Genotypes of Sechium spp. as a Source of Natural Products with Biological Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sechium Compositum
2.2. Obtaining Methanolic and Ethanolic Extracts from Sechium Compositum Fruits
2.3. Obtaining Juice
2.4. Identifying Phenolic Acids by High Performance Liquid Chromatography (HPLC)
2.5. Flavonoids
2.6. Cucurbitacins
2.7. Statistical Analysis
2.8. Obtaining Extracts from a HD-Victor Hybrid and S. edule var. nigrum spinosum
2.9. Extraction Process for HD-Victor Hybrid and S. edule var. nigrum spinosum
2.10. Identification of Secondary Metabolites by High Performance Liquid Chromatography (HPLC)
Extract Preparation for Biological Activity
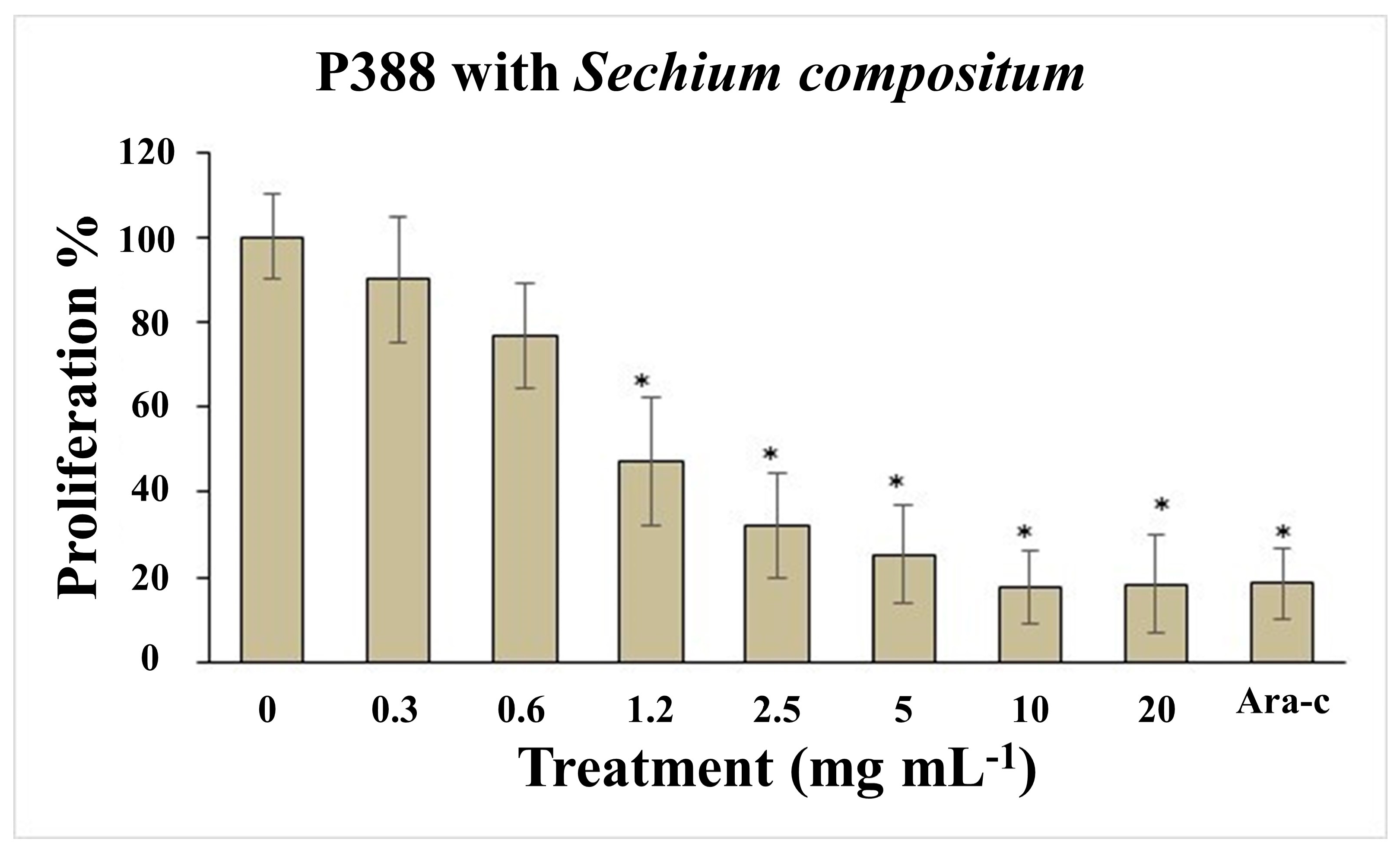
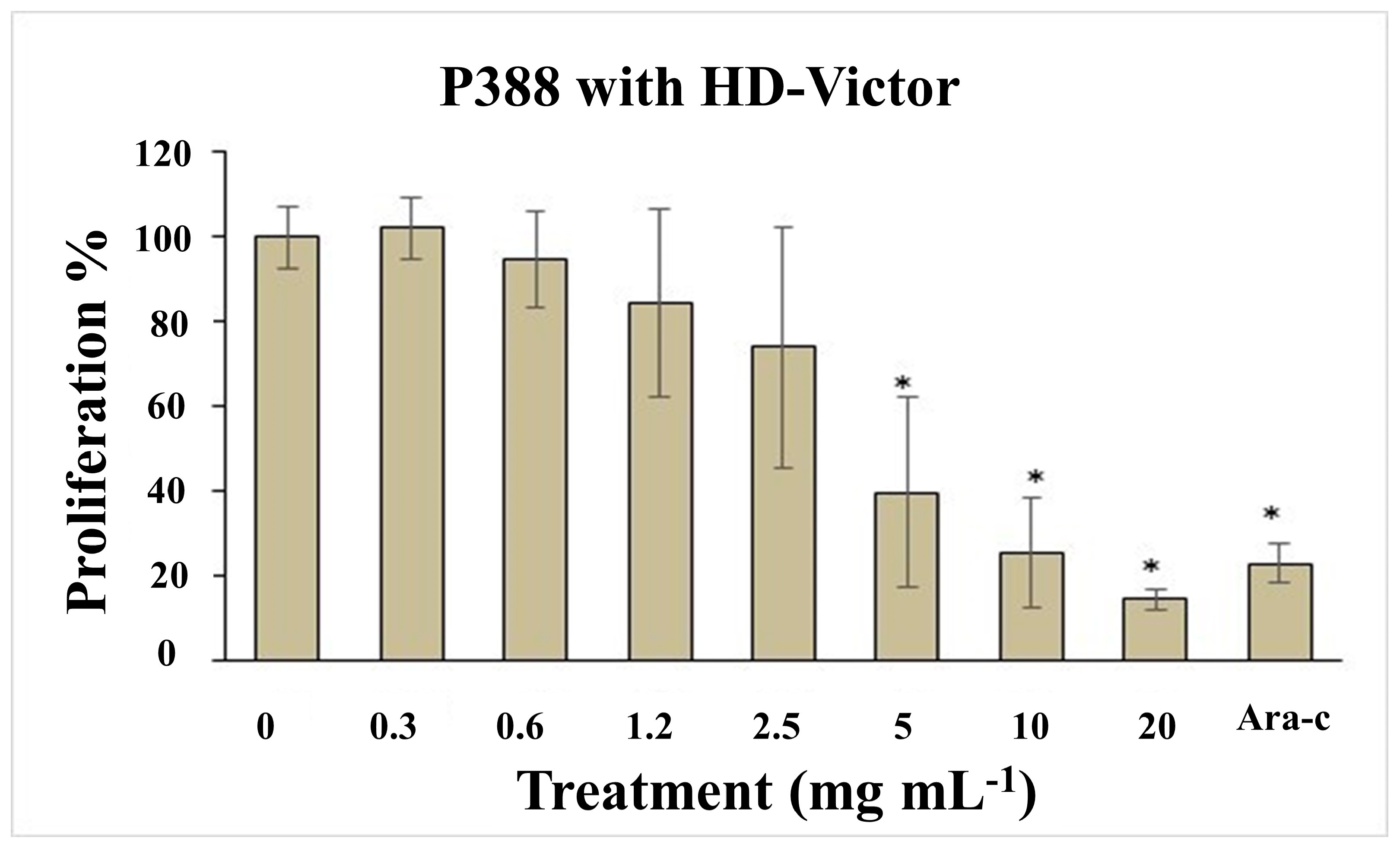
2.11. Biological Activity
2.12. Statistical Analysis
3. Results
3.1. HD-Victor Hybrid (S. compositum x S. edule var. nigrum xalapensis) and var. nigrum spinosum
3.2. Identification of Secondary Metabolites
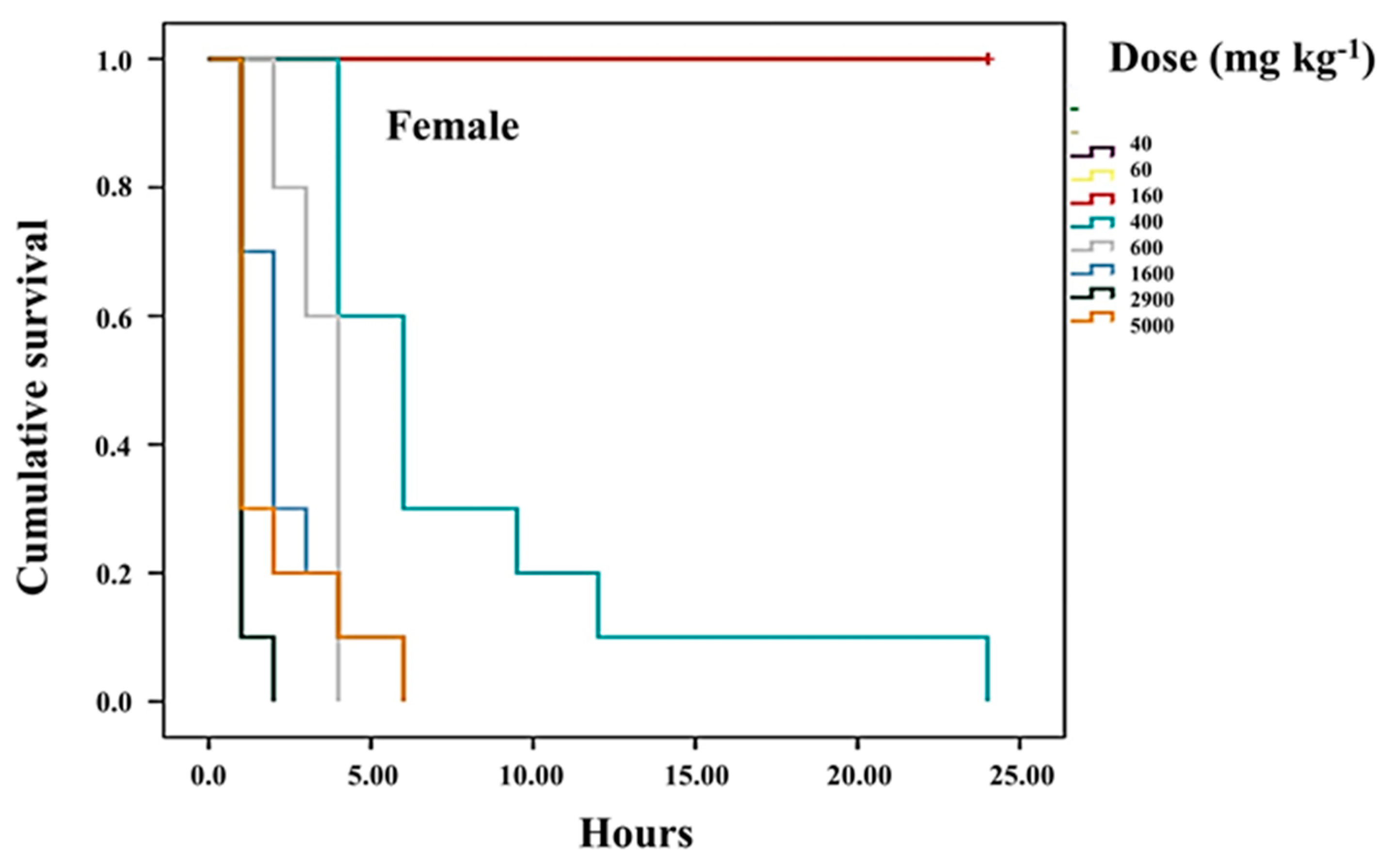
3.3. Survival of Treated Mice Compared to Healthy Mice
3.4. Determination of the Median Lethal Dose (LD50) of the HD-Victor Hybrid
4. Discussion
4.1. Sechium Compositum Juice
4.2. HD-Victor Hybrid (S. compositum x S. edule var. nigrum xalapensis) and S. edule var. nigrum spinosum
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Commission on Genetic Resources for Food and Agriculture Food and Agriculture Organization of the United Nations; FAO. The State of the World’s Bioversity for Food and Agriculture; FAO: Rome, Italy, 2019; 529p, Available online: https://www.fao.org/3/CA3129EN/ca3129en.pdf (accessed on 20 June 2024).
