Neuroimaging-Based Brain Age Estimation: A Promising Personalized Biomarker in Neuropsychiatry
Abstract
:1. Aging, Disease, and the Brain
2. Neuroimaging-Based Brain-Age Estimation
3. Theory and Methodology
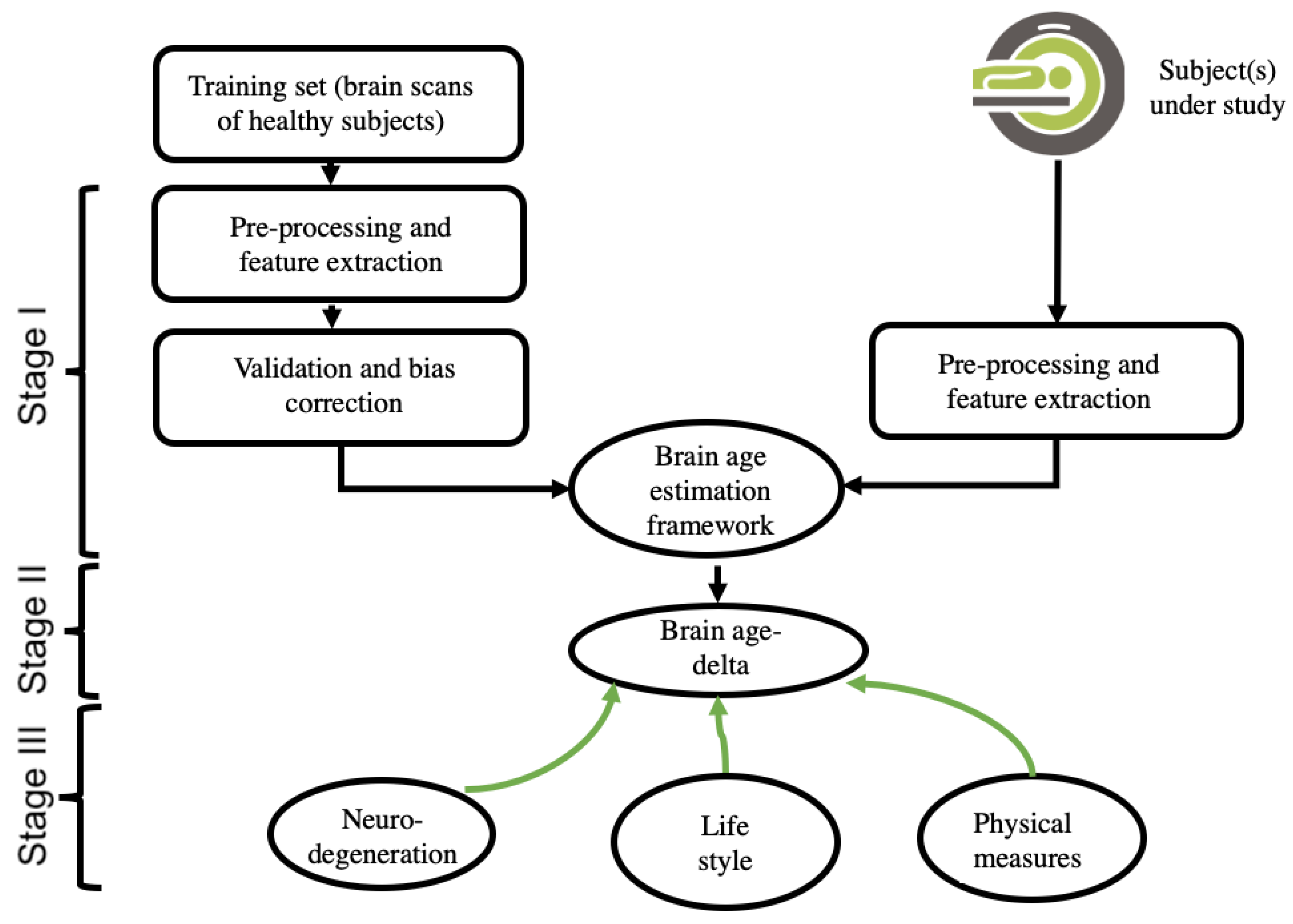
3.1. Theory of Neuroimaging-Based Brain-Age Estimation
3.2. Input Data and Feature-Extraction Methodologies of Neuroimaging
3.3. Data Reduction, Validation, and Bias Adjustment Neuroimaging Methodologies
3.4. Machine-Learning Methodologies
4. Applications in Neuropsychiatry
4.1. Alzheimer’s Disease, Dementia, and Memory Impairment
4.2. Other Neurological Diseases
4.3. Schizophrenia and Psychotic Disorders
4.4. Mood Disorders
4.5. Other Psychiatric Disorders
4.6. Comprehensive Studies
5. Applications to General Populations
6. Strengths, Controversies, and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flatt, T. A New Definition of Aging? Front. Genet. 2012, 3, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Childs, B.G.; Durik, M.; Baker, D.J.; Van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franke, K.; Bublak, P.; Hoyer, D.; Billiet, T.; Gaser, C.; Witte, O.; Schwab, M. In vivo biomarkers of structural and functional brain development and aging in humans. Neurosci. Biobehav. Rev. 2020, 117, 142–164. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Azam, S.; Haque, E.; Balakrishnan, R.; Kim, I.-S.; Choi, D.-K. The Ageing Brain: Molecular and Cellular Basis of Neurodegeneration. Front. Cell Dev. Biol. 2021, 9, 683459. [Google Scholar] [CrossRef] [PubMed]
- Hochla, N.A.N.; Fabian, M.S.; Parsons, O.A. Brain-age quotients in recently detoxified alcoholic, recovered alcoholic and nonalcoholic women. J. Clin. Psychol. 1982, 38, 207–212. [Google Scholar] [CrossRef]
- Franke, K.; Gaser, C. Ten Years of BrainAGE as a Neuroimaging Biomarker of Brain Aging: What Insights Have We Gained? Front. Neurol. 2019, 10, 789. [Google Scholar] [CrossRef] [Green Version]
- Franke, K.; Ziegler, G.; Klöppel, S.; Gaser, C.; Alzheimer’s Disease Neuroimaging Initiative. Estimating the age of healthy subjects from T1-weighted MRI scans using kernel methods: Exploring the influence of various parameters. NeuroImage 2010, 50, 883–892. [Google Scholar] [CrossRef]
- Beheshti, I.; Gravel, P.; Potvin, O.; Dieumegarde, L.; Duchesne, S. A novel patch-based procedure for estimating brain age across adulthood. NeuroImage 2019, 197, 618–624. [Google Scholar] [CrossRef]
- Cole, J.H.; Poudel, R.P.; Tsagkrasoulis, D.; Caan, M.W.; Steves, C.; Spector, T.D.; Montana, G. Predicting brain age with deep learning from raw imaging data results in a reliable and heritable biomarker. NeuroImage 2017, 163, 115–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beheshti, I.; Maikusa, N.; Matsuda, H. The accuracy of T1-weighted voxel-wise and region-wise metrics for brain age estimation. Comput. Methods Programs Biomed. 2022, 214, 106585. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, I.; Nugent, S.; Potvin, O.; Duchesne, S. Bias-adjustment in neuroimaging-based brain age frameworks: A robust scheme. NeuroImage Clin. 2019, 24, 102063. [Google Scholar] [CrossRef]
- Beheshti, I.; Ganaie, M.A.; Paliwal, V.; Rastogi, A.; Razzak, I.; Tanveer, M. Predicting Brain Age Using Machine Learning Algorithms: A Comprehensive Evaluation. IEEE J. Biomed. Health Inform. 2021, 26, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.H. Multimodality neuroimaging brain-age in UK biobank: Relationship to biomedical, lifestyle, and cognitive factors. Neurobiol. Aging 2020, 92, 34–42. [Google Scholar] [CrossRef]
- Sone, D.; Beheshti, I.; Shinagawa, S.; Niimura, H.; Kobayashi, N.; Kida, H.; Shikimoto, R.; Noda, Y.; Nakajima, S.; Bun, S.; et al. Neuroimaging-derived brain age is associated with life satisfaction in cognitively unimpaired elderly: A community-based study. Transl. Psychiatry 2022, 12, 25. [Google Scholar] [CrossRef]
- Beheshti, I.; Maikusa, N.; Matsuda, H. The association between “Brain-Age Score”(BAS) and traditional neuropsychological screening tools in Alzheimer’s disease. Brain Behav. 2018, 8, e01020. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, S.; Hänggi, J.; Mérillat, S.; Jäncke, L. Age prediction on the basis of brain anatomical measures. Hum. Brain Mapp. 2016, 38, 997–1008. [Google Scholar] [CrossRef]
- Sone, D.; Beheshti, I.; Maikusa, N.; Ota, M.; Kimura, Y.; Sato, N.; Koepp, M.; Matsuda, H. Neuroimaging-based brain-age prediction in diverse forms of epilepsy: A signature of psychosis and beyond. Mol. Psychiatry 2019, 26, 825–834. [Google Scholar] [CrossRef] [Green Version]
- Beheshti, I.; Mishra, S.; Sone, D.; Khanna, P.; Matsuda, H. T1-weighted MRI-driven brain age estimation in Alzheimer’s disease and Parkinson’s disease. Aging Dis. 2020, 11, 618. [Google Scholar] [CrossRef]
- Cole, J.; Ritchie, S.J.; Bastin, M.; Hernández, M.C.V.; Maniega, S.M.; Royle, N.; Corley, J.; Pattie, A.; Harris, S.E.; Zhang, Q.; et al. Brain age predicts mortality. Mol. Psychiatry 2017, 23, 1385–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyal, M.S.; Blazey, T.M.; Su, Y.; Couture, L.E.; Durbin, T.J.; Bateman, R.J.; Benzinger, T.L.-S.; Morris, J.C.; Raichle, M.E.; Vlassenko, A.G. Persistent metabolic youth in the aging female brain. Proc. Natl. Acad. Sci. USA 2019, 116, 3251–3255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beheshti, I.; Nugent, S.; Potvin, O.; Duchesne, S. Disappearing metabolic youthfulness in the cognitively impaired female brain. Neurobiol. Aging 2021, 101, 224–229. [Google Scholar] [CrossRef]
- Nemmi, F.; Levardon, M.; Péran, P. Brain-age estimation accuracy is significantly increased using multishell free-water reconstruction. Hum. Brain Mapp. 2022, 43, 2365–2376. [Google Scholar] [CrossRef] [PubMed]
- Butler, E.R.; Chen, A.; Ramadan, R.; Le, T.T.; Ruparel, K.; Moore, T.M.; Satterthwaite, T.D.; Zhang, F.; Shou, H.; Gur, R.C.; et al. Pitfalls in brain age analyses. Hum. Brain Mapp. 2021, 42, 4092–4101. [Google Scholar] [CrossRef]
