Neurological and Olfactory Disturbances After General Anesthesia
Abstract
1. Introduction
2. Review Methodology
3. General Anesthesia: Mechanisms and Neurological Implications
4. Anatomy and Physiology of the Olfactory System
5. Olfactory Dysfunction: Clinical Features and Etiology
6. Neurological and Olfactory Disturbances After General Anesthesia
7. Risk Factors and Vulnerable Populations
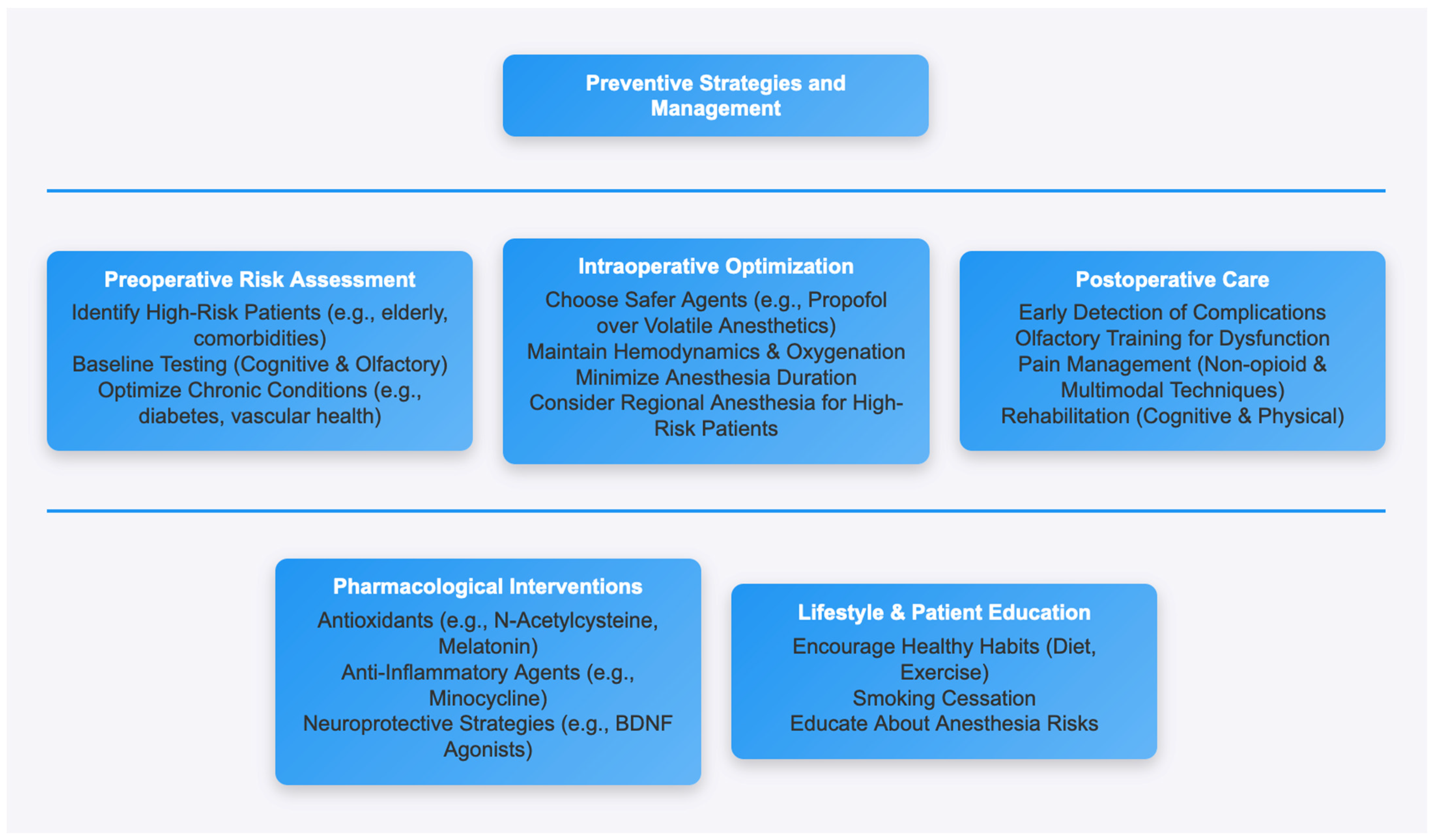
8. Preventive Strategies and Management
9. Future Directions and Research Opportunities
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GA | General anesthesia |
| NSAID | Non-steroidal anti-inflammatory drug |
| POCD | Postoperative cognitive dysfunction |
| GABA | Gamma-aminobutyric acid |
| CNS | Central nervous system |
| ORN | Olfactory receptor neuron |
| ROS | Reactive oxygen species |
| TNF-α | Tumor necrosis factor-Alpha |
| IL-6 | Interleukin 6 |
| APO-E | Apolipoprotein E |
| NMDA | N-methyl-D-aspartate |
References
- Monk, T.G.; Weldon, B.C.; Garvan, C.W.; Dede, D.E.; van der Aa, M.T.; Heilman, K.M.; Gravenstein, J.S. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology 2008, 108, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Evered, L.; Scott, D.A.; Silbert, B.; Maruff, P. Postoperative cognitive dysfunction is independent of type of surgery and anesthetic. Anesth. Analg. 2011, 112, 1179–1185. [Google Scholar] [CrossRef]
- Doty, R.L. Olfactory dysfunction and its measurement in the clinic and workplace. Int. Arch. Occup. Environ. Health 2001, 74, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.A.; Stevenson, R.J. Learning to Smell: Olfactory Perception from Neurobiology to Behavior; Johns Hopkins University Press: Baltimore, MD, USA, 2006. [Google Scholar]
- Schwob, J.E. Neural regeneration and the peripheral olfactory system. Anat. Rec. 2002, 269, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.S.; Johnson, T.; Kuipers, H.M.; Kristensen, D.; Siersma, V.D.; Vila, P.; Jolles, J.; Papaioannou, A.; Abildstrom, H.; Silverstein, J.H.; et al. Does anaesthesia cause postoperative cognitive dysfunction? A randomised study of regional versus general anaesthesia in 438 elderly patients. Acta Anaesthesiol. Scand. 2001, 45, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, J.; Christensen, K.B.; Lund, T.; Lohse, N.; Rasmussen, L.S. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology 2009, 110, 548–555. [Google Scholar] [CrossRef]
- Inouye, S.K.; Westendorp, R.G.; Saczynski, J.S. Delirium in elderly people. Lancet 2014, 383, 911–922. [Google Scholar] [CrossRef]
- Xie, Z.; Dong, Y.; Maeda, U.; Alfille, P.; Culley, D.J.; Crosby, G.; Tanzi, R.E. The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid beta protein levels. Anesthesiology 2006, 104, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Z.; Wang, H.; Dong, Y.; Shi, H.N.; Culley, D.J.; Crosby, G.; Marcantonio, E.R.; Tanzi, R.E.; Xie, Z. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann. Neurol. 2012, 71, 687–698. [Google Scholar] [CrossRef]
- Haxel, B.R.; Grant, L.; Mackay-Sim, A. Olfactory dysfunction after general anesthesia. J. Clin. Neurosci. 2014, 21, 1563–1565. [Google Scholar]
