Lean MASLD and IBD: Exploring the Intersection of Metabolic Dysfunction and the Gut–Liver Axis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Diagnostic Criteria and Definitions
2.3. Liver Steatosis and Fibrosis Evaluation
2.4. Statistical Analysis
3. Results
3.1. Overview of Study Cohort
3.2. Comparison of MASLD vs. Non-MASLD Lean Patients
3.3. Comparison of Clinical Profiles Between IBD and Non-IBD MASLD Patients
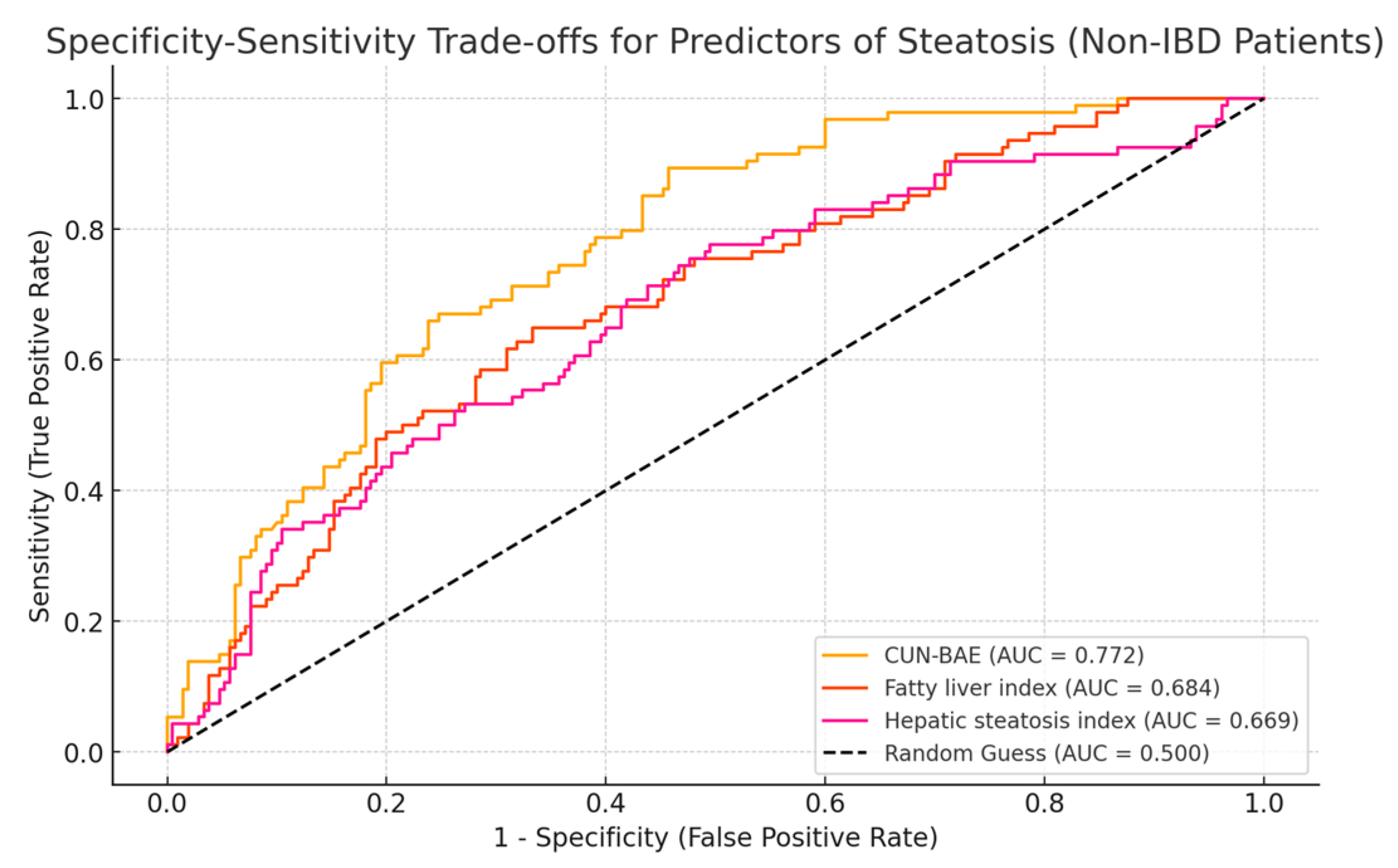
3.4. Accuracy of CUN-BAE for Predicting the Presence of Liver Steatosis
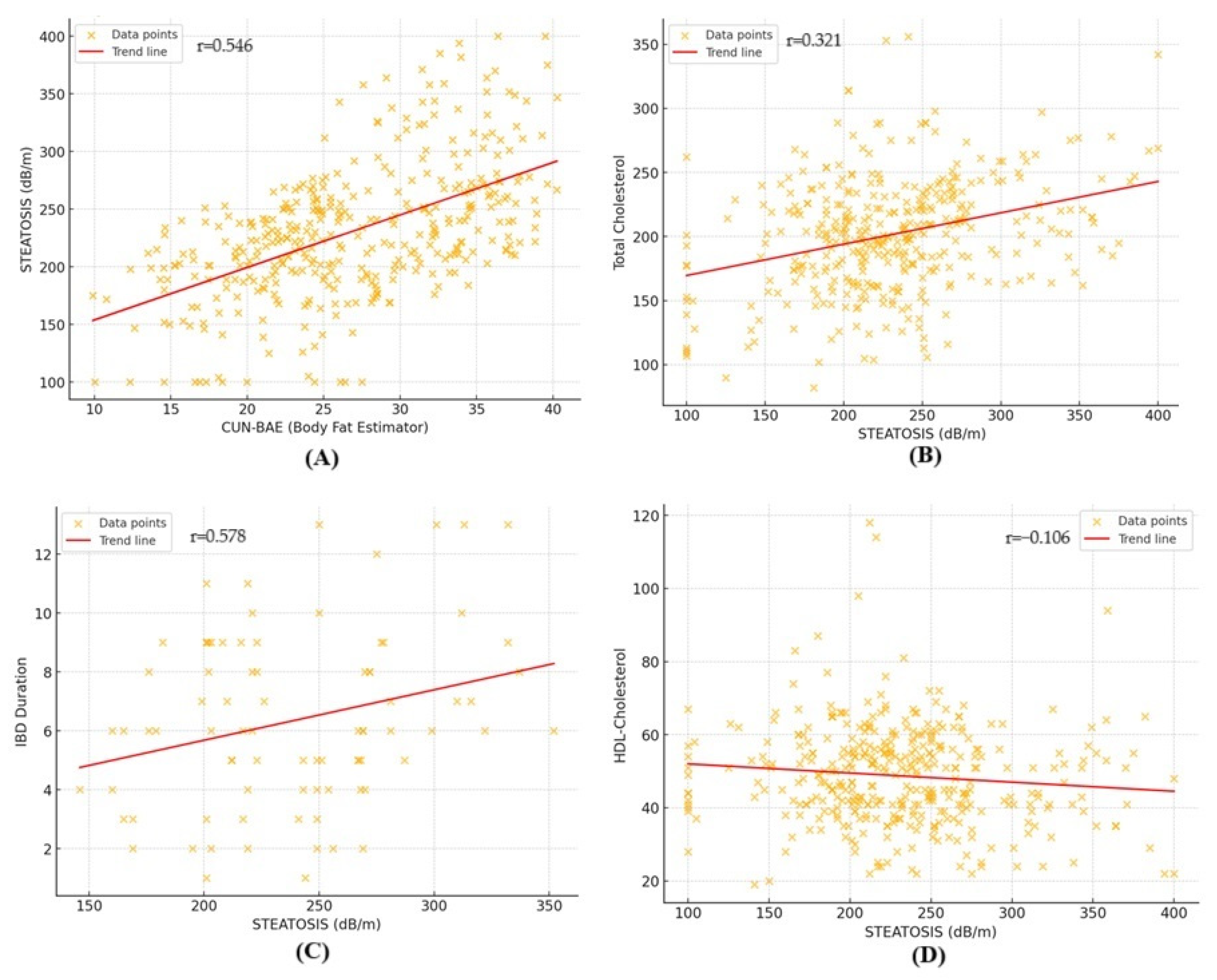
3.5. Factors Associated with Liver Steatosis in Lean Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Younossi, Z.M.; Paik, J.M.; Stepanova, M.; Ong, J.; Alqahtani, S.; Henry, L. Clinical profiles and mortality rates are similar for metabolic dysfunction-associated steatotic liver disease and non-alcoholic fatty liver disease. J. Hepatol. 2024, 80, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Kalligeros, M.; Henry, L. Epidemiology of Metabolic Dysfunction-Associated Steatotic Liver Disease. Clin. Mol. Hepatol. 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Vitellius, C.; Desjonqueres, E.; Lequoy, M.; Amaddeo, G.; Fouchard, I.; N’Kontchou, G.; Canivet, C.M.; Ziol, M.; Regnault, H.; Lannes, A.; et al. MASLD-related HCC: Multicenter study comparing patients with and without cirrhosis. JHEP Rep. 2024, 6, 101160. [Google Scholar] [CrossRef]
- Mantovani, A.; Taverna, A.; Cappelli, D.; Beatrice, G.; Csermely, A.; Sani, E.; Byrne, C.D.; Targher, G. Long-Term Adverse Effect of Liver Stiffness on Glycaemic Control in Type 2 Diabetic Patients with Nonalcoholic Fatty Liver Disease: A Pilot Study. Int. J. Mol. Sci. 2022, 23, 12481. [Google Scholar] [CrossRef] [PubMed]
- Nabi, O.; Lapidus, N.; Boursier, J.; de Ledinghen, V.; Petit, J.M.; Kab, S.; Renuy, A.; Zins, M.; Lacombe, K.; Serfaty, L. Lean individuals with NAFLD have more severe liver disease and poorer clinical outcomes (NASH-CO Study). Hepatology 2023, 78, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Golabi, P.; Paik, J.M.; Arshad, T.; Younossi, Y.; Mishra, A.; Younossi, Z.M. Mortality of NAFLD According to the Body Composition and Presence of Metabolic Abnormalities. Hepatol. Commun. 2020, 4, 1136–1148. [Google Scholar] [CrossRef]
- Younes, R.; Govaere, O.; Petta, S.; Miele, L.; Tiniakos, D.; Burt, A.; David, E.; Vecchio, F.M.; Maggioni, M.; Cabibi, D.; et al. Caucasian lean subjects with non-alcoholic fatty liver disease share long-term prognosis of non-lean: Time for reappraisal of BMI-driven approach? Gut 2022, 71, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Ng, C.H.; Phang, P.H.; Chan, K.E.; Chin, Y.H.; Fu, C.E.; Zeng, R.W.; Xiao, J.; Tan, D.J.H.; Quek, J.; et al. Comparative Burden of Metabolic Dysfunction in Lean NAFLD vs Non-lean NAFLD—A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 1750–1760.e12. [Google Scholar] [CrossRef]
