The Hypotension Prediction Index in Free Flap Transplant in Head and Neck Surgery: Protocol of a Prospective Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Randomization
2.3. Blinding
2.4. Duration
2.5. Study Groups
2.6. Patient Enrolment
2.6.1. Inclusion Criteria
2.6.2. Exclusion Criteria
- Patients under 18 years;
- Lack of health insurance;
- Pregnancy;
- A known history of any of the following conditions:
- -
- congenital heart disease;
- -
- severe aortic and/or mitral stenosis;
- -
- heart failure and ejection fraction < 35%;
- -
- persistent atrial fibrillation and other arrhythmias impairing APCO monitoring.
2.7. Outcomes of the Study
2.7.1. Primary Endpoint
2.7.2. Secondary Endpoints
2.8. Patient and Public Involvement
3. Study Protocol
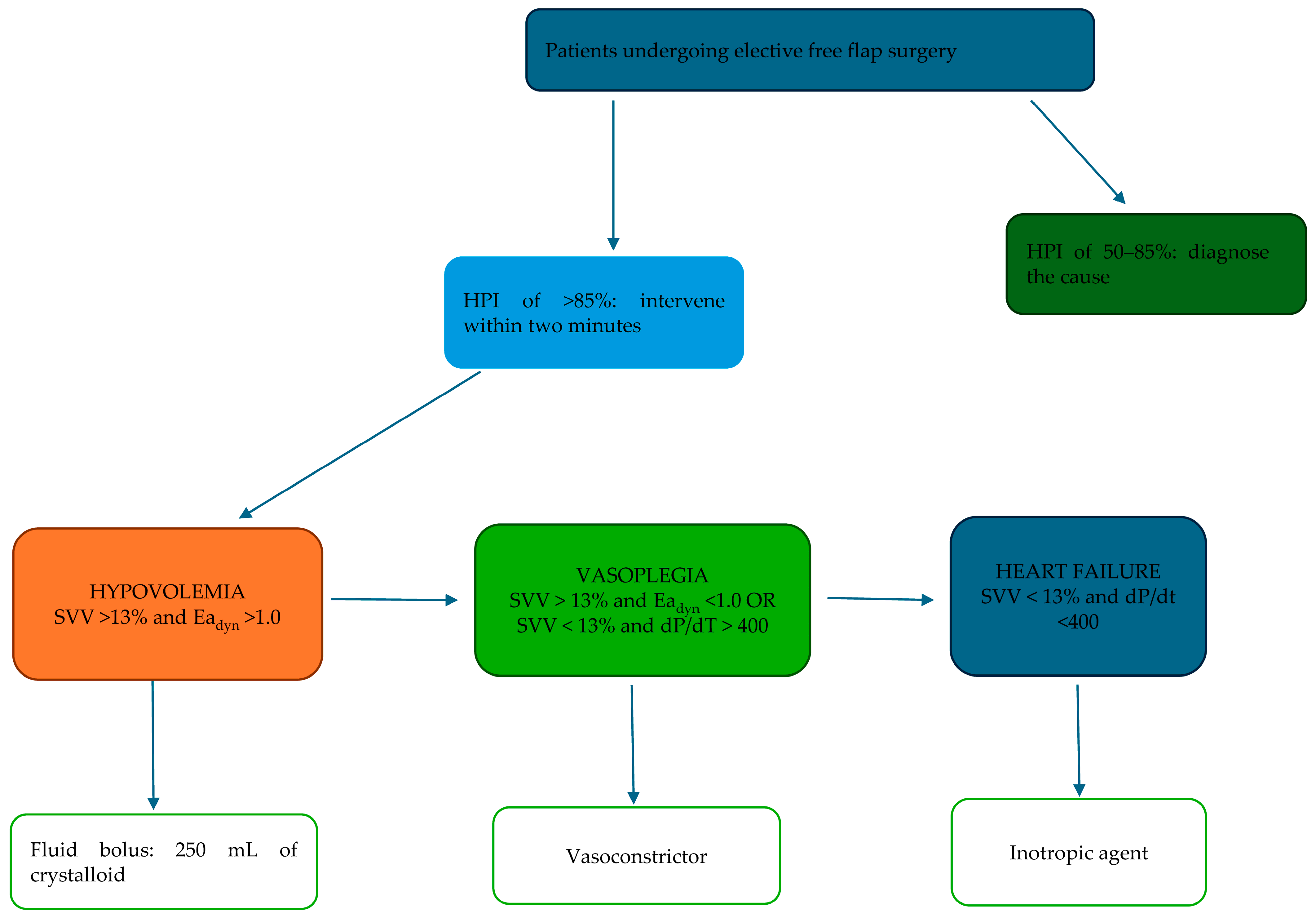
3.1. Interventions
3.1.1. Group A: Standard Therapy
3.1.2. Group B: HPI Monitoring
3.2. Measurements
3.2.1. Preoperative Data
3.2.2. Perioperative Data
3.2.3. Follow-Up
4. Statistical Analysis
4.1. Sample Size Calculation
4.2. Study Populations
4.3. Study Groups
4.4. Statistical Analysis
5. Study Phases
5.1. Enrolment
5.2. Treatment Phase
5.3. Daily Assessment
5.4. Data Management
5.5. Follow-Up
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bijker, J.B.; van Klei, W.A.; Kappen, T.H.; van Wolfswinkel, L.; Moons, K.G.M.; Kalkman, C.J. Incidence of intraoperative hypotension as a function of the chosen definitione: Literature definitions applied to a retrospective cohort using automated data collection. Anesthesiology 2007, 107, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Awad, H.; Alcodray, G.; Raza, A.; Boulos, R.; Essandoh, M.; Bhandary, S.; Dalton, R. Intraoperative Hypotension—Physiologic Basis and Future Directions. J. Cardiothorac. Vasc. Anesth. 2022, 36, 2154–2163. [Google Scholar] [CrossRef]
- Gu, W.-J.; Hou, B.-L.; Kwong, J.S.; Tian, X.; Qian, Y.; Cui, Y.; Hao, J.; Li, J.-C.; Ma, Z.-L.; Gu, X.-P. Association between intraoperative hypotension and 30 day mortality, major adverse cardiac events, and acute kidney injury after non cardiac surgery: A meta-analysis of cohort studies. Int. J. Cardiol. 2018, 258, 68–73. [Google Scholar] [CrossRef]
- Monk, T.G.; Bronsert, M.R.; Henderson, W.G.; Mangione, M.P.; Sum-Ping, S.T.J.; Bentt, D.R.; Nguyen, J.D.; Richman, J.S.; Meguid, R.A.; Hammermeister, K.E. Association between intraoperative hypotension and hypertension and 30-day postoperative mortality in noncardiac surgery. Anesthesiology 2015, 123, 307–319. [Google Scholar] [CrossRef]
- Mascha, E.J.; Yang, D.; Weiss, S.; Sessler, D.I. Intraoperative mean arterial pressure variability and 30-day mortality in patients having noncardiac surgery. Anesthesiology 2015, 123, 79–91. [Google Scholar] [CrossRef]
- Devereaux, P.J.; Yang, H.; Yusuf, S.W.; Guyatt, G.H.; E Leslie, K.; Villar, J.C.; Xavier, D.; Chrolavicius, S.; Greenspan, L.; Pogue, J.; et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): A randomised controlled trial. Lancet 2008, 371, 1839–1847. [Google Scholar] [CrossRef] [PubMed]