- Doty, R.L.; Kamath, V. The influences of age on olfaction: A review. Front. Psychol. 2014, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Ross, G.W.; Petrovitch, H.; Abbott, R.D.; Tanner, C.M.; Popper, J.; Masaki, K.; Launer, L.; White, L.R. Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann. Neurol. 2008, 63, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Planel, E.; Richter, K.E.; Nolan, C.E.; Finley, J.E.; Liu, L.; Wen, Y.; Krishnamurthy, P.; Herman, M.; Wang, L.; Schachter, J.B.; et al. Anesthesia leads to tau hyperphosphorylation through inhibition of phosphatase activity by hypothermia. J. Neurosci. 2008, 28, 3090–3097. [Google Scholar] [CrossRef]
- Murphy, C.; Jernigan, T.L.; Fennema-Notestine, C. Left hippocampal volume loss in Alzheimer’s disease is reflected in performance on odor identification: A structural MRI study. J. Int. Neuropsychol. Soc. 2003, 9, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.N.; Purdon, P.L.; Van Dort, C.J. General anesthesia and altered states of arousal: A systems neuroscience analysis. Annu. Rev. Neurosci. 2011, 34, 601–628. [Google Scholar] [CrossRef] [PubMed]
- Ing, C.; DiMaggio, C.; Whitehouse, A.; Hegarty, M.K.; Brady, J.; von Ungern-Sternberg, B.S.; Davidson, A.; Wood, A.J.J.; Li, G.; Sun, L.S. Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics 2014, 134, e818–e827. [Google Scholar] [CrossRef]
- Franks, N.P. General anaesthesia: From molecular targets to neuronal pathways of sleep and arousal. Nat. Rev. Neurosci. 2008, 9, 370–386. [Google Scholar] [CrossRef] [PubMed]
- Evered, L.; Silbert, B.; Knopman, D.S.; Scott, D.A.; DeKosky, S.T.; Rasmussen, L.S.; Oh, E.S.; Crosby, G.; Berger, M.; Eckenhoff, R.G. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery—2018. Anesthesiology 2018, 129, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Deiner, S.; Luo, X.; Lin, H.M.; Sessler, D.I.; Saager, L.; Sieber, F.E.; Lee, H.B.; Sano, M.; MacCormick Jankowski, C.; Leung, J.M.; et al. Intraoperative infusion of dexmedetomidine for prevention of postoperative delirium and cognitive dysfunction in elderly patients undergoing major elective noncardiac surgery: A randomized clinical trial. Jpn. Automob. Manuf. Assoc. Surg. 2019, 154, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Cibelli, M.; Fidalgo, A.R.; Terrando, N.; Ma, D.; Monaco, C.; Feldmann, M.; Takata, M.; Lever, I.J.; Nanchahal, J.; Fanselow, M.S.; et al. Role of interleukin-1β in postoperative cognitive dysfunction. Ann. Neurol. 2010, 68, 360–368. [Google Scholar] [CrossRef]
- Wei, H.; Liang, G.; Yang, H.; Wang, Q.; Hawkins, B.; Madesh, M.; Wang, S.; Eckenhoff, R.G. The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1,4,5-trisphosphate receptors. Anesthesiology 2010, 113, 1126–1138. [Google Scholar] [CrossRef] [PubMed]
- Mutch, W.A.C.; El-Gabalawy, R.M.; Graham, M.R. Postoperative delirium, learning, and anesthetic neurotoxicity: Some perspectives and directions. Front. Neurol. 2015, 6, 87. [Google Scholar] [CrossRef]