- Villaseñor, J.L. Los géneros de plantas vasculares de la flora de México. Boletín Soc. Botánica México 2004, 75, 105–135. [Google Scholar] [CrossRef]
- León-Lobos, P.; Way, M.; Aranda, D.P.; Lima-Junior, M. The role of ex situ seed banks in the conservation of plant diversity and in ecological restoration in Latin America. Planta Ecol. Divers. 2012, 5, 245–258. [Google Scholar] [CrossRef]
- Boege, E. El Patrimonio Biocultural de Los Pueblos Indígenas de México; Instituto Nacional de Antropología e Historia (INAH); Comisión Nacional Para el Desarrollo de los Pueblos Indígenas (CDI): México City, Mexico, 2008; 344p, Available online: https://www.gob.mx/inpi/documentos/el-patrimonio-biocultural-de-los-pueblos-indigenas-de-mexico (accessed on 24 May 2024).
- Perales, H.R.; Aguirre, J.R. Biodiversidad humanizada. In Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO). Capital Natural de México, Vol. I: Conocimiento Actual de la Biodiversidad; Liga Periférico-Insurgentes Sur 4903, Parques del Pedregal, Tlalpan, c.p 14010; Ciudad de Mexico: Mexico City, Mexico, 2008; pp. 565–603. Available online: http://www2.biodiversidad.gob.mx/pais/pdf/CapNatMex/Vol%20I/I00_PrefacioGuia.pdf (accessed on 2 July 2024).
- Bellón, M.R.; Barrientos-Priego, A.F.; Colunga-García, M.P.; Perales, H.; Reyes-Agüero, J.A.; Rosales-Serna, R.; Zizimbo- Villarreal, D. Diversidad y conservación de recursos genéticos en plantas cultivadas. In Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO) Capital Natural de México, vol. II: Estado de Conservación y Tendencias de Cambio; Liga Periférico-Insurgentes Sur 4903, Parques del Pedregal, Tlalpan, c.p 14010; Ciudad de México: Mexico City, Mexico, 2009; pp. 355–382. Available online: https://bioteca.biodiversidad.gob.mx (accessed on 20 July 2024).
- Kotschi, J.; Von Lossau, A. Agrobiodiversidad, la Clave para la Soberanía Alimentaria y la Adaptación al Cambio Climático; Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH: Friedrich-Ebert-Allee, Germany, 2012; 36p, Available online: https://st11.ning.com/topology/rest/1.0/file/get/2545869653?profile=original (accessed on 10 August 2024).
- Iñiguez-Luna, M.I.; Cadena-Iñiguez, J.; Soto-Hernández, R.M.; Morales-Flores, F.J.; Cortes-Cruz, M.; Watanabe, K.N. Natural Bioactive Compounds of Sechium spp. for Therapeutic and Nutraceutical Supplements. Front. Plant Sci. 2021, 12, 772389. [Google Scholar] [CrossRef] [PubMed]
- Iñiguez-Luna, M.I.; Cadena-Iñiguez, J.; Soto-Hernández, R.M.; Morales-Flores, F.J.; Cortes-Cruz, M.; Watanabe, N.K.; Machida-Hirano, R.; Cadena-Zamudio, J.D. Bioprospecting of Sechium spp. varieties for the selection of characters with pharmacological activity. Sci. Rep. 2021, 11, 6185. [Google Scholar] [CrossRef] [PubMed]
- Commission on Genetic Resources for Food and Agriculture Food and Agriculture Organization of the United Nations FAO. Plan de Acción Mundial para la Conservación y la Utilización Sostenible y el Desarrollo de los Recursos Genéticos Forestales; FAO: Rome, Italy, 2014; 38p, Available online: https://www.fao.org/3/i3849s/i3849s.pdf (accessed on 18 August 2024).