- Salmasi, V.; Maheshwari, K.; Yang, D.; Mascha, E.J.; Singh, A.; Sessler, D.I.; Kurz, A. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: A retrospective cohort analysis. Anesthesiology 2017, 126, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.Y.; Wijeysundera, D.N.; Tait, G.A.; Beattie, W.S. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology 2015, 123, 515–523. [Google Scholar] [CrossRef]
- Kass, J.L.; Lakha, S.; Levin, M.A.; Joseph, T.; Lin, H.; Genden, E.M.; Teng, M.S.; Miles, B.A.; DeMaria, S. Intraoperative hypotension and flap loss in free tissue transfer surgery of the head and neck. Head Neck 2018, 40, 2334–2339. [Google Scholar] [CrossRef]
- Vyas, K.; Wong, L. Intraoperative management of free flaps: Current practice. Ann. Plast. Surg. 2014, 72, S220–S223. [Google Scholar] [CrossRef]
- Ettinger, K.S.; Arce, K.; Lohse, C.M.; Peck, B.W.; Reiland, M.D.; Bezak, B.J.; Moore, E.J. Higher perioperative fluid administration is associated with increased rates of complications following head and neck microvascular reconstruction with fibular free flaps. Microsurgery 2017, 37, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Benes, J. Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: Results of prospective randomized study. Crit. Care 2010, 14, R118. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.; Boldt, J.; Mengistu, A.M.; Röhm, K.D.; Suttner, S. Goal-directed intraoperative therapy based on autocalibrated arterial pressure waveform analysis reduces hospital stay in high-risk surgical patients: A randomized, controlled trial. Crit. Care 2010, 14, R18. [Google Scholar] [CrossRef]
- Lees, N.; Hamilton, M.; Rhodes, A. Clinical review: Goal-directed therapy in high risk surgical patients. Crit. Care 2009, 13, 231. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Chaudhari, S.H.; Nagar, V.; Jain, D.; Bansal, A.; Dutt, A. Prospective analysis of goal-directed fluid therapy vs conventional fluid therapy in perioperative outcome of composite resections of head and neck malignancy with free tissue transfer. Indian J. Anaesth. 2021, 65, 606–611. [Google Scholar] [CrossRef]
- Hatib, F.; Jian, Z.; Buddi, S.; Lee, C.; Settels, J.; Sibert, K.; Rinehart, J.; Cannesson, M. Machine-learning algorithm to predict hypotension based on high-fidelity arterial pressure waveform analysis. Anesthesiology 2018, 129, 663–674. [Google Scholar] [CrossRef]
- Wijnberge, M.; Geerts, B.F.; Hol, L.; Lemmers, N.; Mulder, M.P.; Berge, P.; Schenk, J.; Terwindt, L.E.; Hollmann, M.W.; Vlaar, A.P.; et al. Effect of a Machine Learning-Derived Early Warning System for Intraoperative Hypotension vs Standard Care on Depth and Duration of Intraoperative Hypotension During Elective Noncardiac Surgery: The HYPE Randomized Clinical Trial. JAMA 2020, 323, 1052–1060. [Google Scholar] [CrossRef]