- Saczynski, J.S.; Marcantonio, E.R.; Quach, L.; Fong, T.G.; Gross, A.; Inouye, S.K.; Jones, R.N. Cognitive trajectories after postoperative delirium. N. Engl. J. Med. 2012, 367, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Galletti, B.; Rizzi, A.; Morini, G.; Spataro, G.; Moretti, A. The pathophysiology of olfactory dysfunction and its relationship to cerebral perfusion: A study with MRI. Eur. Arch. Otorhinolaryngol. 2009, 266, 1889–1893. [Google Scholar]
- Fong, T.G.; Davis, D.; Growdon, M.E.; Albuquerque, A.; Inouye, S.K. The interface between delirium and dementia in elderly adults. Lancet Neurol. 2015, 14, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.H.J.; Muniz-Terrera, G.; Keage, H.A.D.; Stephan, B.C.M.; Fleming, J.; Ince, P.G.; Matthews, F.E.; Cunningham, C.; Ely, E.W.; MacLullich, A.M.J.; et al. Association of delirium with cognitive decline in late life: A neuropathologic study of 3 population-based cohort studies. Jpn. Automob. Manuf. Assoc. Psychiatry 2017, 74, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Vacas, S.; Degos, V.; Feng, X.; Maze, M. The neuroinflammatory response of postoperative cognitive decline. Br. Med. Bull. 2013, 106, 161–178. [Google Scholar] [CrossRef]
- Creeley, C.E.; Dikranian, K.T.; Dissen, G.A.; Back, S.A.; Olney, J.W.; Brambrink, A.M. Isoflurane-induced apoptosis of neurons and oligodendrocytes in the fetal rhesus macaque brain. Anesthesiology 2013, 118, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Jevtovic-Todorovic, V.; Hartman, R.E.; Izumi, Y.; Benshoff, N.D.; Dikranian, K.; Zorumski, C.F.; Olney, J.W.; Wozniak, D.F. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J. Neurosci. 2003, 23, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.S.; Li, G.; Miller, T.L.; Salorio, C.; Byrne, M.W.; Bellinger, D.C.; Ing, C.; Park, R.; Radcliffe, J.; Hays, S.R.; et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. Jpn. Automob. Manuf. Assoc. 2014, 315, 2312–2320. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, G.M. The synaptic organization of the brain: An introduction to molecular neuroscience of olfaction. Science 2004, 306, 1776–1777. [Google Scholar]
- Graziadei, P.P.C.; Monti Graziadei, G.A. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J. Neurocytol. 1979, 8, 1–18. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, C.; Wu, X.; Dong, Y.; Culley, D.J.; Crosby, G.; Li, T.; Xie, Z. Different effects of anesthetic isoflurane on caspase-3 activation in young and aged mice. Front. Cell. Neurosci. 2013, 7, 129. [Google Scholar]
- Wilson, D.A.; Sullivan, R.M.; Rennaker, R.L.; Doucette, W.; Strauch, M. Central olfactory processing in health and disease. Trends Neurosci. 2020, 43, 610–624. [Google Scholar]
- Haxel, B.R.; Murrell, W.G.; Mackay-Sim, A. Olfactory dysfunction after general anesthesia: A case-control study. J. Clin. Anesth. 2015, 27, 525–530. [Google Scholar]
- Gottfried, J.A. Central mechanisms of odour object perception. Nat. Rev. Neurosci. 2010, 11, 628–641. [Google Scholar] [CrossRef] [PubMed]
- Platt, M.P.; Agalliu, D.; Cutforth, T. Getting to the heart of the matter: Direct visualization of angiogenesis in the olfactory system. J. Neurosci. 2014, 34, 12222–12223. [Google Scholar]