- IAC-GBC. International Advisory Council of the Global Bioeconomy Summit. Global Bioeconomy Summit Conference Report. Innovation in the Global Bioeconomy for Sustainable and Inclusive Transformation and Wellbeing. 2018. Available online: https://www.biooekonomierat.de/media/pdf/archiv/international-gbs-2018-communique.pdf?m=1637836879& (accessed on 20 May 2024).
- ONU. Future of Food: Toward a 3rd Generation Structural Transformation. No.-1. Nueva York, Estados Unidos de América; CEB Chief Executives Board for Coordination: New York, NY, USA, 2018. [Google Scholar]
- Barrera-Guzmán, L.A.; Legaria-Solano, J.P.; Cadena-Iñiguez, J.; Sahagún-Castellanos, J. Phylogenetics relationships among Mexican species of the genus Sechium (Cucurbitaceae). Turk. J. Bot. 2021, 45, 302–314. [Google Scholar] [CrossRef]
- Barrera-Guzmán, L.A.; Cadena-Iñiguez, J.; Legaria-Solano, J.P.; Sahagún-Castellanos, J. Phylogenetics of the genus Sechium P. Brown: A review. Span. J. Agric. Res. 2021, 19, e07R01. [Google Scholar] [CrossRef]
- Cadena-Iñiguez, J.; Soto-Hernández, R.M.; Arévalo-Galarza, M.L.; Avendaño-Arrazate, C.H.; Aguirre-Medina, J.F.; Ruíz-Posadas, L.M. Caracterización bioquímica de variedades domesticadas de chayote Sechium edule (Jacq.) Sw. comparadas con parientes silvestres. Rev. Chapingo Ser. Hortic. 2011, 17, 45–55. [Google Scholar]
- Cadena-Iñiguez, J.; Arévalo-Galarza, L.; Avendaño-Arrazate, C.H.; Soto-Hernández, M.; Ruiz-Posadas, L.D.M.; Santiago-Osorio, E.; Acosta-Ramos, M.; Cisneros-Solano, V.M.; Aguirre-Medina, J.F.; Ochoa-Martínez, D. Production, genetics, postharvest management and pharmacological characteristics of Sechium edule (Jacq.) Sw. Fresh Prod. 2007, 1, 41–53. [Google Scholar]
- Cadena-Iñiguez, J.; Aguiñiga-Sánchez, I.; Uriostegui-Arias, M.T.; Santiago-Osorio, E.; Ruiz-Posadas, L.D.M.; Soto-Hernández, M. Antiproliferative Effect of Sechium edule (Jacq.) Sw., cv. Madre Negra Extracts on Breast Cancer In Vitro. Separations 2022, 9, 230. [Google Scholar] [CrossRef]
- Monroy-Vázquez, M.E.; Soto-Hernández, M.; Cadena-Iñiguez, J.; Santiago-Osorio, E.; Ruiz-Posadas, L.D.M.; Rosas-Acevedo, H. Estudio biodirigido de un extracto alcohólico de frutos de Sechium edule (Jacq.). Swartz. Agrociencia 2009, 43, 777–790. [Google Scholar]
- Salazar-Aguilar, S.; Ruiz-Posadas, L.D.M.; Cadena-Iñiguez, J.; Soto-Hernández, M.; Santiago-Osorio, E.; Aguiñiga-Sánchez, I.; Cisneros-Solano, V.M.; Aguirre-Medina, J.F.; Olivera, Á.D. Microencapsulation of an extract of Sechium edule (Jacq.) Sw., with antineoplastic activity. Appl. Sci. 2021, 12, 24. [Google Scholar] [CrossRef]
- Aguiñiga-Sánchez, I.; Soto-Hernández, M.; Cadena-Iñiguez, J.; Ruíz-Posadas, L.d.M.; Cadena-Zamudio, J.D.; González-Ugarte, A.K.; Steider, B.W.; Santiago-Osorio, E. Fruit Extract from A Sechium edule Hybrid Induce Apoptosis in Leukaemic Cell Lines but not in Normal Cells. Nutr. Cancer 2015, 67, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Arista-Ugalde, T.L.; Santiago-Osorio, E.; Monroy-García, A.; Rosado-Pérez, J.; Aguiñiga-Sánchez, I.; Cadena-Iñiguez, J.; Mendoza-Núñez, V.M. Antioxidant and anti-inflammatory effect of the consumption of powdered concentrate of Sechium edule var. nigrum spinosum in Mexican older adults with metabolic syndrome. Antioxidants 2022, 11, 1076. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Harborne, J.B. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis; Chapman and Hall: Boca Raton, FL, USA, 1998. [Google Scholar]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Ganetsos, G.; Barker, P.E. (Eds.) Preparative and Production Scale Chromatography; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Khalikova, M.A.; Skarbalius, L.; Naplekov, D.K.; Jadeja, S.; Švec, F.; Lenčo, J. Evaluation of strategies for overcoming trifluoroacetic acid ionization suppression resulted in single-column intact level, middle-up, and bottom-up reversed-phase LC-MS analyses of antibody biopharmaceuticals. Talanta 2021, 233, 122512. [Google Scholar] [CrossRef]