- Marchesini, G.; Vettor, R.; Pinzani, M. MASLD emerging from the fog of fatty liver. J. Hepatol. 2024, 80, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Ann. Hepatol. 2024, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Ishido, S.; Tamaki, N.; Takahashi, Y.; Uchihara, N.; Suzuki, K.; Tanaka, Y.; Miyamoto, H.; Yamada, M.; Matsumoto, H.; Nobusawa, T.; et al. Risk of cardiovascular disease in lean patients with nonalcoholic fatty liver disease. BMC Gastroenterol. 2023, 23, 211. [Google Scholar] [CrossRef]
- Ishido, S.; Tamaki, N.; Takahashi, Y.; Uchihara, N.; Suzuki, K.; Tanaka, Y.; Miyamoto, H.; Yamada, M.; Matsumoto, H.; Nobusawa, T.; et al. Higher mortality among lean patients with non-alcoholic fatty liver disease despite fewer metabolic comorbidities. Aliment. Pharmacol. Ther. 2023, 57, 1014–1027. [Google Scholar]
- Danpanichkul, P.; Suparan, K.; Kim, D.; Wijarnpreecha, K. What Is New in Metabolic Dysfunction-Associated Steatotic Liver Disease in Lean Individuals: From Bench to Bedside. J. Clin. Med. 2024, 13, 278. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Mohan, S. Non-alcoholic Fatty Liver Disease in Lean Subjects: Characteristics and Implications. J. Clin. Transl. Hepatol. 2017, 5, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Romero-Corral, A.; Somers, V.K.; Sierra-Johnson, J.; Thomas, R.J.; Collazo-Clavell, M.L.; Korinek, J.; Allison, T.G.; Batsis, J.A.; Sert-Kuniyoshi, F.H.; Lopez-Jimenez, F. Accuracy of body mass index in diagnosing obesity in the adult general population. Int. J. Obes. 2008, 32, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ambrosi, J.; Silva, C.; Catalán, V.; Rodríguez, A.; Galofré, J.C.; Escalada, J.; Valentí, V.; Rotellar, F.; Romero, S.; Ramírez, B.; et al. Clinical usefulness of a new equation for estimating body fat. Diabetes Care 2012, 35, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Alizadeh-Tabari, S.; Singh, S.; Loomba, R. Meta-analysis: Prevalence of, and risk factors for, non-alcoholic fatty liver disease in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2022, 55, 894–907. [Google Scholar] [CrossRef] [PubMed]