- Le, T.T.; Kuplicki, R.T.; McKinney, B.A.; Yeh, H.-W.; Thompson, W.K.; Paulus, M.P.; Tulsa 1000 Investigators; Aupperle, R.L.; Bodurka, J.; Cha, Y.-H.; et al. A Nonlinear Simulation Framework Supports Adjusting for Age When Analyzing BrainAGE. Front. Aging Neurosci. 2018, 10, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Lipton, Z.C.; Yang, J.; Small, S.A.; Provenzano, F.A.; Alzheimer’s Disease Neuroimaging Initiative; Australian Imaging Biomarkers and Lifestyle flagship study of ageing; Frontotemporal Lobar Degeneration Neuroimaging Initiative. Estimating brain age based on a uniform healthy population with deep learning and structural magnetic resonance imaging. Neurobiol. Aging 2020, 91, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Ning, K.; Duffy, B.A.; Franklin, M.; Matloff, W.; Zhao, L.; Arzouni, N.; Sun, F.; Toga, A.W. Improving brain age estimates with deep learning leads to identification of novel genetic factors associated with brain aging. Neurobiol. Aging 2021, 105, 199–204. [Google Scholar] [CrossRef]
- Popescu, S.G.; Glocker, B.; Sharp, D.J.; Cole, J.H. Local Brain-Age: A U-Net Model. Front. Aging Neurosci. 2021, 13, 761954. [Google Scholar] [CrossRef]
- Levakov, G.; Rosenthal, G.; Shelef, I.; Raviv, T.R.; Avidan, G. From a deep learning model back to the brain—Identifying regional predictors and their relation to aging. Hum. Brain Mapp. 2020, 41, 3235–3252. [Google Scholar] [CrossRef]
- Gaser, C.; Franke, K.; Klöppel, S.; Koutsouleris, N.; Sauer, H.; Alzheimer’s Disease Neuroimaging Initiative. BrainAGE in Mild Cognitive Impaired Patients: Predicting the Conversion to Alzheimer’s Disease. PLoS ONE 2013, 8, e67346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Löwe, L.C.; Gaser, C.; Franke, K.; Alzheimer’s Disease Neuroimaging Initiative. The Effect of the APOE Genotype on Individual BrainAGE in Normal Aging, Mild Cognitive Impairment, and Alzheimer’s Disease. PLoS ONE 2016, 11, e0157514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Knol, M.J.; Tiulpin, A.; Dubost, F.; de Bruijne, M.; Vernooij, M.W.; Adams, H.H.H.; Ikram, M.A.; Niessen, W.J.; Roshchupkin, G.V. Gray Matter Age Prediction as a Biomarker for Risk of Dementia. Proc. Natl. Acad. Sci. USA 2019, 116, 21213–21218. [Google Scholar] [CrossRef] [Green Version]
- Ly, M.; Yu, G.Z.; Karim, H.T.; Muppidi, N.R.; Mizuno, A.; Klunk, W.E.; Aizenstein, H.J. Improving brain age prediction models: Incorporation of amyloid status in Alzheimer’s disease. Neurobiol. Aging 2019, 87, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Mohajer, B.; Abbasi, N.; Mohammadi, E.; Khazaie, H.; Osorio, R.S.; Rosenzweig, I.; Eickhoff, C.R.; Zarei, M.; Tahmasian, M.; Eickhoff, S.B.; et al. Gray matter volume and estimated brain age gap are not linked with sleep-disordered breathing. Hum. Brain Mapp. 2020, 41, 3034–3044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habes, M.; Pomponio, R.; Shou, H.; Doshi, J.; Mamourian, E.; Erus, G.; Nasrallah, I.; Launer, L.J.; Rashid, T.; Bilgel, M.; et al. The Brain Chart of Aging: Machine-learning analytics reveals links between brain aging, white matter disease, amyloid burden, and cognition in the iSTAGING consortium of 10,216 harmonized MR scans. Alzheimer’s Dement. 2020, 17, 89–102. [Google Scholar] [CrossRef]
- Millar, P.R.; Luckett, P.H.; Gordon, B.A.; Benzinger, T.L.; Schindler, S.E.; Fagan, A.M.; Cruchaga, C.; Bateman, R.J.; Allegri, R.; Jucker, M.; et al. Predicting brain age from functional connectivity in symptomatic and preclinical Alzheimer disease. NeuroImage 2022, 256, 119228. [Google Scholar] [CrossRef]
- Hill, N.T.M.; Mowszowski, L.; Naismith, S.L.; Chadwick, V.L.; Valenzuela, M.; Lampit, A. Computerized Cognitive Training in Older Adults With Mild Cognitive Impairment or Dementia: A Systematic Review and Meta-Analysis. Am. J. Psychiatry 2017, 174, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Sherman, D.S.; Mauser, J.; Nuno, M.; Sherzai, D. The Efficacy of Cognitive Intervention in Mild Cognitive Impairment (MCI): A Meta-Analysis of Outcomes on Neuropsychological Measures. Neuropsychol. Rev. 2017, 27, 440–484. [Google Scholar] [CrossRef] [Green Version]
- Nousia, A.; Siokas, V.; Aretouli, E.; Messinis, L.; Aloizou, A.-M.; Martzoukou, M.; Karala, M.; Koumpoulis, C.; Nasios, G.; Dardiotis, E. Beneficial Effect of Multidomain Cognitive Training on the Neuropsychological Performance of Patients with Early-Stage Alzheimer’s Disease. Neural Plast. 2018, 2018, 2845176. [Google Scholar] [CrossRef]
- Counts, S.E.; Ikonomovic, M.D.; Mercado, N.; Vega, I.E.; Mufson, E.J. Biomarkers for the Early Detection and Progression of Alzheimer’s Disease. Neurotherapeutics 2016, 14, 35–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eickhoff, C.R.; Hoffstaedter, F.; Caspers, J.; Reetz, K.; Mathys, C.; Dogan, I.; Amunts, K.; Schnitzler, A.; Eickhoff, S.B. Advanced brain ageing in Parkinson’s disease is related to disease duration and individual impairment. Brain Commun. 2021, 3, fcab191. [Google Scholar] [CrossRef] [PubMed]
- Charissé, D.; Erus, G.; Pomponio, R.; Gorges, M.; Schmidt, N.; Schneider, C.; Liepelt-Scarfone, I.; Riedel, O.; Reetz, K.; Schulz, J.B.; et al. Brain age and Alzheimer’s-like atrophy are domain-specific predictors of cognitive impairment in Parkinson’s disease. Neurobiol. Aging 2021, 109, 31–42. [Google Scholar] [CrossRef]
- Pardoe, H.R.; Cole, J.H.; Blackmon, K.; Thesen, T.; Kuzniecky, R.; Human Epilepsy Project Investigators. Structural brain changes in medically refractory focal epilepsy resemble premature brain aging. Epilepsy Res. 2017, 133, 28–32. [Google Scholar] [CrossRef]
- Hwang, G.; Hermann, B.; Nair, V.A.; Conant, L.L.; Dabbs, K.; Mathis, J.; Cook, C.J.; Rivera-Bonet, C.N.; Mohanty, R.; Zhao, G.; et al. Brain aging in temporal lobe epilepsy: Chronological, structural, and functional. NeuroImage Clin. 2020, 25, 102183. [Google Scholar] [CrossRef]
- de Bézenac, C.E.; Adan, G.; Weber, B.; Keller, S.S. Association of Epilepsy Surgery With Changes in Imaging-Defined Brain Age. Neurology 2021, 97, e554–e563. [Google Scholar] [CrossRef]
- Cole, J.H.; Raffel, J.; Friede, T.; Eshaghi, A.; Brownlee, W.J.; Chard, D.; De Stefano, N.; Enzinger, C.; Pirpamer, L.; Filippi, M.; et al. Longitudinal Assessment of Multiple Sclerosis with the Brain-Age Paradigm. Ann. Neurol. 2020, 88, 93–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, S.A.H.; Muraro, P.A.; Cencioni, M.T.; Knowles, S.; Cole, J.H.; Nicholas, R. Worse Physical Disability Is Associated With the Expression of PD-1 on Inflammatory T-Cells in Multiple Sclerosis Patients With Older Appearing Brains. Front. Neurol. 2022, 12, 801097. [Google Scholar] [CrossRef]