- Vasileiadou, A.; Karapanagiotis, I.; Zotou, A. Development and validation of a liquid chromatographic method with diode array detection for the determination of anthraquinones, flavonoids and other natural dyes in aged silk. J. Chromatogr. A 2021, 1651, 462312. [Google Scholar] [CrossRef]
- Dong, M.W. Modern HPLC for Practicing Scientists; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Cadena-Iñiguez, J.; Avendaño-Arrazate, C.H.; Soto-Hernández, M.; Ruiz-Posadas, L.M.; Aguirre-Medina, J.F.; Arévalo-Galarza, L. Infraspecific variation of Sechium edule (Jacq.) Sw. in the state of Veracruz, México. Genet. Resour. Crop Evol. 2008, 55, 835–847. [Google Scholar] [CrossRef]
- Afifi, M.S.; Ross, S.A.; ElSohly, M.A.; Naeem, Z.E.; Halaweish, F.T. Cucurbitacins of Cucumis prophetarum and Cucumis prophetarum. J. Chem. Ecol. 1999, 25, 847–859. [Google Scholar] [CrossRef]
- Cadena-Iñiguez, J. Caracterización Morfoestructural, Fisiológica, Química y Genética de Diferentes Tipos de Chayote (Sechium edule (Jacq.) Sw.) (TESIS); Colegio de Postgraduados, Campus Montecillo, Centro Botánica: Texcoco, Mexico, 2005. [Google Scholar]
- Lorke, D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983, 54, 275–287. [Google Scholar] [CrossRef]
- Salazar-Aguilar, S.; Ruiz-Posadas, L.D.M.; Cadena-Iñiguez, J.; Soto-Hernández, M.; Santiago-Osorio, E.; Aguiñiga-Sánchez, I.; Rivera-Martínez, A.R.; Aguirre-Medina, J.F. Chemical analyses and in vitro and in vivo toxicity of fruit methanol extract of Sechium edule var. nigrum spinosum. Pharm. Biol. 2017, 55, 1638–1645. [Google Scholar] [CrossRef]
- Salazar-Aguilar, S.; Ruiz-Posadas, L.D.M.; Cadena-Iñiguez, J.; Soto-Hernández, M.; Santiago-Osorio, E.; Aguiñiga-Sánchez, I.; Rivera-Martínez, A.R.; Aguirre-Medina, J.F. Sechium edule (Jacq.) Swartz, a new cultivar with antiproliferative potential in a human cervical cancer HeLa cell line. Nutrients 2017, 9, 798. [Google Scholar] [CrossRef] [PubMed]
- Hatano, T.; Takagi, M.; Ito, H.; Yoshida, T. Acylated flavonoid glycosides and accompanying phenolics from licorice. Phytochemistry 1998, 47, 287–293. [Google Scholar] [CrossRef]
- Villamizar, L.F.; Martínez, F. Estudio fisicoquímico de la solubilidad del Eudragit s100® en algunos medios acuosos y orgánicos. Rev. Colomb. Química 2008, 37, 173–187. [Google Scholar]
- Sharma, K.; Ko, E.Y.; Assefa, A.D.; Ha, S.; Nile, S.H.; Lee, E.T.; Park, S.W. Temperature-dependent studies on the total phenolics, flavonoids, antioxidant activities, and sugar content in six onion varieties. J. Food Drug Anal. 2015, 23, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Tiho, T.; Yao, N.J.C.; Brou, C.Y.; Adima, A.A. Drying temperature effect on total phenols and flavonoids content, and antioxidant activity of Borassus aethiopum Mart ripe fruits pulp. J. Food Res. 2017, 6, 50–64. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Le, M.D. Influence of various drying conditions on phytochemical compounds and antioxidant activity of carrot peel. Beverages 2018, 4, 80. [Google Scholar] [CrossRef]
- Kaushik, U.; Aeri, V.; Mir, S.R. Cucurbitacins—An insight into medicinal leads from nature. Pharmacogn. Rev. 2015, 9, 12–18. [Google Scholar] [CrossRef]
- Ávalos, G.Á.; Pérez, U.C.E. Metabolismo secundario de plantas. Reduca 2011, 2, 119–145. [Google Scholar]