- Lamperti, M.; Romero, C.S.; Guarracino, F.; Cammarota, G.; Vetrugno, L.; Tufegdzic, B.; Lozsan, F.; Macias Frias, J.J.; Duma, A.; Bock, M.; et al. Preoperative assessment of adults undergoing elective noncardiac surgery: Updated guidelines from the European Society of Anaesthesiology and Intensive Care. Eur. J. Anaesthesiol. 2025, 42, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; McAuliffe, S.; Swain, C.A.; Ward, S.A.; Crosby, G.; Zheng, H.; Sherman, J.; Dong, Y.; Zhang, Y.; Sunder, N.; et al. Cerebrospinal fluid Aβ to tau ratio and postoperative cognitive change. Ann. Surg. 2014, 259, 155–161. [Google Scholar] [CrossRef]
- Wei, H.; Xie, Z. Anesthesia, calcium homeostasis and Alzheimer’s disease. Curr. Alzheimer Res. 2013, 10, 618–626. [Google Scholar]
- Doty, R.L.; Shaman, P.; Applebaum, S.L.; Giberson, R.; Siksorski, L.; Rosenberg, L. Smell identification ability: Changes with age. Science 1984, 226, 1441–1443. [Google Scholar] [CrossRef] [PubMed]
- Vennemann, M.M.; Hummel, T.; Berger, K. The association between smoking and smell and taste impairment in the general population. J. Neurol. 2008, 255, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Kobal, G.; Gudziol, H.; Mackay-Sim, A. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: An upgrade based on a group of more than 3,000 subjects. Eur. Arch. Otorhinolaryngol. 2007, 264, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef]
- Henkin, R.I.; Levy, L.M.; Lin, C.S. Taste and smell phantoms revealed by brain functional MRI (fMRI). J. Comput. Assist. Tomogr. 2003, 27, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Culley, D.J.; Dong, Y.; Zhang, G.; Zhang, B.; Moir, R.D.; Frosch, M.P.; Crosby, G.; Tanzi, R.E. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid beta-protein level in vivo. Ann. Neurol. 2010, 64, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Platt, M.P.; Hoang, A.N.; Goldstein, B.J. The role of inflammation in neuronal injury in the olfactory system. Laryngoscope 2011, 121, 2485–2492. [Google Scholar]
- Cattano, D.; Young, C.; Straiko, M.M.; Olney, J.W. Subanesthetic doses of propofol induce neuroapoptosis in the infant mouse brain. Anesth. Analg. 2008, 106, 1712–1714. [Google Scholar] [CrossRef]
- Gudziol, H.; Graul, J.; Bitter, T.; Guntinas-Lichius, O. Olfactory epithelial changes and inflammatory markers in the nasal mucosa after total laryngectomy. Chemosens. Percept. 2005, 2, 1–8. [Google Scholar]
- Lavalle, S.; Masiello, E.; Iannella, G.; Magliulo, G.; Pace, A.; Lechien, J.R.; Calvo-Henriquez, C.; Cocuzza, S.; Parisi, F.M.; Favier, V.; et al. Unraveling the Complexities of Oxidative Stress and Inflammation Biomarkers in Obstructive Sleep Apnea Syndrome: A Comprehensive Review. Life 2024, 14, 425. [Google Scholar] [CrossRef]
- Temmel, A.F.; Quint, C.; Schickinger-Fischer, B.; Klimek, L.; Stoller, E.; Hummel, T. Characteristics of olfactory disorders in relation to major causes of olfactory loss. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C. Age-related effects on the olfactory system. Microsc. Res. Tech. 2019, 82, 900–906. [Google Scholar]