- Gan, M.S.; Shi, W.; Wang, M.S.; Sun, M.Y.; Yin, B.; Bai, G.; Jia, X.; Sun, C.; Niu, M.X.; Wang, Z.; et al. Accelerated Brain Aging in Mild Traumatic Brain Injury: Longitudinal Pattern Recognition with White Matter Integrity. J. Neurotrauma 2021, 38, 2549–2559. [Google Scholar] [CrossRef]
- Hellstrøm, T.; Andelic, N.; de Lange, A.-M.; Helseth, E.; Eiklid, K.; Westlye, L. Apolipoprotein ɛ4 Status and Brain Structure 12 Months after Mild Traumatic Injury: Brain Age Prediction Using Brain Morphometry and Diffusion Tensor Imaging. J. Clin. Med. 2021, 10, 418. [Google Scholar] [CrossRef]
- Yu, G.Z.; Ly, M.; Karim, H.T.; Muppidi, N.; Aizenstein, H.J.; Ibinson, J.W. Accelerated brain aging in chronic low back pain. Brain Res. 2021, 1755, 147263. [Google Scholar] [CrossRef] [PubMed]
- Hung, P.S.-P.; Zhang, J.Y.; Noorani, A.; Walker, M.R.; Huang, M.; Zhang, J.W.; Laperriere, N.; Rudzicz, F.; Hodaie, M. Differential expression of a brain aging biomarker across discrete chronic pain disorders. Pain 2022, 163, 1468–1478. [Google Scholar] [CrossRef]
- Azor, A.M.; Cole, J.H.; Holland, A.J.; Dumba, M.; Patel, M.C.; Sadlon, A.; Goldstone, A.P.; Manning, K.E. Increased brain age in adults with Prader-Willi syndrome. NeuroImage Clin. 2019, 21, 101664. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.H.; Underwood, J.; Caan, M.W.; De Francesco, D.; Van Zoest, R.A.; Leech, R.; Wit, F.W.; Portegies, P.; Geurtsen, G.J.; Schmand, B.A.; et al. Increased brain-predicted aging in treated HIV disease. Neurology 2017, 88, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T.D. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014, 29, 1583–1590. [Google Scholar] [CrossRef]
- Oh, J.; Vidal-Jordana, A.; Montalban, X. Multiple sclerosis: Clinical aspects. Curr. Opin. Neurol. 2018, 31, 752–759. [Google Scholar] [CrossRef]
- Koutsouleris, N.; Davatzikos, C.; Borgwardt, S.; Gaser, C.; Bottlender, R.; Frodl, T.; Falkai, P.; Riecher-Rössler, A.; Möller, H.-J.; Reiser, M.; et al. Accelerated Brain Aging in Schizophrenia and Beyond: A Neuroanatomical Marker of Psychiatric Disorders. Schizophr. Bull. 2013, 40, 1140–1153. [Google Scholar] [CrossRef] [Green Version]
- Schnack, H.G.; Van Haren, N.E.; Nieuwenhuis, M.; Pol, H.H.; Cahn, W.; Kahn, R.S. Accelerated Brain Aging in Schizophrenia: A Longitudinal Pattern Recognition Study. Am. J. Psychiatry 2016, 173, 607–616. [Google Scholar] [CrossRef] [Green Version]
- Nenadić, I.; Dietzek, M.; Langbein, K.; Sauer, H.; Gaser, C. BrainAGE score indicates accelerated brain aging in schizophrenia, but not bipolar disorder. Psychiatry Res. Neuroimaging 2017, 266, 86–89. [Google Scholar] [CrossRef]
- Kolenic, M.; Franke, K.; Hlinka, J.; Matejka, M.; Capkova, J.; Pausova, Z.; Uher, R.; Alda, M.; Spaniel, F.; Hajek, T. Obesity, dyslipidemia and brain age in first-episode psychosis. J. Psychiatr. Res. 2018, 99, 151–158. [Google Scholar] [CrossRef]
- Chung, Y.; Addington, J.; Bearden, C.E.; Cadenhead, K.; Cornblatt, B.; Mathalon, D.H.; McGlashan, T.; Perkins, D.; Seidman, L.J.; Tsuang, M.; et al. Adding a neuroanatomical biomarker to an individualized risk calculator for psychosis: A proof-of-concept study. Schizophr. Res. 2019, 208, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Hajek, T.; Franke, K.; Kolenic, M.; Capkova, J.; Matejka, M.; Propper, L.; Uher, R.; Stopkova, P.; Novak, T.; Paus, T.; et al. Brain Age in Early Stages of Bipolar Disorders or Schizophrenia. Schizophr. Bull. 2017, 45, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Shahab, S.; Mulsant, B.H.; Levesque, M.L.; Calarco, N.; Nazeri, A.; Wheeler, A.L.; Foussias, G.; Rajji, T.K.; Voineskos, A.N. Brain structure, cognition, and brain age in schizophrenia, bipolar disorder, and healthy controls. Neuropsychopharmacology 2018, 44, 898–906. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.-Y.; Lee, P.-L.; Hung, S.-C.; Liu, L.-K.; Lee, W.-J.; Chung, C.-P.; Yang, A.C.; Tsai, S.-J.; Wang, P.-N.; Chen, L.-K.; et al. Large-Scale Structural Covariance Networks Predict Age in Middle-to-Late Adulthood: A Novel Brain Aging Biomarker. Cereb. Cortex 2020, 30, 5844–5862. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, S.; Kaufmann, T.; de Lange, A.-M.G.; Richard, G.; Doan, N.T.; Alnæs, D.; van der Meer, D.; Rokicki, J.; Moberget, T.; Maximov, I.I.; et al. Brain Age Prediction Reveals Aberrant Brain White Matter in Schizophrenia and Bipolar Disorder: A Multisample Diffusion Tensor Imaging Study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2020, 5, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Antoniades, M.; Schnack, H.G.; Kahn, R.S.; Frangou, S. Brain age prediction in schizophrenia: Does the choice of machine learning algorithm matter? Psychiatry Res. Neuroimaging 2021, 310, 111270. [Google Scholar] [CrossRef]
- Lieslehto, J.; Jääskeläinen, E.; Kiviniemi, V.; Haapea, M.; Jones, P.B.; Murray, G.K.; Veijola, J.; Dannlowski, U.; Grotegerd, D.; Meinert, S.; et al. The progression of disorder-specific brain pattern expression in schizophrenia over 9 years. npj Schizophr. 2021, 7, 32. [Google Scholar] [CrossRef]
- McWhinney, S.; Kolenic, M.; Franke, K.; Fialova, M.; Knytl, P.; Matejka, M.; Spaniel, F.; Hajek, T. Obesity as a Risk Factor for Accelerated Brain Ageing in First-Episode Psychosis—A Longitudinal Study. Schizophr. Bull. 2021, 47, 1772–1781. [Google Scholar] [CrossRef]
- Teeuw, J.; Ori, A.P.; Brouwer, R.M.; de Zwarte, S.M.; Schnack, H.G.; Pol, H.E.H.; Ophoff, R.A. Accelerated aging in the brain, epigenetic aging in blood, and polygenic risk for schizophrenia. Schizophr. Res. 2021, 231, 189–197. [Google Scholar] [CrossRef]
- Wang, J.; Kochunov, P.; Sampath, H.; Hatch, K.S.; Ryan, M.C.; Xue, F.; Neda, J.; Paul, T.; Hahn, B.; Gold, J.; et al. White matter brain aging in relationship to schizophrenia and its cognitive deficit. Schizophr. Res. 2021, 230, 9–16. [Google Scholar] [CrossRef]
- Xi, Y.-B.; Wu, X.-S.; Cui, L.-B.; Bai, L.-J.; Gan, S.-Q.; Jia, X.-Y.; Li, X.; Xu, Y.-Q.; Kang, X.-W.; Guo, F.; et al. Neuroimaging-based brain-age prediction of first-episode schizophrenia and the alteration of brain age after early medication. Br. J. Psychiatry 2021, 220, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Demro, C.; Shen, C.; Hendrickson, T.J.; Arend, J.L.; Disner, S.G.; Sponheim, S.R. Advanced Brain-Age in Psychotic Psychopathology: Evidence for Transdiagnostic Neurodevelopmental Origins. Front. Aging Neurosci. 2022, 14, 872867. [Google Scholar] [CrossRef] [PubMed]