- Shadung, K.G.; Mashela, P.W.; Mphosi, M.S. Suitable drying temperature for preserving cucurbitacins in fruit of wild cucumber and wild watermelon. HortTechnology 2016, 26, 816–819. [Google Scholar] [CrossRef]
- Shadung, K.G.; Mashela, P.W.; Mulaudzi, V.L. Responses of cucurbitacin A and B concentrations from fruits of Cucumis myriocarpus and Cucumis africanus to drying method. Res. Crops 2017, 18, 569–573. [Google Scholar] [CrossRef]
- Kumar, R.S.; Gupta, M.; Mazumdar, U.K.; Rajeshwar, Y.; Kumar, T.S.; Gomathi, P.; Roy, R. Effects of methanol extracts of Caesalpinia bonducella and Bauhinia racemosa on hematology and hepatorenal function in mice. J. Toxicol. Sci. 2005, 30, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Espinoza, J.A.; Loraine, S. Las plantas medicinales en la lucha contra el cáncer, relevancia para México. Rev. Mex. Cienc. Farm. 2010, 41, 18–27. [Google Scholar]
- O’Shea, J.J.; Paul, W.E. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science 2010, 327, 1098–1102. [Google Scholar] [CrossRef]
- Salama, A.M.; Polo, A.E.; Contreras, C.R.; Maldonado, L. Analisis fitoquimico preliminar y determinacion de las actividades anti-inflamatoria y cardiaca de los frutos de Sechium edule. Rev. Colomb. Cienc. Químico-Farm. 1986, 15, 79–82. [Google Scholar]
- Jahan, F.; Nanji, K.; Qidwai, W.; Qasim, R. Fibromyalgia syndrome: An overview of pathophysiology, diagnosis and management. Oman Med. J. 2012, 27, 192–195. [Google Scholar] [CrossRef]
- Nagarajan, N.; Morden, A.; Bischof, D.; King, E.A.; Kosztowski, M.; Wick, E.C.; Stein, E.M. The role of fiber supplementation in the treatment of irritable bowel syndrome: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2015, 27, 1002–1010. [Google Scholar] [CrossRef]
- Cadena-Iñiguez, J.; Soto-Hernandez, M.; Torres-Salas, A.; Aguiñiga-Sánchez, I.; Ruiz-Posadas, L.M.; Rivera-Martinez, A.R.; Avendaño-Arrazate, C.H.; Santiago-Osorio, E. The antiproliferative effect of chayote varieties (Sechium edule (Jacq.) Sw.) on tumour cell lines. J. Med. Plant Res. 2013, 7, 455–460. [Google Scholar] [CrossRef]
- Lira Saade, R. Chayote, Sechium Edule (Jacq.) Sw. Promoting the Conservation and Use of Underutilized and Neglected Crops; Bioversity International: Rome, Italy, 1996. [Google Scholar]
- Siciliano, T.; De Tommasi, N.; Morelli, I.; Braca, A. Study of flavonoids of Sechium edule (Jacq) Swartz (Cucurbitaceae) different edible organs by liquid chromatography photodiode array mass spectrometry. J. Agric. Food Chem. 2004, 52, 6510–6515. [Google Scholar] [CrossRef]
- Kamble, M.B.; Dumbre, R.K.; Rangari, V.D. Hepatoprotective activity studies of herbal formulations. Int. J. Green Pharm. (IJGP) 2008, 2, 147–151. [Google Scholar] [CrossRef]
- Firdous, S.; Sravanthi, K.; Debnath, R.; Neeraja, K.A. Protective effect of ethanolic extract and its ethylacetate and n-butanol fractions of Sechium edule fruits against carbon tetrachloride induced hepatic injury in rats. Int. J. Pharm. Pharm. Sci. 2012, 4, 354–359. [Google Scholar]
- Neeraja, K.; Rabindra, D.; Firdous, S.M. Cardioprotective activity of fruits of Sechium edule. Bangladesh J. Pharmacol. 2015, 10, 125–130. [Google Scholar] [CrossRef]
- Escorcia-Gutiérrez, N.; Molina-Galán, J.D.; Castillo-González, F.; Mejía-Contreras, J.A. Rendimiento, heterosis y depresión endogámica de cruzas simples de maíz. Rev. Fitotec. Mex. 2010, 33, 271–279. [Google Scholar] [CrossRef]