- Solitano, V.; Moschetta, A. Metabolic Dysfunction-Associated Steatotic Liver Disease in Inflammatory Bowel Disease: A Proposed Stepwise Approach. Clin. Gastroenterol. Hepatol. 2024, 22, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Mouries, J.; Brescia, P.; Silvestri, A.; Spadoni, I.; Sorribas, M.; Wiest, R.; Mileti, E.; Galbiati, M.; Invernizzi, P.; Adorini, L.; et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J. Hepatol. 2019, 71, 1216–1228. [Google Scholar] [CrossRef]
- Lin, A.; Roth, H.; Anyane-Yeboa, A.; Rubin, D.T.; Paul, S. Prevalence of Nonalcoholic Fatty Liver Disease in Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Inflamm. Bowel Dis. 2021, 27, 947–955. [Google Scholar] [CrossRef]
- Martínez-Domínguez, S.J.; García-Mateo, S.; Gargallo-Puyuelo, C.J.; Gallego-Llera, B.; Callau, P.; Mendi, C.; Arroyo-Villarino, M.T.; Simón-Marco, M.Á.; Ampuero, J.; Gomollón, F. Inflammatory Bowel Disease Is an Independent Risk Factor for Metabolic Dysfunction-Associated Steatotic Liver Disease in Lean Individuals. Inflamm. Bowel Dis. 2024, 30, 1274–1283. [Google Scholar] [CrossRef] [PubMed]
- Mózes, F.E.; Lee, J.A.; Selvaraj, E.A.; Jayaswal, A.N.A.; Trauner, M.; Boursier, J.; Fournier, C.; Staufer, K.; Stauber, R.E.; Bugianesi, E.; et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: An individual patient data meta-analysis. Gut 2022, 71, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 17, 374–443. [Google Scholar]
- Kaneva, A.M.; Bojko, E.R. Fatty liver index (FLI): More than a marker of hepatic steatosis. J. Physiol. Biochem. 2024, 80, 11–26. [Google Scholar] [CrossRef]
- Fennoun, H.; Mansouri, S.E.; Tahiri, M.; Haraj, N.E.; El Aziz, S.; Hadad, F.; Hliwa, W.; Badr, W.; Chadli, A. Interest of hepatic steatosis index (HSI) in screening for metabolic steatopathy in patients with type 2 diabetes. Pan Afr. Med. J. 2020, 37, 270. [Google Scholar] [PubMed]
- Young, S.; Tariq, R.; Provenza, J.; Satapathy, S.K.; Faisal, K.; Choudhry, A.; Friedman, S.L.; Singal, A.K. Prevalence and Profile of Nonalcoholic Fatty Liver Disease in Lean Adults: Systematic Review and Meta-Analysis. Hepatol. Commun. 2020, 4, 953–972. [Google Scholar] [CrossRef]
- Ye, Q.; Zou, B.; Yeo, Y.H.; Li, J.; Huang, D.Q.; Wu, Y.; Yang, H.; Liu, C.; Kam, L.Y.; Tan, X.X.E.; et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Sato-Espinoza, K.; Chotiprasidhi, P.; Huaman, M.R.; Díaz-Ferrer, J. Update in lean metabolic dysfunction-associated steatotic liver disease. World J. Hepatol. 2024, 16, 452–464. [Google Scholar]
- Semmler, G.; Wernly, S.; Bachmayer, S.; Wernly, B.; Schwenoha, L.; Huber-Schönauer, U.; Stickel, F.; Niederseer, D.; Aigner, E.; Datz, C. Nonalcoholic Fatty Liver Disease in Lean Subjects: Associations With Metabolic Dysregulation and Cardiovascular Risk-A Single-Center Cross-Sectional Study. Clin. Transl. Gastroenterol. 2021, 12, e00326. [Google Scholar] [CrossRef]