- Besteher, B.; Gaser, C.; Nenadić, I. Machine-learning based brain age estimation in major depression showing no evidence of accelerated aging. Psychiatry Res. Neuroimaging 2019, 290, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Van Gestel, H.; Franke, K.; Petite, J.; Slaney, C.; Garnham, J.; Helmick, C.; Johnson, K.; Uher, R.; Alda, M.; Hajek, T. Brain age in bipolar disorders: Effects of lithium treatment. Aust. N. Z. J. Psychiatry 2019, 53, 1179–1188. [Google Scholar] [CrossRef]
- De Nooij, L.; Harris, M.A.; Hawkins, E.L.; Clarke, T.-K.; Shen, X.; Chan, S.W.Y.; Ziermans, T.B.; McIntosh, A.M.; Whalley, H.C. Longitudinal trajectories of brain age in young individuals at familial risk of mood disorder from the Scottish Bipolar Family Study. Wellcome Open Res. 2020, 4, 206. [Google Scholar] [CrossRef]
- Christman, S.; Bermudez, C.; Hao, L.; Landman, B.A.; Boyd, B.; Albert, K.; Woodward, N.; Shokouhi, S.; Vega, J.; Andrews, P.; et al. Accelerated brain aging predicts impaired cognitive performance and greater disability in geriatric but not midlife adult depression. Transl. Psychiatry 2020, 10, 317. [Google Scholar] [CrossRef]
- Ahmed, R.; Ryan, C.; Christman, S.; Elson, D.; Bermudez, C.; Landman, B.A.; Szymkowicz, S.M.; Boyd, B.D.; Kang, H.; Taylor, W.D. Structural MRI-Based Measures of Accelerated Brain Aging do not Moderate the Acute Antidepressant Response in Late-Life Depression. Am. J. Geriatr. Psychiatry 2021, 30, 1015–1025. [Google Scholar] [CrossRef]
- Ballester, P.L.; Suh, J.S.; Nogovitsyn, N.; Hassel, S.; Strother, S.C.; Arnott, S.R.; Minuzzi, L.; Sassi, R.B.; Lam, R.W.; Milev, R.; et al. Accelerated brain aging in major depressive disorder and antidepressant treatment response: A CAN-BIND report. NeuroImage Clin. 2021, 32, 102864. [Google Scholar] [CrossRef]
- Han, L.K.M.; Dinga, R.; Hahn, T.; Ching, C.R.K.; Eyler, L.T.; Aftanas, L.; Aghajani, M.; Aleman, A.; Baune, B.T.; Berger, K.; et al. Brain aging in major depressive disorder: Results from the ENIGMA major depressive disorder working group. Mol. Psychiatry 2020, 26, 5124–5139. [Google Scholar] [CrossRef]
- Han, L.K.M.; Schnack, H.G.; Brouwer, R.M.; Veltman, D.J.; van der Wee, N.J.A.; van Tol, M.-J.; Aghajani, M.; Penninx, B.W.J.H. Contributing factors to advanced brain aging in depression and anxiety disorders. Transl. Psychiatry 2021, 11, 402. [Google Scholar] [CrossRef]
- Dunlop, K.; Victoria, L.W.; Downar, J.; Gunning, F.M.; Liston, C. Accelerated brain aging predicts impulsivity and symptom severity in depression. Neuropsychopharmacology 2021, 46, 911–919. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.; Yang, L.; Wen, B.; Zhang, X.; Cheng, J.; Han, S.; Zhang, Y.; Cheng, J. Accelerated Brain Aging in Patients With Obsessive-Compulsive Disorder. Front. Psychiatry 2022, 13, 852479. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Taylor, A.; Shinohara, R.T.; Kounios, J.; Zhang, F. Multidimensional Brain-Age Prediction Reveals Altered Brain Developmental Trajectory in Psychiatric Disorders. Cereb. Cortex 2022. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.C.; Hong, L.E.; Hatch, K.S.; Gao, S.; Chen, S.; Haerian, K.; Wang, J.; Goldwaser, E.L.; Du, X.; Adhikari, B.M.; et al. The additive impact of cardio-metabolic disorders and psychiatric illnesses on accelerated brain aging. Hum. Brain Mapp. 2022, 43, 1997–2010. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, T.; van der Meer, D.; Doan, N.T.; Schwarz, E.; Lund, M.J.; Agartz, I.; Alnæs, D.; Barch, D.M.; Baur-Streubel, R.; Bertolino, A.; et al. Common brain disorders are associated with heritable patterns of apparent aging of the brain. Nat. Neurosci. 2019, 22, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Bashyam, V.M.; Erus, G.; Doshi, J.; Habes, M.; Nasrallah, I.M.; Truelove-Hill, M.; Srinivasan, D.; Mamourian, L.; Pomponio, R.; Fan, Y.; et al. MRI signatures of brain age and disease over the lifespan based on a deep brain network and 14 468 individuals worldwide. Brain 2020, 143, 2312–2324. [Google Scholar] [CrossRef]
- Kolbeinsson, A.; Filippi, S.; Panagakis, Y.; Matthews, P.M.; Elliott, P.; Dehghan, A.; Tzoulaki, I. Accelerated MRI-predicted brain ageing and its associations with cardiometabolic and brain disorders. Sci. Rep. 2020, 10, 19940. [Google Scholar] [CrossRef]
- Rokicki, J.; Wolfers, T.; Nordhøy, W.; Tesli, N.; Quintana, D.S.; Alnæs, D.; Richard, G.; de Lange, A.G.; Lund, M.J.; Norbom, L.; et al. Multimodal imaging improves brain age prediction and reveals distinct abnormalities in patients with psychiatric and neurological disorders. Hum. Brain Mapp. 2020, 42, 1714–1726. [Google Scholar] [CrossRef]
- Franke, K.; Gaser, C.; Manor, B.; Novak, V. Advanced BrainAGE in older adults with type 2 diabetes mellitus. Front. Aging Neurosci. 2013, 5, 90. [Google Scholar] [CrossRef]
- Efranke, K.; Eristow, M.; Egaser, C. Gender-specific impact of personal health parameters on individual brain aging in cognitively unimpaired elderly subjects. Front. Aging Neurosci. 2014, 6, 94. [Google Scholar] [CrossRef]
- Franke, K.; Hagemann, G.; Schleussner, E.; Gaser, C. Changes of individual BrainAGE during the course of the menstrual cycle. NeuroImage 2015, 115, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Luders, E.; Cherbuin, N.; Gaser, C. Estimating brain age using high-resolution pattern recognition: Younger brains in long-term meditation practitioners. NeuroImage 2016, 134, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Franke, K.; Gaser, C.; Roseboom, T.J.; Schwab, M.; de Rooij, S.R. Premature brain aging in humans exposed to maternal nutrient restriction during early gestation. NeuroImage 2018, 173, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Hatton, S.N.; Franz, C.E.; Elman, J.; Panizzon, M.S.; Hagler, D.J.; Fennema-Notestine, C.; Eyler, L.T.; McEvoy, L.K.; Lyons, M.J.; Dale, A.M.; et al. Negative fateful life events in midlife and advanced predicted brain aging. Neurobiol. Aging 2018, 67, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kiehl, K.A.; Anderson, N.E.; Aharoni, E.; Maurer, J.; Harenski, K.A.; Rao, V.; Claus, E.D.; Harenski, C.; Koenigs, M.; Decety, J.; et al. Age of gray matters: Neuroprediction of recidivism. NeuroImage Clin. 2018, 19, 813–823. [Google Scholar] [CrossRef]