- Tannin-Spitz, T.; Bergman, M.; Grossman, S. Cucurbitacin glucosides: Antioxidant and free-radical scavenging activities. Biochem. Biophys. Res. Commun. 2007, 364, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Elberry, A.A.; Mufti, S.; Al-Maghrabi, J.; Abdel Sattar, E.; Ghareib, S.A.; Mosli, H.A.; Gabr, S.A. Immunomodulatory effect of red onion (Allium cepa Linn) scale extract on experimentally induced atypical prostatic hyperplasia in Wistar rats. Mediat. Inflamm. 2014, 2014, 640746. [Google Scholar] [CrossRef]
- Chagas, M.D.S.S.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Flavonols and Flavones as Potential anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxid. Med. Cell. Longev. 2022, 2022, 9966750. [Google Scholar] [CrossRef]
- Mishra, A.; Kumar, S.; Pandey, A.K. Scientific validation of the medicinal efficacy of Tinospora cordifolia. Sci. World J. 2013, 2013, 292934. [Google Scholar] [CrossRef]
- Watanabe, M.A.E.; Amarante, M.K.; Conti, B.J.; Sforcin, J.M. Cytotoxic constituents of propolis inducing anticancer effects: A review. J. Pharm. Pharmacol. 2011, 63, 1378–1386. [Google Scholar] [CrossRef]
- Manivel, P.; Chen, X. Chlorogenic, Caffeic, and Ferulic Acids and Their Derivatives in Foods. In Handbook of Dietary Phytochemicals; Xiao, J., Sarker, S.D., Asakawa, Y., Eds.; Springer: Singapore, 2021; pp. 1033–1063. [Google Scholar] [CrossRef]
- Setzer, W.N.; Setzer, M.C. Plant-derived triterpenoids as potential antineoplastic agents. Mini Rev. Med. Chem. 2003, 3, 540–556. [Google Scholar] [CrossRef]
- Ishii, T.; Kira, N.; Yoshida, T.; Narahara, H. Cucurbitacin D induces growth inhibition, cell cycle arrest, and apoptosis in human endometrial and ovarian cancer cells. Tumor Biol. 2013, 34, 285–291. [Google Scholar] [CrossRef]
- Hernández-Aquino, E.; Muriel, P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J. Gastroenterol. 2018, 24, 1679. [Google Scholar] [CrossRef]
- Aguiñiga-Sánchez, I. Efecto Antitumoral In Vivo de Sechium, P. Browne (Cucurbitaceae). Ph.D. Thesis, Colegio de Postgraduados, Campus Montecillo, Texcoco, Estado de México, Mexico City, Mexico, 2012; 262p. [Google Scholar]
- Parra, J.; Hernández, P.; Ocampo-Maroto, F.; Álvarez-Valverde, V.; Carvajal-Miranda, Y.; Rodríguez-Rodríguez, G.; Herrera, C. Phytochemical characterization and antioxidant profile of Sechium edule (Jacq) Swartz (Cucurbitaceae) varieties grown in Costa Rica. J. Pharm. Pharmacogn. Res. 2018, 6, 448–457. [Google Scholar] [CrossRef]
- Pott, D.M.; Vallarino, J.G.; Osorio, S. Metabolite changes during postharvest storage: Effects on fruit quality traits. Metabolites 2020, 10, 187. [Google Scholar] [CrossRef] [PubMed]
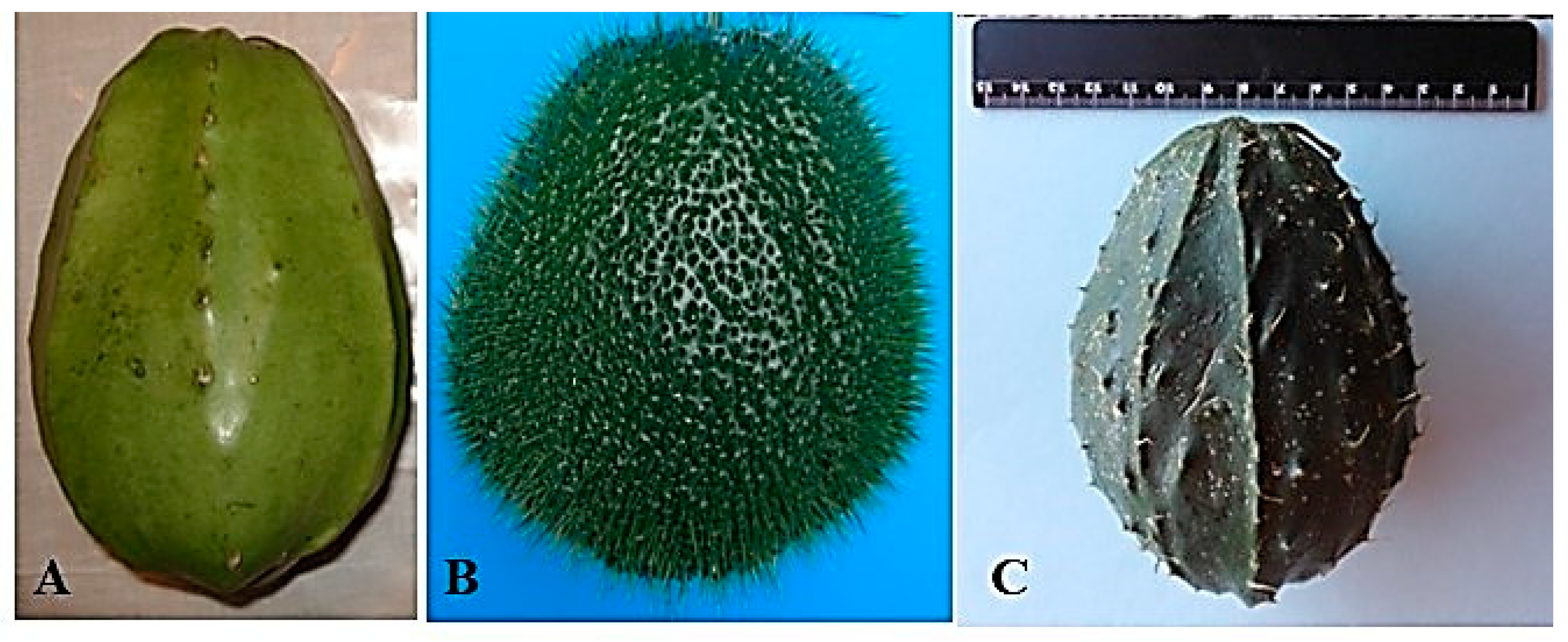

| Name | Genotypes | Varietal Distinction | Flavor |
|---|---|---|---|
| 631-12 | Sechium edule var. nigrum minor | variety | bitter |
| 632-12 | S. compositum (Donn. Sm.) C. Jeffrey | wild type | bitter |
| 633-12 | S. compositum x S. edule var. nigrum maxima | hybrid | bitter |
| 635-12 | S. compositum x S. edule var. nigrum spinosum | hybrid | bitter |
| 636-12 | S. chinantlense Lira & Chiang | wild type | bitter |
| 639-12 | S. edule var. amarus sylvestris x S. edule var. virens levis | 639-12-H-D | bitter |
| 641-12 | S. edule var. nigrum xalapensis x S. edule ver. amarus sylvestris | hybrid | bitter |
| 643-12 | S. compositum x S. edule var. nigrum xalapensis | H-D-Victor | bitter |
| H387 | S. edule var. amarus sylvestris x S. edule var. virens levis | H-387 | bitter |
| Campiña | Sechum edule (Jacq) Sw. | Campiña Reg. 0855 | edible |
| Cañitas | Sechum edule (Jacq) Sw. | Cañitas Reg. 0856 | edible |
| Ventlali | Sechum edule (Jacq) Sw. | Ventlali Reg. 0857 | edible |
| Phenolic Acids (mg kg−1) | |||
|---|---|---|---|
| Compound | Methanolic Extract (Dry Biomass) | Ethanolic Extract (Dry Biomass) | Juice (Fresh Biomass) |