- Hudetz, J.A.; Iqbal, Z.; Gandhi, S.D.; Patterson, K.M.; Byrne, A.J.; Pagel, P.S. Postoperative Delirium and Short-Term Cognitive Dysfunction Occur More Frequently in Patients Undergoing Valve Surgery with or without Coronary Artery Bypass Graft Surgery Compared with Coronary Artery Bypass Graft Surgery Alone: Results of a Pilot Study. J. Cardiothorac. Vasc. Anesth. 2011, 25, 811–816. [Google Scholar] [CrossRef]
- Jiang, H.; Scheet, P.; Kendall, G.S.; Liu, H.; Scherer, S.; Hu, H.; Wang, L.; Crawford, D. Genetic susceptibility to olfactory impairment: A genome-wide association study. Brain Behav. 2020, 10, e01583. [Google Scholar]
- Evered, L.; Silbert, B.; Scott, D.A.; Ames, D.; Maruff, P.; Blennow, K. Cerebrospinal fluid biomarker for Alzheimer disease predicts postoperative cognitive dysfunction. Anesthesiology 2017, 127, 765–775. [Google Scholar]
- Brown, C.H.; Probert, J.; Healy, R.; Parish, M.; Nomura, Y.; Yamaguchi, A.; Tian, J.; Zehr, K.; Mandal, K.; Hogue, C.W. Cognitive decline after delirium in patients undergoing cardiac surgery. Anesthesiology 2016, 125, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.W.; Roses, A.D.; Haines, J.L.; Pericak-Vance, M.A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993, 261, 921–923. [Google Scholar] [CrossRef]
- Calder, P.C.; Bosco, N.; Bourdet-Sicard, R.; Capuron, L.; Delzenne, N.; Doré, J.; Franceschi, C.; Lehtinen, M.J.; Recker, T.; Salvioli, S.; et al. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res. Rev. 2017, 40, 95–119. [Google Scholar] [CrossRef]
- Guay, J.; Parker, M.J.; Griffiths, R.; Kopp, S. Peripheral nerve blocks for hip fractures. Cochrane Database Syst. Rev. 2020, 11, CD001159. [Google Scholar] [CrossRef]
- Casati, A.; Fanelli, G.; Pietropaoli, P.; Proietti, R.; Tufano, R.; Danelli, G.; Fierro, G.; De Cosmo, G.; Servillo, G. Continuous monitoring of cerebral oxygen saturation in elderly patients undergoing major abdominal surgery minimizes brain exposure to potential hypoxia. Anesth. Analg. 2005, 101, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Ebert, T.J.; Hall, J.E.; Barney, J.A.; Uhrich, T.D.; Colinco, M.D. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology 2011, 93, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Rissom, K.; Reden, J.; Hähner, A.; Weidenbecher, M.; Hüttenbrink, K.B. Effects of olfactory training in patients with olfactory loss. Laryngoscope 2009, 119, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Kehlet, H.; Jensen, T.S.; Woolf, C.J. Persistent postsurgical pain: Risk factors and prevention. Lancet 2006, 367, 1618–1625. [Google Scholar] [CrossRef]
- Xiong, W.X.; Zhou, G.X.; Wang, B.; Xue, Z.G.; Wang, L.; Sun, H.C.; Ge, S.J. Impaired spatial learning and memory after sevoflurane-nitrous oxide anesthesia in aged rats is associated with down-regulated cAMP/CREB signaling. PLoS ONE 2013, 8, e79408. [Google Scholar] [CrossRef] [PubMed]
- Maze, M.; Virtanen, R.; Daunt, D.; Banks, S.J.; Stover, E.P.; Feldman, D. Effects of dexmedetomidine, a novel imidazole sedative-anesthetic agent, on adrenal steroidogenesis: In vivo and in vitro studies. Anesth. Analg. 2008, 73, 204–208. [Google Scholar] [CrossRef]