- Le, T.T.; Kuplicki, R.; Yeh, H.-W.; Aupperle, R.L.; Khalsa, S.S.; Simmons, W.K.; Paulus, M.P. Effect of Ibuprofen on BrainAGE: A Randomized, Placebo-Controlled, Dose-Response Exploratory Study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 836–843. [Google Scholar] [CrossRef]
- Luders, E.; Gingnell, M.; Poromaa, I.S.; Engman, J.; Kurth, F.; Gaser, C. Potential Brain Age Reversal after Pregnancy: Younger Brains at 4–6 Weeks Postpartum. Neuroscience 2018, 386, 309–314. [Google Scholar] [CrossRef]
- Rogenmoser, L.; Kernbach, J.; Schlaug, G.; Gaser, C. Keeping brains young with making music. Anat. Embryol. 2017, 223, 297–305. [Google Scholar] [CrossRef]
- Scheller, E.; Schumacher, L.; Peter, J.; Lahr, J.; Wehrle, J.; Kaller, C.; Gaser, C.; Klöppel, S. Brain Aging and APOE ε4 Interact to Reveal Potential Neuronal Compensation in Healthy Older Adults. Front. Aging Neurosci. 2018, 10, 74. [Google Scholar] [CrossRef] [Green Version]
- de Lange, A.-M.G.; Kaufmann, T.; van der Meer, D.; Maglanoc, L.A.; Alnæs, D.; Moberget, T.; Douaud, G.; Andreassen, O.A.; Westlye, L.T. Population-based neuroimaging reveals traces of childbirth in the maternal brain. Proc. Natl. Acad. Sci. USA 2019, 116, 22341–22346. [Google Scholar] [CrossRef]
- Cruz-Almeida, Y.; Fillingim, R.; Riley, J.L., III; Woods, A.J.; Porges, E.; Cohen, R.; Cole, J. Chronic pain is associated with a brain aging biomarker in community-dwelling older adults. Pain 2019, 160, 1119–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Lange, A.-M.G.; Anatürk, M.; Suri, S.; Kaufmann, T.; Cole, J.H.; Griffanti, L.; Zsoldos, E.; Jensen, D.E.; Filippini, N.; Singh-Manoux, A.; et al. Multimodal brain-age prediction and cardiovascular risk: The Whitehall II MRI sub-study. NeuroImage 2020, 222, 117292. [Google Scholar] [CrossRef] [PubMed]
- de Lange, A.G.; Barth, C.; Kaufmann, T.; Anatürk, M.; Suri, S.; Ebmeier, K.; Westlye, L.T. The maternal brain: Region-specific patterns of brain aging are traceable decades after childbirth. Hum. Brain Mapp. 2020, 41, 4718–4729. [Google Scholar] [CrossRef] [PubMed]
- Henneghan, A.; Rao, V.; Harrison, R.A.; Karuturi, M.; Blayney, D.W.; Palesh, O.; Kesler, S.R. Cortical Brain Age from Pre-treatment to Post-chemotherapy in Patients with Breast Cancer. Neurotox. Res. 2020, 37, 788–799. [Google Scholar] [CrossRef]
- Reuben, A.; Elliott, M.L.; Abraham, W.C.; Broadbent, J.; Houts, R.M.; Ireland, D.; Knodt, A.R.; Poulton, R.; Ramrakha, S.; Hariri, A.R.; et al. Association of Childhood Lead Exposure With MRI Measurements of Structural Brain Integrity in Midlife. JAMA 2020, 324, 1970–1979. [Google Scholar] [CrossRef]
- Seidel, G.; Gaser, C.; Götz, T.; Günther, A.; Hamzei, F. Accelerated brain ageing in sepsis survivors with cognitive long-term impairment. Eur. J. Neurosci. 2020, 52, 4395–4402. [Google Scholar] [CrossRef]
- Anatürk, M.; Kaufmann, T.; Cole, J.H.; Suri, S.; Griffanti, L.; Zsoldos, E.; Filippini, N.; Singh-Manoux, A.; Kivimäki, M.; Westlye, L.T.; et al. Prediction of brain age and cognitive age: Quantifying brain and cognitive maintenance in aging. Hum. Brain Mapp. 2020, 42, 1626–1640. [Google Scholar] [CrossRef]
- Bittner, N.; Jockwitz, C.; Franke, K.; Gaser, C.; Moebus, S.; Bayen, U.J.; Amunts, K.; Caspers, S. When your brain looks older than expected: Combined lifestyle risk and BrainAGE. Anat. Embryol. 2021, 226, 621–645. [Google Scholar] [CrossRef]
- Cherbuin, N.; Walsh, E.I.; Shaw, M.; Luders, E.; Anstey, K.J.; Sachdev, P.S.; Abhayaratna, W.P.; Gaser, C. Optimal Blood Pressure Keeps Our Brains Younger. Front. Aging Neurosci. 2021, 13, 694982. [Google Scholar] [CrossRef]
- Dunås, T.; Wåhlin, A.; Nyberg, L.; Boraxbekk, C.-J. Multimodal Image Analysis of Apparent Brain Age Identifies Physical Fitness as Predictor of Brain Maintenance. Cereb. Cortex 2021, 31, 3393–3407. [Google Scholar] [CrossRef]
- Elliott, M.L.; Belsky, D.W.; Knodt, A.R.; Ireland, D.; Melzer, T.R.; Poulton, R.; Ramrakha, S.; Caspi, A.; Moffitt, T.E.; Hariri, A.R. Brain-age in midlife is associated with accelerated biological aging and cognitive decline in a longitudinal birth cohort. Mol. Psychiatry 2019, 26, 3829–3838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedderich, D.M.; Menegaux, A.; Schmitz-Koep, B.; Nuttall, R.; Zimmermann, J.; Schneider, S.C.; Bäuml, J.G.; Daamen, M.; Boecker, H.; Wilke, M.; et al. Increased Brain Age Gap Estimate (BrainAGE) in Young Adults After Premature Birth. Front. Aging Neurosci. 2021, 13, 653365. [Google Scholar] [CrossRef] [PubMed]
- Karim, H.T.; Ly, M.; Yu, G.; Krafty, R.; Tudorascu, D.L.; Aizenstein, H.J.; Andreescu, C. Aging faster: Worry and rumination in late life are associated with greater brain age. Neurobiol. Aging 2021, 101, 13–21. [Google Scholar] [CrossRef]
- Rakesh, D.; Cropley, V.; Zalesky, A.; Vijayakumar, N.; Allen, N.B.; Whittle, S. Neighborhood disadvantage and longitudinal brain-predicted-age trajectory during adolescence. Dev. Cogn. Neurosci. 2021, 51, 101002. [Google Scholar] [CrossRef] [PubMed]
- Rosemann, S.; Thiel, C.M. No association between age-related hearing loss and brain age derived from structural neuroimaging data. Neuroimage Rep. 2021, 1, 100020. [Google Scholar] [CrossRef]
- Salih, A.; Galazzo, I.B.; Raisi-Estabragh, Z.; Rauseo, E.; Gkontra, P.; Petersen, S.E.; Lekadir, K.; Altmann, A.; Radeva, P.; Menegaz, G. Brain age estimation at tract group level and its association with daily life measures, cardiac risk factors and genetic variants. Sci. Rep. 2021, 11, 20563. [Google Scholar] [CrossRef] [PubMed]
- Sanders, A.-M.; Richard, G.; Kolskår, K.; Ulrichsen, K.M.; Kaufmann, T.; Alnæs, D.; Beck, D.; Dørum, E.S.; de Lange, A.-M.G.; Nordvik, J.E.; et al. Linking objective measures of physical activity and capability with brain structure in healthy community dwelling older adults. NeuroImage Clin. 2021, 31, 102767. [Google Scholar] [CrossRef] [PubMed]
- Subramaniapillai, S.; Rajagopal, S.; Snytte, J.; Otto, A.R.; Einstein, G.; Rajah, M.N. Sex differences in brain aging among adults with family history of Alzheimer’s disease and APOE4 genetic risk. NeuroImage Clin. 2021, 30, 102620. [Google Scholar] [CrossRef]