| gallic acid | 42.82 ± 0.022 c | 62.35 ± 0.021 b | 115.44 ± 0.034 a |
| chlorogenic acid | 3.56 ± 0.01 b | 0. 00 ± 0.0 c | 451.20 ± 0.024 a |
| syringic acid | 1.94 ± 0.01 b | 1.93 ± 0.012 b | 12.86 ± 0.026 a |
| vanillinic acid | 0.00 ± 0.0 b | 1.01 ± 0.013 b | 19.61 ± 0.032 a |
| p-hydroxybenzoic acid | 2.42 ± 0.031 b | 0.82 ± 0.013 b | 36.66 ± 0.025 a |
| caffeic acid | 1.31 ± 0.011 b | 2.88 ± 0.03 b | 76.89 ± 0.015 a |
| ferulic acid | 3.32 ± 0.023 a | 1.84 ± 0.013 ab | 0.00 ± 0.0 b |
| p-coumaric acid | 0.68 ± 0.01 b | 0.50 ± 0.01 b | 19.51 ± 0.0.31 a |
| Sum of quantified compounds | 56.05 | 71.34 | 732.16 |
| Flavonoids (mg kg−1) | |||
|---|---|---|---|
| Compound | Methanolic Extract (Dry Biomass) | Ethanolic Extract (Dry Biomass) | Juice (Fresh Biomass) |
| rutin | 42.45 ± 0.001 b | 1.49 ± 0.002 c | 62.70 ± 0.0023 a |
| phloridzin | 6.20 ± 0.003 b | 5.18 ± 0.001 b | 85.46 ± 0.0011 a |
| mirectin | 11.01 ± 0.004 b | 0.59 ± 0.003 b | 145.83 ± 0.00151 a |
| quercetin | 0.56 ± 0.001 b | 1.90 ± 0.004 b | 15.37 ± 0.0014 a |
| naringenin | 303.69 ± 0.005 a | 58.51 ± 0.0012 c | 182.70 ± 0.002 b |
| phloretin | 51.84 ± 0.0021 a | 10.39 ± 0.0032 a | 25.44 ± 0.0031 a |
| galangin | 6.55 ± 00.27 a | 5.23 ± 0.0026 a | 4.08 ± 0.0015 a |
| Sum of quantified compounds | 422.30 | 83.29 | 521.59 |
| Cucurbitacins (mg kg−1) | |||
|---|---|---|---|
| Compound | Methanolic Extract (Dry Biomass) | Ethanolic Extract (Dry Biomass) | Juice (Fresh Biomass) |
| cucurbitacin D | 17.24 ± 0.001 a | 0.11 ± 0.001 b | 7.93 ± 0.0031 ab |
| cucurbitacin I | 25.45 ± 0.0031 b | 0.121 ± 0.003 b | 1723.39 ± 0.0021 a |
| cucurbitacin B | 1.03 ± 0.0042 a | 0.50 ± 0.003 a | 1.888 ± 0.0016 a |
| cucurbitacin E | 24.99 ± 0.0012 a | 2.57 ± 0.0014 b | 15.24 ± 0.0014 a |
| Sum of quantified compounds | 68.78 | 3.31 | 1748.45 |
| Variable | S. edule var. nigrum spinosum | HD-Víctor |
|---|---|---|
| Fresh weight (kg) | 80.00 | 15.00 |
| Dry weight (kg) | 5.24 | 1.56 |
| Water (%) | 93.44 | 89.60 |
| Dry matter yield (%) | 6.55 | 10.40 |
| Extract yield (%) | 6.65 | 14.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cadena-Iñiguez, J.; Arévalo-Galarza, M.d.L.; Santiago-Osorio, E.; Aguiñiga-Sánchez, I.; Cadena-Zamudio, J.D.; Soto-Hernández, M.; Ramírez-Rodas, Y.C.; Ruiz-Posadas, L.d.M.; Salazar-Aguilar, S.; Cisneros-Solano, V.M. Genotypes of Sechium spp. as a Source of Natural Products with Biological Activity. Life 2025, 15, 15. https://doi.org/10.3390/life15010015
Cadena-Iñiguez J, Arévalo-Galarza MdL, Santiago-Osorio E, Aguiñiga-Sánchez I, Cadena-Zamudio JD, Soto-Hernández M, Ramírez-Rodas YC, Ruiz-Posadas LdM, Salazar-Aguilar S, Cisneros-Solano VM. Genotypes of Sechium spp. as a Source of Natural Products with Biological Activity. Life. 2025; 15(1):15. https://doi.org/10.3390/life15010015
Chicago/Turabian StyleCadena-Iñiguez, Jorge, Ma. de Lourdes Arévalo-Galarza, Edelmiro Santiago-Osorio, Itzen Aguiñiga-Sánchez, Jorge David Cadena-Zamudio, Marcos Soto-Hernández, Yeimy C. Ramírez-Rodas, Lucero del Mar Ruiz-Posadas, Sandra Salazar-Aguilar, and Víctor Manuel Cisneros-Solano. 2025. "Genotypes of Sechium spp. as a Source of Natural Products with Biological Activity" Life 15, no. 1: 15. https://doi.org/10.3390/life15010015
APA StyleCadena-Iñiguez, J., Arévalo-Galarza, M. d. L., Santiago-Osorio, E., Aguiñiga-Sánchez, I., Cadena-Zamudio, J. D., Soto-Hernández, M., Ramírez-Rodas, Y. C., Ruiz-Posadas, L. d. M., Salazar-Aguilar, S., & Cisneros-Solano, V. M. (2025). Genotypes of Sechium spp. as a Source of Natural Products with Biological Activity. Life, 15(1), 15. https://doi.org/10.3390/life15010015