- Kawanaka, M.; Nishino, K.; Kawada, M.; Ishii, K.; Tanikawa, T.; Katsumata, R.; Urata, N.; Nakamura, J.; Suehiro, M.; Haruma, K.; et al. Lean nonalcoholic fatty liver disease: Age-dependent differences in pathology, prognosis, and liver-related events. Hepatol. Res. 2023, 53, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.; Eder, S.K.; Felder, T.K.; Kedenko, L.; Paulweber, B.; Stadlmayr, A.; Huber-Schönauer, U.; Niederseer, D.; Stickel, F.; Auer, S.; et al. Clinical and Metabolic Characterization of Lean Caucasian Subjects With Non-alcoholic Fatty Liver. Am. J. Gastroenterol. 2017, 112, 102–110. [Google Scholar] [CrossRef]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist circumference as a vital sign in clinical practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Vilarinho, S.; Ajmera, V.; Zheng, M.; Loomba, R. Emerging Role of Genomic Analysis in Clinical Evaluation of Lean Individuals With NAFLD. Hepatology 2021, 74, 2241–2250. [Google Scholar] [CrossRef] [PubMed]
- Milić, S.; Lulić, D.; Štimac, D. Non-alcoholic fatty liver disease and obesity: Biochemical, metabolic and clinical presentations. World J. Gastroenterol. 2014, 20, 9330–9337. [Google Scholar] [CrossRef] [PubMed]
- Moyana, T.N. Metabolic dysfunction-associated steatotic liver disease: The question of long-term high-normal alanine aminotransferase as a screening test. World J. Gastroenterol. 2024, 30, 4576–4582. [Google Scholar] [CrossRef] [PubMed]
- Ampuero, J.; Aller, R.; Gallego-Durán, R.; Crespo, J.; Calleja, J.L.; García-Monzón, C.; Gómez-Camarero, J.; Caballería, J.; Iacono, O.L.; Ibañez, L.; et al. The biochemical pattern defines MASLD phenotypes linked to distinct histology and prognosis. J. Gastroenterol. 2024, 59, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Esmaili, S.; Rogers, G.B.; Bugianesi, E.; Petta, S.; Marchesini, G.; Bayoumi, A.; Metwally, M.; Azardaryany, M.K.; Coulter, S.; et al. Lean NAFLD: A Distinct Entity Shaped by Differential Metabolic Adaptation. Hepatology 2020, 71, 1213–1227. [Google Scholar] [CrossRef]
- Benedé-Ubieto, R.; Cubero, F.J.; Nevzorova, Y.A. Breaking the barriers: The role of gut homeostasis in Metabolic-Associated Steatotic Liver Disease (MASLD). Gut Microbes 2024, 16, 2331460. [Google Scholar] [CrossRef]