- Vidal-Pineiro, D.; Wang, Y.; Krogsrud, S.K.; Amlien, I.K.; Baaré, W.F.; Bartres-Faz, D.; Bertram, L.; Brandmaier, A.M.; Drevon, C.A.; Düzel, S.; et al. Individual variations in ‘brain age’ relate to early-life factors more than to longitudinal brain change. eLife 2021, 10, e69995. [Google Scholar] [CrossRef]
- Weihs, A.; Frenzel, S.; Wittfeld, K.; Obst, A.; Stubbe, B.; Habes, M.; Szentkirályi, A.; Berger, K.; Fietze, I.; Penzel, T.; et al. Associations between sleep apnea and advanced brain aging in a large-scale population study. Sleep 2020, 44, zsaa204. [Google Scholar] [CrossRef]
- Angebrandt, A.; Abulseoud, O.A.; Kisner, M.; Diazgranados, N.; Momenan, R.; Yang, Y.; Stein, E.A.; Ross, T.J. Dose-dependent relationship between social drinking and brain aging. Neurobiol. Aging 2021, 111, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Beck, D.; Lange, A.G.; Pedersen, M.L.; Alnæs, D.; Maximov, I.I.; Voldsbekk, I.; Richard, G.; Sanders, A.; Ulrichsen, K.M.; Dørum, E.S.; et al. Cardiometabolic risk factors associated with brain age and accelerate brain ageing. Hum. Brain Mapp. 2021, 43, 700–720. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, K.J.; Moffitt, T.E.; Ambler, A.; Hariri, A.R.; Harrington, H.; Houts, R.M.; Ireland, D.; Knodt, A.; Poulton, R.; Ramrakha, S.; et al. Association of Treatable Health Conditions During Adolescence With Accelerated Aging at Midlife. JAMA Pediatr. 2022, 176, 392. [Google Scholar] [CrossRef]
- Giannakopoulos, P.; Montandon, M.-L.; Herrmann, F.R.; Hedderich, D.; Gaser, C.; Kellner, E.; Rodriguez, C.; Haller, S. Alzheimer resemblance atrophy index, BrainAGE, and normal pressure hydrocephalus score in the prediction of subtle cognitive decline: Added value compared to existing MR imaging markers. Eur. Radiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Linli, Z.; Feng, J.; Zhao, W.; Guo, S. Associations between smoking and accelerated brain ageing. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2021, 113, 110471. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, B.A.; Simon, J.E.; Grooms, D.R.; Clark, L.A.; Wages, N.P.; Clark, B.C. Brain-Predicted Age Difference Moderates the Association Between Muscle Strength and Mobility. Front. Aging Neurosci. 2022, 14, 808022. [Google Scholar] [CrossRef]
- Wang, M.; Ren, Q.; Shi, Y.; Shu, H.; Liu, D.; Gu, L.; Xie, C.; Zhang, Z.; Wu, T.; Wang, Z. The effect of Alzheimer’s disease risk factors on brain aging in normal Chineses: Cognitive aging and cognitive reserve. Neurosci. Lett. 2021, 771, 136398. [Google Scholar] [CrossRef]
- Whitsel, N.; Reynolds, C.A.; Buchholz, E.J.; Pahlen, S.; Pearce, R.C.; Hatton, S.N.; Elman, J.A.; Gillespie, N.A.; Gustavson, D.E.; Puckett, O.K.; et al. Long-term associations of cigarette smoking in early mid-life with predicted brain aging from mid- to late life. Addiction 2021, 117, 1049–1059. [Google Scholar] [CrossRef]
- Zheng, Y.; Habes, M.; Gonzales, M.; Pomponio, R.; Nasrallah, I.; Khan, S.; Vaughan, D.E.; Davatzikos, C.; Seshadri, S.; Launer, L.; et al. Mid-life epigenetic age, neuroimaging brain age, and cognitive function: Coronary artery risk development in young adults (CARDIA) study. Aging 2022, 14, 1691–1712. [Google Scholar] [CrossRef]
| First Author [ref.] | Year | Cohort | Imaging Modality | ML Algorithm | Main Findings |
|---|---|---|---|---|---|
| Alzheimer’s Disease and Cognitive Impairment | |||||
| Gaser [31] | 2013 | 133 pMCI, 62 sMCI | T1WI | RVR | BAG predicts conversion to AD, 10% greater risk of developing AD by each 1 additional yr of BAG |
| Lowe [32] | 2016 | 150 AD, 112 pMCI, 36 sMCI, 107HC | T1WI | RVR | Effect of APOEe4 on BrainAGE changing rates over time |
| Beheshti [17] | 2018 | 147 AD, 112 pMCI, 102 sMCI, 146 HCs | T1WI | SVR | BAG: +5.36 yr in AD, +3.15 yr in pMCI, +2.38 yr in sMCI. Correlation with cognitive function |
| Wang [33] | 2019 | 3688 people (middle age to elderly) | T1WI | CNN | BAG: related to incident dementia |
| Mohajer [35] | 2020 | 48 AD, 222 MCI, 60 HCs | T1WI | SVR | BAG was elevated in MCI and AD but was not associated with sleep-disordered breathing. |
| Ly [34] | 2020 | 74 AD, 283 MCI, 51 preclinical AD, 83 HCs | T1WI | GPR | BAG differentiated cognitively unimpaired Amyloid (+) from Amyloid (−). |
| Beheshti [23] | 2021 | 292 AD, 440 MCI, 548 HCs | FDG-PET | SVR | Younger BAG in females than in males in HCs group but not in MCI or AD groups |
| Habes [36] | 2021 | 1932 MCI/AD, 8284 HCs | T1WI | RBF-kernel | BAG associated with WMH as well as cognitive function |
| Parkinson’s disease | |||||
| Beheshti [20] | 2020 | 160 PD, 129 AD, 839 HCs | T1WI | SVR | GM-based BAG: +1.50 yr in PD, +9.29 yr in AD. WM-based BAG: +2.47 yr in PD, +8.85 yr in AD. WM-based BAG > GM-based BAG in PD |
| Eickhoff [42] | 2021 | 372 PD, 172 HCs | T1WI | SVR | BAG: +2.9 yr in PD. Associated with disease duration and cognitive and motor impairment. |
| Charisse [43] | 2022 | 83 PD-NC, 78 PD-MCI, 17 PD-D, 84 HCs | T1WI | SVR | RBA: +2.38 yr in PD-NC, +1.90 yr in PD-MCI, +3.52 yr in PD-D. Associated with attention deficits and working memory |
| Epilepsy | |||||
| Pardoe [44] | 2017 | 42 new FE, 94 refractory FE, 74 HCs | T1WI | GPR | BAG: +4.5yr in refractory FE, no significance in new FE |
| Hwang [45] | 2020 | 104 TLE, 151 HCs | T1WI, fMRI | SVR | T1-based BAG: +6.6 yr in TLE. fMRI-based BAG: +8.3 yr in TLE Association with clinical data |
| Sone [19] | 2021 | 318 epilepsy, 1,196 HCs | T1WI | SVR | BAG: >+4 yr in all types of epilepsies, +10.9 yr in TLE with psychosis |
| de Bézenac [46] | 2022 | 48 TLE, 37 HCs | T1WI | GPR | BAG: +7.97 yr in TLE, postsurgical reduction of BAG |
| Multiple sclerosis | |||||
| Cole [47] | 2020 | 1204 MS/CIS, 150 HCs | T1WI | GPR | BAG: +10.3 yr in MS, +13.3 yr in SPMS, predictive value for progression |
| Jacobs [48] | 2021 | 179 MS | T1WI | GPR | BAG: +6.54 yr in MS, associated with a physical disability |
| Traumatic brain injury | |||||
| Gan [49] | 2021 | 116 mTBI, 63 HCs | DTI | RVR | BAG: +2.59 yr in mTBI, associated with post-concussion complaints |
| Hellstrom [50] | 2021 | 123 mTBI | T1WI, DTI | XGBoost | No significant difference in BAG between APOEe4 carriers and non-carriers after mTBI |
| Pain | |||||
| Yu [51] | 2021 | 31 CLBP, 32 HCs | T1WI | GPR | Discrepancy in BAG between HCs and CLBP was greater in older individuals |
| Hung [52] | 2022 | 45 TN, 52 OA, 50 CLBP, 812 HCs | T1WI | GPR | BAG: +6.48 yr in TN, +9.80 yr in OA, no significance in BP. Female-driven elevation in BAG |