- Maheshwari, K.; Shimada, T.; Yang, D.; Khanna, S.; Cywinski, J.B.; Irefin, S.A.; Ayad, S.; Turan, A.; Ruetzler, K.; Qiu, Y.; et al. Hypotension Prediction index for orevention of hypotension during moderate to high risk noncardiac surgery. Anesthesiology 2020, 133, 1214–1222. [Google Scholar] [CrossRef]
- Shin, B.; Maler, S.A.; Reddy, K.; Fleming, N.W. Use of the hypotension prediction index during cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2021, 35, 1769–1775. [Google Scholar] [CrossRef]
- Grundmann, C.D.; Wischermann, J.M.; Fassbender, P.; Bischoff, P.; Frey, U.H. Hemodynamic monitoring wiht hypotension prediction index versus arterial waveform analysis alone and incidence of perioperative hypotension. Acta Anesthesiol. Scand. 2021, 65, 1404–1412. [Google Scholar] [CrossRef]
- Schenk, J.; Wijnberge, M.; Maaskant, J.M.; Hollmann, M.W.; Hol, L.; Immink, R.V.; Vlaar, A.P.; van der Ster, B.J.; Geerts, B.F.; Veelo, D.P. Effect of hypotension Predicition Index-Guided intraoperative haemodynamic care on depth and duration of postoperative hypotension: A sub-study of the Hypotension Prediction Trial. Br. J. Anesth. 2021, 127, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.J.; Vistisen, S.T.; Jian, Z.; Hatib, F.; Scheeren, T.W.L. Ability of an Arterial Waveform Analysis—Derived Hypotension Prediction Index to predict Future Hypotensive Events in Surgical Patients. Anesth. Analg. 2020, 130, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Schneck, E.; Schulte, D.; Habig, L.; Ruhrmann, S.; Edinger, F.; Markmann, M.; Habicher, M.; Rickert, M.; Koch, C.; Sander, M. Hypotension Prediction Index based protocolized haemodynamic management reduces the incidence and duration of ontraoperative hypotension in primary total hip arthroplasty: A single centre feasibility randomised blinded prospective interventional trial. J. Clin. Monit. Comput. 2020, 34, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Frassanito, L.; Sonnino, C.; Piersanti, A.; Zanfini, B.A.; Catarci, S.; Giuri, P.P.; Scorzoni, M.; Gonnella, G.L.; Antonelli, M.; Draisci, G. Performance of the Hypotension Prediction Index with Noninvasive Arterial Pressure Waveforms in Awake Cesarean Delivery Patients under spinal Anesthesia. Anesth. Analg. 2022, 134, 633–643. [Google Scholar] [CrossRef]
- Lorente, J.V.; Ripollés-Melchor, J.; Jiménez, I.; Becerra, A.I.; Mojarro, I.; Fernández-Valdes-Bango, P.; Fuentes, M.A.; Moreno, A.; Agudelo, M.E.; de la Vega, A.V.-P.; et al. Intraoperative hemodynamic optimization using the hypotension prediction index vs. goal-directed hemodynamic therapy during elective major abdominal surgery: The Predict-H multicenter randomized controlled trial. Front. Anesthesiol. 2023, 2, 1193886. [Google Scholar] [CrossRef]