- Michalak, A.; Mosińska, P.; Fichna, J. Common links between metabolic syndrome and inflammatory bowel disease: Current overview and future perspectives. Pharmacol. Rep. 2016, 68, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dan, L.; Tu, X.; Sun, Y.; Deng, M.; Chen, X.; Hesketh, T.; Li, R.; Wang, X.; Li, X. Metabolic dysfunction-associated fatty liver disease and liver function markers are associated with Crohn’s disease but not Ulcerative Colitis: A prospective cohort study. Hepatol. Int. 2023, 17, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Duque, J.C.; Calleja, J.L.; Iruzubieta, P.; Hernández-Conde, M.; Rivas-Rivas, C.; Vera, M.I.; Garcia, M.J.; Pascual, M.; Castro, B.; García-Blanco, A.; et al. Increased risk of MAFLD and Liver Fibrosis in Inflammatory Bowel Disease Independent of Classic Metabolic Risk Factors. Clin. Gastroenterol. Hepatol. 2023, 21, 406–414.e7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Y.; Liu, S.; Zhu, S.; Li, P.; Wu, S. Mortality risk associated with MASLD, MASLD type and different cardiometabolic risk factors in IBD patients: A long-term prospective cohort study. Dig. Liver Dis. 2024, 23, S1590–S8658. [Google Scholar] [CrossRef] [PubMed]
- Sartini, A.; Gitto, S.; Bianchini, M.; Verga, M.C.; Di Girolamo, M.; Bertani, A.; Del Buono, M.; Schepis, F.; Lei, B.; De Maria, N.; et al. Non-alcoholic fatty liver disease phenotypes in patients with inflammatory bowel disease. Cell Death Dis. 2018, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Veltkamp, C.; Lan, S.; Korompoki, E.; Weiss, K.H.; Schmidt, H.; Seitz, H.K. Hepatic Steatosis and Fibrosis in Chronic Inflammatory Bowel Disease. J. Clin. Med. 2022, 11, 2623. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Konieczna, J.; Reynés, B.; Martín, M.; Fiol, M.; Palou, A.; Romaguera, D.; Oliver, P. CUN-BAE Index as a Screening Tool to Identify Increased Metabolic Risk in Apparently Healthy Normal-Weight Adults and Those with Obesity. J. Nutr. 2021, 151, 2215–2225. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, X.; He, S.; Kuang, M.; Xie, G.; Sheng, G.; Zou, Y. The Clínica Universidad de Navarra-Body Adiposity Estimator index is a reliable tool for screening metabolic dysfunction-associated steatotic liver disease: An analysis from a gender perspective. Lipids Health Dis. 2024, 23, 311. [Google Scholar] [CrossRef]
- Chen, X.; Geng, S.; Shi, Z.; Ding, J.; Li, H.; Su, D.; Cheng, Y.; Shi, S.; Tian, Q. Association of the CUN-BAE body adiposity estimator and other obesity indicators with cardiometabolic multimorbidity: A cross-sectional study. Sci. Rep. 2024, 14, 10557. [Google Scholar] [CrossRef]
- Kanwal, F.; Neuschwander-Tetri, B.A.; Loomba, R.; Rinella, M.E. Metabolic dysfunction-associated steatotic liver disease: Update and impact of new nomenclature on the American Association for the Study of Liver Diseases practice guidance on nonalcoholic fatty liver disease. Hepatology 2024, 79, 1212–1219. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Overall Cohort (Mean ± Std) n = 387 | IBD Patients (Mean ± Std) n = 82 | Non-IBD Patients (Mean ± Std) n = 305 | p-Value |