| Others | |||||
| Azor [53] | 2019 | 20 PWS, 40 HCs | T1WI | GPR | BAG: +7.24 yr in PWS, Not associated with IQ, hormonal or psychotropic medications, or abnormal behaviors |
| Cole [54] | 2017 | 162 HIV(+), 105 HIV(−) | T1WI | GPR | BAG: +2.15 yr in HIV(+), associated with cognitive performance |
| First Author [ref.] | Year | Cohort | Imaging Modality | ML Algorithm | Main Findings |
|---|---|---|---|---|---|
| Schizophrenia and Psychosis | |||||
| Koutsouleris [57] | 2014 | 141 SZ, 104 MDD, 57B PD, 89 ARMS, 127 HCs | T1WI | SVR | BAG: +5.5 yr in SZ, +4.0 yr in MDD, +3.1 yr in BPD, +1.7 yr in ARMS. |
| Schnack [58] | 2016 | 341 SZ, 386 HCs | T1WI | SVR | BAG: +3.36 yr in SZ, acceleration just after illness onset |
| Nenadic [59] | 2017 | 45 SZ, 22 BPAD, 70 HCs | T1WI | RVR | BAG: +2.56 yr in SZ, no significance in BPAD |
| Kolenic [60] | 2018 | 120 FEP, 114 HCs | T1WI | RVR | BAG: +2.64 yr in FES, associated with obesity |
| Hajek [62] | 2019 | 43 FES, 43 HCs, 96 offspring of BPAD (48 affected, 48 unaffected), 60 HCs | T1WI | RVR | BAG: +2.64 yr in FES, no significance in early BPAD |
| Chung [61] | 2019 | 476 CHR | N/A | N/A | BAG predicts conversion to psychosis in a univariate analysis but not in a multivariate analysis |
| Shahab [63] | 2019 | 81 SZ, 53 BPAD, 91 HCs | T1WI, DTI | RF | BAG: +7.8–8.2 yr in SZ, no significance in BPAD |
| Kuo [64] | 2020 | 26 SZ, 30 MDD, 19AD, 109 HCs | T1WI | LASSO, ICA | BAG: +5.69 yr in SCZ, +3.25 yr in AD, no significance in MDD. Association with large-scale structural covariance network |
| Tønnesen [65] | 2020 | 668 SZ, 185 BPAD, 990 HCs | DTI | XGBoost | Increased BAG in SZ (Cohen’s d = −0.29) and BPAD (Cohen’s d = 0.18) |
| Lee [66] | 2021 | 90 SZ, 200 HCs, 76 SZ, 87 HCs | T1WI | OLS, Ridge, LASSO, Elastic-Net, SVR, RVR | BAG: +3.8–5.2yr in SZ cohort 1, +4.5–11.7 yr in SZ cohort 2. Algorithm choice can be a cause of inter-study variability. |
| Lieslehto [67] | 2021 | 29 SZ, 61 HCs | T1WI | SVR | BAG: +1.3 yr at baseline, +7.7 yr at follow-up in SZ. It was suggested that BA captured treatment-related and global brain alterations. |
| McWhinney [68] | 2021 | 183FEP, 155 HCs | T1WI | RVR | BAG: +3.39 yr in FEP at baseline, longitudinal worsening was associated with clinical outcomes or higher baseline BMI |
| Teeuw [69] | 2021 | 193 SZ, 218 HCs | T1WI | SVR | BAG: correlation with polygenic risk, no correlation with epigenetic aging |
| Wang [70] | 2021 | 166 SZ, 107 HCs | DTI | RF | BAG: +5.903 in SZ >30 yrs old. Association with working memory and processing speed |
| Xi [71] | 2021 | 60 FES, 60 HCs | DTI | RVR | BAG: +4.932 yr in FES, +2.718. Decreased BAG after early medication |
| Demro [72] | 2022 | 163 psychosis, 103 relatives, 66 HCs | T1WI | SVR/RF | BAG increase in psychosis more than HCs or relatives. Associated with cognition or schizotypal symptoms in relatives |
| Mood disorders | |||||
| Bestteher [73] | 2019 | 38 MDD, 40 HCs | T1WI | RVR | BAG: no significant change in MDD |
| Van Gestel [74] | 2019 | 84 BPAD, 45 HCs | T1WI | RVR | BAG: +4.28 yr in BPAD without Li treatment, no significance in BPAD with Li treatment or HCs |
| de Nooij [75] | 2019 | 283AYA | T1WI | RVR | Reduction of BAG in young high-risk individuals who developed a mood disorder over 2-yr follow-up |
| Christman [76] | 2020 | 76 MDD (middle-age), 118 MDD (elderly), 130 HCs | T1WI | CNN | BAG: +3.69 yrs in geriatric MDD, no increase in mid-life MDD. Associated with cognitive and functional deficits in elderly |
| Ahmed [77] | 2021 | 95 late-life depression | T1WI | CNN | BAG: +4.36 yrs in late-life depression. Not associated with treatment response. |
| Ballester [78] | 2021 | 160 MDD, 111 HCs | T1WI | GPR | BAG: higher in older MDD than in younger MDD, associated with BMI in MDD, not associated with treatment response |
| Han [79] | 2021 | 2675 MDD, 4314 HCs | T1WI | Ridge regression | BAG: +1.08 yr in MDD with no specific association with clinical characteristics |
| Han [80] | 2021 | 220 MDD/Anxiety, 65 HCs | T1WI | Ridge regression | BAG: +2.78 yr in MDD, +2.91 yr in Anxiety. Association with somatic symptoms (+4.21 yr) and antidepressant use (−2.53 yr) |
| Dunlop [81] | 2021 | 109 MDD, 710 HCs | fMRI | SVR | BAG: +2.11 yr in MDD, associated with impulsivity and symptom severity |
| Others | |||||
| Liu [82] | 2022 | 90 OCD, 106 HCs | T1WI | GPR | BAP: +0.826 yr in OCD, associated with disease duration |
| Niu [83] | 2022 | 70 SP, 77 SAD, 70 MDD, 44 PTSD, 48 ODD, 81 ADHD | T1WI | Ridge regression | Multidimensional brain-age index is sensitive to distinct regional change patterns |
| Ryan [84] | 2022 | 1618 SMI, 11,849 HCs | DTI | RF, gradient boosting regression, LASSO | Additive effect of SMI and cardiometabolic disorders on brain aging, the greater effect of SMI than CMD |
| Comprehensive | |||||
| Kaufmann [85] | 2019 | 10,141 patients, 35,474 HCs | T1WI | XGBoost | BAG: d = +1.03 in dementia, +0.41 in MCI, +0.10 in MDD, +0.74 in MS, +0.29 in BPAD, +0.51 in SZ, +0.06 in ADHD, +0.07 in ASD |
| Bashyam [86] | 2020 | 353 AD, 833 MCI, 387 SZ, 12,689 HCs | T1WI | CNN | Successful discrimination for neuropsychiatric disorders |
| Kolbeinsson [87] | 2020 | 12,196 people who had not been stratified for health | T1WI | CNN | Identified risk factors, e.g., MS, diabetes, and beneficial factors, e.g., physical strength |
| Rokicki [88] | 2021 | 54 AD, 90 MCI, 56 SCI, 159 SZ, 135 BPAD, 750 HCs | T1WI, T2WI, ASL | RF | Highest accuracy by multimodal imaging model |
| First Author [ref.] | Year | Cohort | Imaging Modality | ML Algorithm | Main Findings |
|---|---|---|---|---|---|
| Franke [89] | 2013 | 185 people | T1WI | RVR | BAG: +4.6 yr in T2DM, Acceleration by +0.2 yr per year |
| Franke [90] | 2014 | 228 elderly | T1WI | RVR | BAG associated with health markers with gender-specific pattern |
| Franke [91] | 2015 | 8 women | T1WI | RVR | BAG changes during the course of the menstrual cycle |
| Luders [92] | 2016 | 50 LTM, 50 HCs | T1WI | RVR | BAG: −7.5 yr in LTM |
| Cole [21] | 2018 | 669 people | T1WI | GPR | Higher BAG was associated with weaker grip strength, poorer lung function, slower walking speed, lower fluid intelligence, higher allostatic load, and increased mortality risk. |
| Franke [93] | 2018 | 118 elderly | T1WI | RVR | BAG: +4.3 yr in males whose mothers were exposed to famine in early gestation |
