coagulation. Pseudanabaena and Anabaena were efficiently removed from the water by filtration and clarification. | [64] |
| Canada | Coagulation | 90% of intracellular MCs were removed. Removal of sludge after coagulation was recommended to avoid the re-release of MCs to water. | [65] |
| Egypt | Rapid sand filtration and disinfection methods | Rapid sand filtration of drinking water partially removed Microcystis and Pseudanabaena limnetica (formerly Oscillatoria limnetica) (ranging from 25 to 34%). O. limnetica cells were reported to resist disinfection, which is applied in the final stages of water treatment, and disinfection only reduced O. limnetica by between 25 and 66.7%. | [66] |
| Portugal | Ozonation and coagulation | 2.5–7.9% and 6.8–11.7% of microcystins (MCs) were removed by coagulation before and after ozonation, respectively. | [67] |
| China | Ferrate oxidation | Ferrate oxidation removed up to 98% of cyanotoxins, with efficiency influenced by contact time, dosage, and pH. A 30 min contact period at 40 mg/L ferrate and pH 6–10 yielded optimal results. | [68] |
| China | Chlorination and UV application | Chlorine (0.2–2.0 mg/L) and ultraviolet (UV) treatment damaged up to 96.6% of M. aeruginosa cells and degraded microcystin-LR (MC-LR_ by 69–83%. Combined UV/chlorine treatment was most effective with removal percentages of MC-LR between 68.9 and 82.7% in 15 min. | [69] |
| Belgium | Dissolved air flotation | Air flotation removed 40–80% of MCs, 90–100% of Anabaena, and 30% of Planktothrix cells. | [70] |
| Canada | Sand and graphitized sand filtration | Graphitized sand filtration reduced MC-LR to <0.61 μg/L (within WHO limits), outperforming standard sand filtration at a lower cost (CAD 160 vs. 6000). | [71] |
| France | Rapid and slow sand filtration | Bacteria-assisted filtration removed up to 99% of algal cells and toxins, offering a low-cost potable water solution. | [72] |
| South Australia | Biological sand filtration | MC-LR and microcystin-LA MC-LA were completely removed from drinking water. | [73] |
| Membrane-based methods/Adsorption (carbon-based methods) | |||
| Germany | Ultrafiltration | Membranes removed 98% of intracellular MCs and >96% anatoxin-a, eliminating both toxins and toxin-producing microbes. | [74] |
| Canada | Granular activated carbon (GAC) and powdered activated carbon (PAC) adsorbents | GAC reduced MC-LR from up to 47 to 1 μg/L; PAC (100 mg/L) removed 86%, lowering MC-LR from 22 to 3 μg/L. | [75] |
| South Africa | PAC (tyre-based | Tyre-based PAC achieved 100% MC-LR removal at pH 4, 10,000 mg/L carbon, in 34 min. | [76] |
| Switzerland | Gravity-driven membrane (GDM) | GDM systems removed M. aeruginosa and lowered cyanotoxins to <1 μg/L within 15 days. | [77] |
| Australia | Microfiltration and ultrafiltration | Membranes removed >98% of intracellular M. aeruginosa cyanotoxins. | [78] |
| China | Nanomaterial algacidal sheet | Algaecidal sheets removed 56–89% of M. aeruginosa and Anabaena within one day. | [79] |
| Australia | GAC | GAC removed up to 95% of anatoxin and extracellular MCs. | [80] |
| Brazil | GAC coupled with Fenton oxidation/flocculation/coagulation/sedimentation | GAC columns adsorbed 4.15 μg/g MCs; combined Fenton oxidation and GAC effectively purified water to safe limits. | [81] |
| Germany | Ultrafiltration | Membranes removed 98% of MCs and >96% anatoxin-a, eliminating toxins and producers. | [74] |
| Canada | Granular activated carbon (GAC) and powdered activated carbon (PAC) adsorbents | GAC decreased MC-LR to 1 μg/L; PAC (100 mg/L) reduced MC-LR from 22 to 3 μg/L. | [75] |
| South Africa | PAC (tyre-based) | Tyre-based PAC removed 100% MC-LR at optimal conditions (pH 4, 10,000 mg/L, 34 min). | [76] |
| Advanced oxidation technologies for the degradation of cyanotoxins | |||
| Canada | Potassium permanganate oxidation | Up to 95% of MCs were removed from drinking water. | [65] |