|---|---|---|---|---|
| Age (years) | 50.9 ± 15.52 | 40.68 ± 13.62 | 53.66 ± 14.85 | <0.001 |
| Female, n (%) | 174 (44.9%) | 46 (56.1%) | 128 (41.5%) | 0.064 |
| Man, n (%) | 213 (55.1%) | 36 (43.9%) | 177 (58.5%) | 0.058 |
| Height (cm) | 169.13 ± 9.01 | 166.79 ± 10.06 | 169.77 ± 8.61 | 0.016 |
| Weight (kg) | 64.83 ± 8.97 | 64.07 ± 8.78 | 65.04 ± 9.02 | 0.378 |
| BMI (kg/m2) | 22.59 ± 1.79 | 22.94 ± 1.23 | 22.49 ± 1.91 | 0.011 |
| Waist circumference (cm) | 85.59 ± 4.77 | 85.770 ± 4.08 | 85.541 ± 4.94 | 0.669 |
| MASLD, n (%) | 132 (34.1%) | 38 (46.3%) | 94 (30.9%) | 0.013 |
| CAP value (dB/m) | 230.29 ± 57.39 | 239.35 ± 47.13 | 227.84 ± 59.69 | 0.067 |
| LSM (kPa) | 6.22 ± 2.85 | 6.65 ± 1.9 | 6.11 ± 3.05 | 0.054 |
| Platelet count (109/L) | 236.15 ± 75.69 | 252.26 ± 60.91 | 231.81 ± 78.73 | 0.013 |
| CRP (mg/dL) | 0.53 ± 2.31 | 0.72 ± 1.68 | 0.51 ± 1.22 | 0.033 |
| Ferritin (mg/dL) | 141.78 ± 98.36 | 153.57 ± 81.23 | 138.61 ± 102.37 | 0.165 |
| Fasting plasma glucose (mg/dL) | 101.96 ± 29.88 | 108.48 ± 25.57 | 100.20 ± 30.74 | 0.014 |
| Urea (mg/dL) | 36.77 ± 12.21 | 38.78 ± 12.15 | 36.23 ± 12.19 | 0.094 |
| Creatinine (mg/dL) | 0.82 ± 0.2 | 0.88 ± 0.5 | 0.83 ± 0.4 | 0.673 |
| ALT (IU/L) | 41.45 ± 39.41 | 42.28 ± 37.68 | 41.230 ± 39.92 | 0.825 |
| AST (IU/L) | 37.24 ± 31.24 | 32.79 ± 24.16 | 38.45 ± 32.83 | 0.085 |
| GGT (IU/L) | 69.54 ± 154.98 | 37.35 ± 25.09 | 48.23 ± 173.19 | 0.031 |
| ALP (IU/L) | 93.26 ± 50.14 | 84.74 ± 36.17 | 95.55 ± 53.11 | 0.033 |
| Total cholesterol (mg/dL) | 201.46 ± 43.72 | 214.31 ± 32.67 | 198 ± 45.68 | <0.001 |
| Triglycerides (mg/dL) | 126.83 ± 64.19 | 145.65 ± 69.81 | 121.75 ± 61.73 | 0.006 |
| LDL-Col (mg/dL) | 128.78 ± 38.92 | 127.69 ± 38.98 | 129.07 ± 38.96 | 0.777 |
| HDL-Col (mg/dL) | 48.76 ± 13.45 | 43.92 ± 12.39 | 50.06 ± 13.45 | <0.001 |
| Lipid-lowering treatment, n (%) | 53 (13.7%) | 23 (28%) | 30 (9.9%) | <0.001 |
| Hypertension, n (%) | 110 (28.5%) | 20 (24.4%) | 90 (29.6%) | 0.425 |
| T2DM, n (%) | 59 (15.3%) | 11 (13.4%) | 48 (15.8%) | 0.721 |
| Active smoker, n (%) | 86 (22.3%) | 28 (34.1%) | 58 (19.1%) | 0.006 |
| Previous smoker, n (%) | 64 (16.6%) | 21 (25.6%) | 43 (14.1%) | 0.021 |
| CUN-BAE | 26.84 ± 6.89 | 28.15 ± 6.61 | 26.48 ± 6.94 | 0.046 |
| Fatty liver index | 31.49 ± 18.25 | 31.41 ± 14.30 | 31.51 ± 19.20 | 0.956 |
| Hepatic steatosis index | 32.04 ± 4.25 | 34.52 ± 3.56 | 31.37 ± 4.17 | <0.001 |
| Parameter | MASLD Patients (Mean ± SD) n = 132 | Non-MASLD Patients (Mean ± SD) n = 255 | p-Value |
|---|---|---|---|