| Hatton [94] | 2018 | 359 men | T1WI | SVR | BAG associated with negative fateful life events in midlife |
| Kiehl [95] | 2018 | 1332 incarcerated males | T1WI | ICA | Brain age predicts recidivism, particularly when combined with other data. |
| Le [96] | 2018 | 20 healthy people | T1WI | SVR | BAG: −1.15 or −1.18 yr by taking ibuprofen |
| Luders [97] | 2018 | 14 healthy women after childbirth | T1WI | RVR | Brain age became younger in late postpartum by 5.4 yr. |
| Rogenmoser [98] | 2018 | 42 pro-musician, 45 amateurs, 38HCs | T1WI | RVR | BAG: −3.70 to −4.51 yr in musicians |
| Scheller [99] | 2018 | 34 elderly | T1WI | RVR | interaction of BAG and APOE variants, suggesting a compensation mechanism in the elderly |
| de Lange [100] | 2019 | 12,021 women | T1WI | XGBoost | BAG decrease with the number of previous childbirths |
| Cruz-Almeida [101] | 2019 | 47 elderly | T1WI | GPR | Increased BAG in elderly with chronic pain |
| Cole [15] | 2020 | 14,701 people | T1WI, FLAIR, T2*, DTI, fMRI | LASSO | BAS associated with stroke history, diabetes, smoking, alcohol, and cognitive measures |
| de Lange [102] | 2020 | 473 people | T1WI, DTI, fMRI | XGBoost | Associated with cardiovascular risk |
| de Lange [103] | 2020 | 19,787 women | T1WI | XGBoost | BAG decrease with the number of previous childbirths. Involvement of brain subcortical regions |
| Henneghan [104] | 2020 | 43 breast cancer with chemotherapy, 50 HCs | T1WI | SVR/RF | Trend-level increase on BAG after chemotherapy for breast cancer |
| Reuben [105] | 2020 | 564 people at 45 yr | T1WI | SVR/RF | BAG: +0.77 yr in those who had lead exposure in childhood |
| Seidel [106] | 2020 | 20 sepsis survivors with cognitive deficits, 44 HCs | T1WI | Kernel regression | BAG: +4.5 yr in sepsis survivor, associated with the severity of dyscognition |
| Anaturk [107] | 2021 | 537 elderly | T1WI, DTI, FLAIR | XGBoost | Relationship with cumulative lifestyle measures independent of cognitive age |
| Bittner [108] | 2021 | 622 elderly | T1WI | RVR | BAG: +5.04 months by combined lifestyle risk, +0.6 month by smoking, −0.55 month by physical activity |
| Cherbuin [109] | 2021 | 335 middle age, 351 elderly | T1WI | RVR | BAG: +51.1–65.7days by every additional 10-mmHg increase in BP |
| Dunas [110] | 2021 | 351 people | T1WI, DTI, fMRI | OLS, BRR, LASSO, ENET, SVR, RVR, GPR | BAG associated with current and past physical fitness and cognitive ability |
| Elliott [111] | 2021 | 869 middle-age | T1WI | SVR/RF | Associated with cognitive function, impaired brain health at age 3, and other signs of aging |
| Hedderich [112] | 2021 | 101 premature-born adults, 111 full-term controls | T1WI | RVR | BAG: +1.4 yr in premature-born adults, associated with low gestational age, low birth weight, and increased neonatal treatment intensity |
| Karim [113] | 2021 | 78 older adults | T1WI, T2WI, FLAIR | GPR | BAG associated with male sex, worry, and rumination |
| Rakesh [114] | 2021 | 166 adolescents | T1WI | SVR | increased BAG by neighborhood disadvantage, modulated by effortful control |
| Rosemann [115] | 2021 | 169 elderly | T1WI | GPR | No association with age-related hearing loss |
| Salih [116] | 2021 | 15,335 HCs | DTI | Bayesian ridge regression | Limbic tract-based BAG was most accurate and associated with daily life factors. Two SNPs were associated with BAG. |
| Sanders [117] | 2021 | 122 elderly | T1WI | XGBoost | BAG decrease in more physically active women but not men |
| Subramaniapillai [118] | 2021 | 1067 elderly | T1WI | Elastic net regression | Brain age was more associated with AD risk factors in women than in men. |
| Vidal-Pineiro [119] | 2021 | 6950 people | T1WI | LASSO, XGBoost | No association between cross-sectional brain age and longitudinal change. Association with congenital factors, suggesting a lifelong influence on brain structure from early life |
| Weihs [120] | 2021 | 690 people | T1WI | OLS | Brain age associated with AHI and ODI in PSG data |
| Angebrandt [121] | 2022 | 240 HCs, 231 HCs (middle age) | T1WI | SVR/RF | Dose-dependent relation between 90-day alcohol consumption and BAG |
| Beck [122] | 2022 | 790 healthy people | T1WI, DTI | XGBoost | T1-based BAG: associated with sBP, smoking, pulse, and CRP. DTI-based BAG: associated with phosphate, MCV |
| Bourassa [123] | 2022 | 910 people (midlife) | T1WI | SVR/RF | BAG in midlife is associated with smoking, obesity, and psychological problems during adolescence. |
| Giannakopoulos [124] | 2022 | 80 elderly | T1WI | RVR | BAG predicted a decrease in executive function over time. |
| Linli [125] | 2022 | 33,293 people | T1WI | XGBoost | BAG: +1.19 yr in active regular smokers, associated with the amount of smoking |
| Sone [16] | 2022 | 773 elderly | T1WI | SVR | BAG: associated with life satisfaction, alcohol use, and diabetes |
| Vaughan [126] | 2022 | 57 elderly | T1WI | GPR | BAG: associated with leg strength, moderating the relationship between strength and mobility |
| Wang [127] | 2022 | 165 elderly | T1WI | RVR | BAG: associated with female gender, higher education but not with APOE-e4 or family history of dementia |
| Whistel [128] | 2022 | 712 people | T1WI | SVR | Association of BAG in mid- to late-life with heavier smoking and alcohol consumption in early mid-life |
| Zheng [129] | 2022 | 1676 HCs | T1WI | RBF-kernel | BAG associated with worse cognitive outcomes over time |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sone, D.; Beheshti, I. Neuroimaging-Based Brain Age Estimation: A Promising Personalized Biomarker in Neuropsychiatry. J. Pers. Med. 2022, 12, 1850. https://doi.org/10.3390/jpm12111850
Sone D, Beheshti I. Neuroimaging-Based Brain Age Estimation: A Promising Personalized Biomarker in Neuropsychiatry. Journal of Personalized Medicine. 2022; 12(11):1850. https://doi.org/10.3390/jpm12111850
Chicago/Turabian StyleSone, Daichi, and Iman Beheshti. 2022. "Neuroimaging-Based Brain Age Estimation: A Promising Personalized Biomarker in Neuropsychiatry" Journal of Personalized Medicine 12, no. 11: 1850. https://doi.org/10.3390/jpm12111850
APA StyleSone, D., & Beheshti, I. (2022). Neuroimaging-Based Brain Age Estimation: A Promising Personalized Biomarker in Neuropsychiatry. Journal of Personalized Medicine, 12(11), 1850. https://doi.org/10.3390/jpm12111850