- Szrama, J.; Gradys, A.; Bartkowiak, T.; Woźniak, A.; Nowak, Z.; Zwoliński, K.; Lohani, A.; Jawień, N.; Smuszkiewicz, P.; Kusza, K. The Incidence of Perioperative Hypotension in Patients Undergoing Major Abdominal Surgery with the Use of Arterial Waveform Analysis and the Hypotension Prediction Index Hemodynamic Monitoring-A Retrospective Analysis. J. Pers. Med. 2024, 14, 174. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sample Size Calculator: Determines the Minimum Number of Subjects for Adequate Study Power. Available online: https://clincalc.com/stats/samplesize.aspx (accessed on 26 September 2023).
- Keller, A. A new diagnostic algorithm for early prediction of vascular compromise in 208 microsurgical flaps using tissue oxygen saturation measurements. Ann. Plast. Surg. 2009, 62, 538–543. [Google Scholar] [CrossRef]
- Steele, M.H. Three-Year Experience Using Near Infrared Spectroscopy Tissue Oximetry Monitoring of Free Tissue Transfers. Ann. Plast. Surg. 2011, 66, 540–545. [Google Scholar] [CrossRef]
| Enrollment | Study Period | ||||
|---|---|---|---|---|---|
| Timepoint ** | D−1 | Surgical Day (d0) | Daily Follow-Up (d0 TO dhd) | Hospital Discharge (dhd) | Follow-Up (d30) |
| Enrollment: | |||||
| Eligibility screen | ✗ | ||||
| Written informed consent | ✗ | ||||
| Written and oral project explanation | ✗ | ||||
| Collection of patient demographic and comorbidity data | ✗ | ||||
| Allocation | ✗ | ||||
| Interventions: | |||||
| Group A | ✗ | ||||
| Group B (HPI) | ✗ | ||||
| ASSESSMENTS: | |||||
| Primary outcomes | ✗ | ||||
| Secondary outcomes | ✗ | ✗ | ✗ | ✗ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szrama, J.; Gradys, A.; Woźniak, A.; Nowak, Z.; Bartkowiak, T.; Lohani, A.; Zwoliński, K.; Koszel, T.; Kusza, K. The Hypotension Prediction Index in Free Flap Transplant in Head and Neck Surgery: Protocol of a Prospective Randomized Controlled Trial. Life 2025, 15, 400. https://doi.org/10.3390/life15030400
Szrama J, Gradys A, Woźniak A, Nowak Z, Bartkowiak T, Lohani A, Zwoliński K, Koszel T, Kusza K. The Hypotension Prediction Index in Free Flap Transplant in Head and Neck Surgery: Protocol of a Prospective Randomized Controlled Trial. Life. 2025; 15(3):400. https://doi.org/10.3390/life15030400
Chicago/Turabian StyleSzrama, Jakub, Agata Gradys, Amadeusz Woźniak, Zuzanna Nowak, Tomasz Bartkowiak, Ashish Lohani, Krzysztof Zwoliński, Tomasz Koszel, and Krzysztof Kusza. 2025. "The Hypotension Prediction Index in Free Flap Transplant in Head and Neck Surgery: Protocol of a Prospective Randomized Controlled Trial" Life 15, no. 3: 400. https://doi.org/10.3390/life15030400
APA StyleSzrama, J., Gradys, A., Woźniak, A., Nowak, Z., Bartkowiak, T., Lohani, A., Zwoliński, K., Koszel, T., & Kusza, K. (2025). The Hypotension Prediction Index in Free Flap Transplant in Head and Neck Surgery: Protocol of a Prospective Randomized Controlled Trial. Life, 15(3), 400. https://doi.org/10.3390/life15030400