| Age (years) | 52.29 ± 14.67 | 50.19 ± 15.93 | 0.197 |
| Height (cm) | 169.75 ± 8.71 | 168.82 ± 9.17 | 0.329 |
| Weight (km) | 67.34 ± 8.51 | 63.54 ± 8.95 | <0.001 |
| BMI (kg/m2) | 23.30 ± 1.50 | 22.22 ± 1.83 | <0.001 |
| Waist circumference (cm) | 87.42 ± 3.99 | 84.64 ± 4.88 | <0.001 |
| CAP value (dB/m) | 290.48 ± 40.30 | 199.01 ± 36.03 | <0.001 |
| LSM (kPa) | 6.93 ± 2.39 | 5.86 ± 3.01 | <0.001 |
| Platelet count (109/L) | 232.23 ± 86.31 | 238.20 ± 69.64 | 0.493 |
| Ferritin (mg/dL) | 148.71 ± 114.77 | 138.19 ± 88.68 | 0.359 |
| CRP (mg/dL) | 0.97 ± 1.23 | 0.84 ± 1.46 | 0.164 |
| Fasting plasma glucose (mg/dL) | 107.94 ± 35.09 | 98.86 ± 26.33 | 0.014 |
| Urea (mg/dL) | 37.21 ± 10.60 | 36.54 ± 12.98 | 0.587 |
| Creatinine (mg/dL) | 0.88 ± 1.89 | 0.82 ± 2.15 | 0.443 |
| ALT (IU/L) | 45.17 ± 36.40 | 39.52 ± 40.82 | 0.166 |
| AST (IU/L) | 39.92 ± 36.50 | 35.86 ± 28.12 | 0.266 |
| GGT (IU/L) | 77.55 ± 159.16 | 65.39 ± 152.93 | 0.472 |
| ALP (IU/L) | 89.11 ± 42.59 | 95.42 ± 53.61 | 0.209 |
| Total cholesterol (mg/dL) | 216.48 ± 40.25 | 193.67 ± 43.49 | 0.018 |
| Triglycerides (mg/dL) | 150.91 ± 74.61 | 114.32 ± 54.12 | <0.001 |
| LDL-Col (mg/dL) | 142.42 ± 35 | 121.69 ± 39.04 | <0.001 |
| HDL-Col (mg/dL) | 46.42 ± 12.82 | 49.98 ± 13.65 | 0.012 |
| Lipid-lowering treatment, n (%) | 34 (25.7%) | 19 (7.45%) | <0.001 |
| Hypertension, n (%) | 52 (39.3%) | 58 (22.7%) | <0.001 |
| T2DM, n (%) | 30 (22.7%) | 29 (11.3%) | <0.001 |
| Active smoker, n (%) | 42 (31.8%) | 44 (17.2%) | 0.014 |
| Previous smoker, n (%) | 26 (19.7%) | 38 (14.9%) | 0.094 |
| CUN-BAE | 31.21 ± 5.42 | 24.57 ± 6.49 | <0.001 |
| Fatty liver index | 38.90 ± 18.03 | 27.65 ± 17.19 | <0.001 |
| Hepatic steatosis index | 33.76 ± 4.39 | 31.15 ± 3.90 | <0.001 |
| Fibrosis stage, n (%) | |||
| F0 | 55 (41.6%) | 112 (43.9%) | |
| F1 | 41 (31.1%) | 97 (38.1%) | |
| F2 | 27 (20.4%) | 38 (14.9%) | |
| F3 | 7 (5.3%) | 8 (3.1%) | |
| F4 | 2 (1.5%) | 0 |
| Parameter | MASLD and IBD Patients (Mean ± Std) n = 38 | MASLD Patients Without IBD (Mean ± Std) n = 94 | p-Value |
|---|---|---|---|
| Age (years) | 47.18 ± 13.24 | 54.35 ± 14.78 | 0.008 |
| Height (cm) | 165.05 ± 9.66 | 171.64 ± 7.55 | <0.001 |
| Weight (kg) | 64.73 ± 8.29 | 68.39 ± 8.41 | 0.025 |
| BMI (kg/m2) | 23.68 ± 0.97 | 23.14 ± 1.64 | 0.021 |
| Waist circumference (cm) | 88.43 ± 3.24 | 87.01 ± 4.21 | 0.038 |
| CAP value (dB/m) | 281.31 ± 28.45 | 273.19 ± 43.78 | 0.048 |
| LSM (kPa) | 6.94 ± 1.81 | 6.931 ± 2.58 | 0.977 |
| Platelet count (109/L) | 247.92 ± 55.62 | 225.89 ± 95.51 | 0.101 |
| Ferritin (mg/dL) | 150.31 ± 77.73 | 148.06 ± 127.08 | 0.901 |
| CRP (mg/dL) | 0.93 ± 1.21 | 0.77 ± 1.54 | 0.043 |