| China | KMnO4 pre-oxidation | MnO4 combined with sand filtration or Mn-oxidizing bacteria (e.g., Pseudomonas) enhanced the removal of MC-LR and BP-4 via transformation of Mn2+ to Mn oxides. | [82] |
| China | •OH oxidation | •OH (1 mg/L) pre-treatment, alone or with 0.5 mg/L NaClO, inactivated blooms and maintained disinfection byproducts (DBPs) within Chinese drinking water safety limits. | [83] |
| China | •OH oxidation | In a 480 m3/day plant, •OH (0.88 mg/L) rapidly inactivated algae; combined with ClO2 and sand filtration, it met all Chinese water quality standards. | [84] |
| German | Ozonation | Ozone (1.5 mg/L) degraded M. aeruginosa toxins; as little as 0.2 mg/L oxidized MC-LR below detection within seconds to minutes, reducing toxicity. | [85] |
| Australia | Ozonation | Ozone (0.2 mg/L) degraded MC-LR below high-performance liquid chromatography (HPLC) detection limits within a short period of time (sec to min). | [86] |
| United Kingdom | TiO2 and UV | TiO2 and UV removed MCs in under 45 min. | [87] |
| China | Ferrate (VI) and UV | Ferrate (VI) (0.08–0.17 mmol/L) with UV degraded up to 100% of MC-LR. | [88] |
| Biodegradation | |||
| Egypt | Biodegradation by Bacillus sp. | Up to 300 μg/L of CYN was biodegraded at rates from 1.25 to 50 μg/L/day. | [89] |
| Poland | Biodegradation by Aeromonas sp. | CYN biodegradation by Aeromonas sp. was 100% at pH 7 and 25–30 °C but dropped to 0% at pH 11 and colder temperatures. | [90] |
| New Zealand | Biodegradation by Sphingomonas bacteria | Sphingomonas species in a ceramic-supported bioreactor removed up to 100% of MCs. | [91] |
| Methods based on plant coagulants | |||
| Vietnam | Use of Moringa oleifera (drumstik tree) | Plant-based coagulants removed up to 97% of turbidity (15 g/L, pH 6.8) and 86–94% at pH 6.2. | [92] |
| Canada | Use of cactus | Cactus species reduced turbidity by 92–98% and lowered pH from 8.89 to 6. | [93] |
| Morocco | Opuntia ficus-indica and Vicia faba seed coagulants | At 0.5–1 g/L and pH 5, plant coagulants removed up to 85% of M. aeruginosa and about 80% of intracellular cyanotoxins. | [94] |
| Brazil | Coagulation using M. oleifera/flocculation/dissolved air flotation | Combining plant-based methods effectively removed M. aeruginosa from water. | [95] |
| Brazil | M. oleifera coagulants | M. oleifera coagulants reduced turbidity, suspended matter, and chlorophyll-a by 40–60%. | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marumure, J.; Gwenzi, W.; Makuvara, Z.; Simbanegavi, T.T.; Alufasi, R.; Goredema, M.; Gufe, C.; Karidzagundi, R.; Rzymski, P.; Halabowski, D. Global Occurrence of Cyanotoxins in Drinking Water Systems: Recent Advances, Human Health Risks, Mitigation, and Future Directions. Life 2025, 15, 825. https://doi.org/10.3390/life15050825
Marumure J, Gwenzi W, Makuvara Z, Simbanegavi TT, Alufasi R, Goredema M, Gufe C, Karidzagundi R, Rzymski P, Halabowski D. Global Occurrence of Cyanotoxins in Drinking Water Systems: Recent Advances, Human Health Risks, Mitigation, and Future Directions. Life. 2025; 15(5):825. https://doi.org/10.3390/life15050825
Chicago/Turabian StyleMarumure, Jerikias, Willis Gwenzi, Zakio Makuvara, Tinoziva T. Simbanegavi, Richwell Alufasi, Marvelous Goredema, Claudious Gufe, Rangarirayi Karidzagundi, Piotr Rzymski, and Dariusz Halabowski. 2025. "Global Occurrence of Cyanotoxins in Drinking Water Systems: Recent Advances, Human Health Risks, Mitigation, and Future Directions" Life 15, no. 5: 825. https://doi.org/10.3390/life15050825
APA StyleMarumure, J., Gwenzi, W., Makuvara, Z., Simbanegavi, T. T., Alufasi, R., Goredema, M., Gufe, C., Karidzagundi, R., Rzymski, P., & Halabowski, D. (2025). Global Occurrence of Cyanotoxins in Drinking Water Systems: Recent Advances, Human Health Risks, Mitigation, and Future Directions. Life, 15(5), 825. https://doi.org/10.3390/life15050825