- Tanabe, S.; Yamashita, T. The role of immune cells in brain development and neurodevelopmental diseases. Int. Immunol. 2019, 31, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Hudetz, J.A.; Patterson, K.M.; Iqbal, Z.; Gandhi, S.D.; Byrne, A.J.; Hudetz, A.G.; Pagel, P.S.; Warltier, D.C. Ketamine attenuates delirium after cardiac surgery with cardiopulmonary bypass. J. Cardiothorac. Vasc. Anesth. 2009, 23, 651–657. [Google Scholar] [CrossRef]
- Vivar, C.; Potter, M.C.; van Praag, H. All about running: Synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr. Top. Behav. Neurosci. 2013, 15, 189–210. [Google Scholar]
- Deiner, S.; Westlake, B.; Dutton, R.P. Patterns of surgical care and complications in elderly adults. J. Am. Geriatr. Soc. 2020, 62, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.T.V.; Cheng, B.C.P.; Lee, T.M.C.; Gin, T.; CODA Trial Group. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J. Neurosurg. Anesthesiol. 2019, 25, 33–42. [Google Scholar] [CrossRef] [PubMed]

| Risk Factor/Vulnerable Population | Description | Associated Complications | Key References |
|---|---|---|---|
| Advanced Age | Reduced neuronal plasticity, vascular function, and immune regulation | Higher incidence of POCD, prolonged recovery of olfactory function | [20,55,56] |
| Pediatric Populations | Developing brain sensitive to neurotoxic effects | Potential long-term cognitive and sensory deficits | [7,30] |
| Neurodegenerative Diseases | Pre-existing conditions like Alzheimer’s or Parkinson’s | Exacerbation of cognitive and sensory dysfunctions | [31,41] |
| Chronic Conditions | Diabetes, cardiovascular disease, COPD | Increased risk due to systemic inflammation and vascular dysfunction | [22] |
| Genetic Predispositions | e.g., APOE-ε4 allele | Increased risk of POCD and neurodegeneration | [53,57] |
| Prolonged Anesthesia Exposure | Extended duration of surgery and anesthesia | Higher risk of cumulative neurotoxic effects | [42,55] |
| Environmental Factors | Chronic exposure to toxins, smoking | Impaired baseline olfactory function, reduced neural resilience | [26,45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maniaci, A.; Lentini, M.; Trombadore, R.; Gruppuso, L.; Milardi, S.; Scrofani, R.; Cuttone, G.; Sorbello, M.; Modica, R.; Lechien, J.R.; et al. Neurological and Olfactory Disturbances After General Anesthesia. Life 2025, 15, 344. https://doi.org/10.3390/life15030344
Maniaci A, Lentini M, Trombadore R, Gruppuso L, Milardi S, Scrofani R, Cuttone G, Sorbello M, Modica R, Lechien JR, et al. Neurological and Olfactory Disturbances After General Anesthesia. Life. 2025; 15(3):344. https://doi.org/10.3390/life15030344
Chicago/Turabian StyleManiaci, Antonino, Mario Lentini, Rosario Trombadore, Loris Gruppuso, Santo Milardi, Rosario Scrofani, Giuseppe Cuttone, Massimiliano Sorbello, Rodolfo Modica, Jerome R. Lechien, and et al. 2025. "Neurological and Olfactory Disturbances After General Anesthesia" Life 15, no. 3: 344. https://doi.org/10.3390/life15030344
APA StyleManiaci, A., Lentini, M., Trombadore, R., Gruppuso, L., Milardi, S., Scrofani, R., Cuttone, G., Sorbello, M., Modica, R., Lechien, J. R., Boscolo-Rizzo, P., Paternò, D. S., & La Via, L. (2025). Neurological and Olfactory Disturbances After General Anesthesia. Life, 15(3), 344. https://doi.org/10.3390/life15030344