| Fasting plasma glucose (mg/dL) | 106.97 ± 23.39 | 108.33 ± 38.94 | 0.806 |
| Urea (mg/dL) | 39.05 ± 12.12 | 36.46 ± 9.89 | 0.248 |
| Creatinine (mg/dL) | 1.02 ± 1.6 | 0.96 ± 2.48 | 0.195 |
| ALT (IU/L) | 38.68 ± 27.01 | 47.79 ± 39.39 | 0.130 |
| AST (IU/L) | 29.13 ± 16.27 | 44.27 ± 41.28 | 0.003 |
| GGT (IU/L) | 33.52 ± 17.41 | 95.34 ± 185.61 | 0.001 |
| ALP (IU/L) | 82.52 ± 37.17 | 91.77 ± 44.49 | 0.225 |
| Total cholesterol (mg/dL) | 218.52 ± 31.37 | 215.64 ± 43.45 | 0.672 |
| Triglycerides (mg/dL) | 155.15 ± 73.45 | 149.19 ± 75.38 | 0.676 |
| LDL-Col (mg/dL) | 142.94 ± 37.69 | 142.21 ± 34.06 | 0.916 |
| HDL-Col (mg/dL) | 43.61 ± 12.16 | 47.56 ± 12.96 | 0.101 |
| Lipid-lowering treatment, n (%) | 14 (36.8%) | 20 (58.8%) | 0.102 |
| Hypertension, n (%) | 16 (42.1%) | 36 (38.4%) | 0.724 |
| T2DM, n (%) | 9 (23.6%) | 21 (22.3%) | 0.892 |
| Active smoker, n (%) | 11 (28.9%) | 31 (32.9%) | 0.135 |
| Previous smoker, n (%) | 7 (18.4%) | 19 (20.2%) | 0.836 |
| CUN-BAE | 31.85 ± 4.96 | 30.94 ± 5.59 | 0.362 |
| Fatty liver index | 36.45 ± 14.26 | 39.88 ± 19.32 | 0.264 |
| Hepatic steatosis index | 35.65 ± 3.86 | 32.99 ± 4.37 | <0.001 |
| Fibrosis stage, n (%) | |||
| F0 | 15 (39.5%) | 30 (31.9%) | |
| F1 | 12 (31.5%) | 39 (41.5%) | |
| F2 | 8 (21.1%) | 19 (20.3%) | |
| F3 | 3 (7.9%) | 4 (4.2%) | |
| F4 | 0 | 2 (2.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotaru, A.; Stafie, R.; Stratina, E.; Zenovia, S.; Nastasa, R.; Minea, H.; Huiban, L.; Cuciureanu, T.; Muzica, C.; Chiriac, S.; et al. Lean MASLD and IBD: Exploring the Intersection of Metabolic Dysfunction and the Gut–Liver Axis. Life 2025, 15, 288. https://doi.org/10.3390/life15020288
Rotaru A, Stafie R, Stratina E, Zenovia S, Nastasa R, Minea H, Huiban L, Cuciureanu T, Muzica C, Chiriac S, et al. Lean MASLD and IBD: Exploring the Intersection of Metabolic Dysfunction and the Gut–Liver Axis. Life. 2025; 15(2):288. https://doi.org/10.3390/life15020288
Chicago/Turabian StyleRotaru, Adrian, Remus Stafie, Ermina Stratina, Sebastian Zenovia, Robert Nastasa, Horia Minea, Laura Huiban, Tudor Cuciureanu, Cristina Muzica, Stefan Chiriac, and et al. 2025. "Lean MASLD and IBD: Exploring the Intersection of Metabolic Dysfunction and the Gut–Liver Axis" Life 15, no. 2: 288. https://doi.org/10.3390/life15020288
APA StyleRotaru, A., Stafie, R., Stratina, E., Zenovia, S., Nastasa, R., Minea, H., Huiban, L., Cuciureanu, T., Muzica, C., Chiriac, S., Girleanu, I., Singeap, A.-M., Sfarti, C., Stanciu, C., & Trifan, A. (2025). Lean MASLD and IBD: Exploring the Intersection of Metabolic Dysfunction and the Gut–Liver Axis. Life, 15(2), 288. https://doi.org/10.3390/life15020288











