The Effect of Intravenous Tranexamic Acid on Myomectomy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Eligibility Criteria
2.2. Information Sources
2.3. Search
- (“tranexamic acid” [MeSH Terms] OR (“tranexamic” [All Fields] AND “acid” [All Fields]) OR “tranexamic acid” [All Fields]) AND (“uterine myomectomy” [MeSH Terms] OR (“uterine” [All Fields] AND “myomectomy” [All Fields]) OR “uterine myomectomy” [All Fields] OR “myomectomies” [All Fields] OR “myomectomy” [All Fields])
- (“tranexamic acid” [MeSH Terms] OR (“tranexamic” [All Fields] AND “acid” [All Fields]) OR “tranexamic acid” [All Fields]) AND (“fibroid s” [All Fields] OR “leiomyoma” [MeSH Terms] OR “leiomyoma” [All Fields] OR “fibroid” [All Fields] OR “fibroids” [All Fields])
2.4. Selection of Sources of Evidence
2.5. Data-Charting Process, Data Items and Synthesis of Results
2.6. Quality Assessment
2.7. Statistical Analysis
3. Results
3.1. Excluded Studies
3.2. Included Studies
3.3. Quality Assessment
3.4. Patient Characteristics
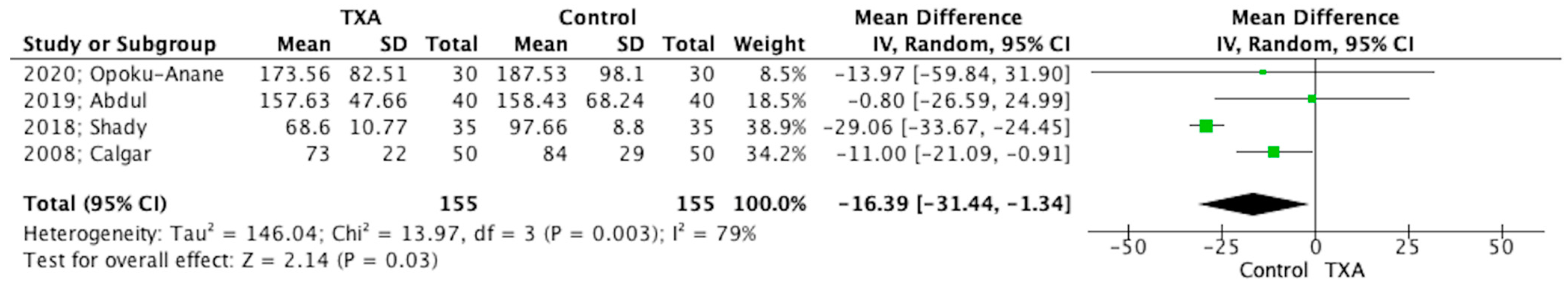
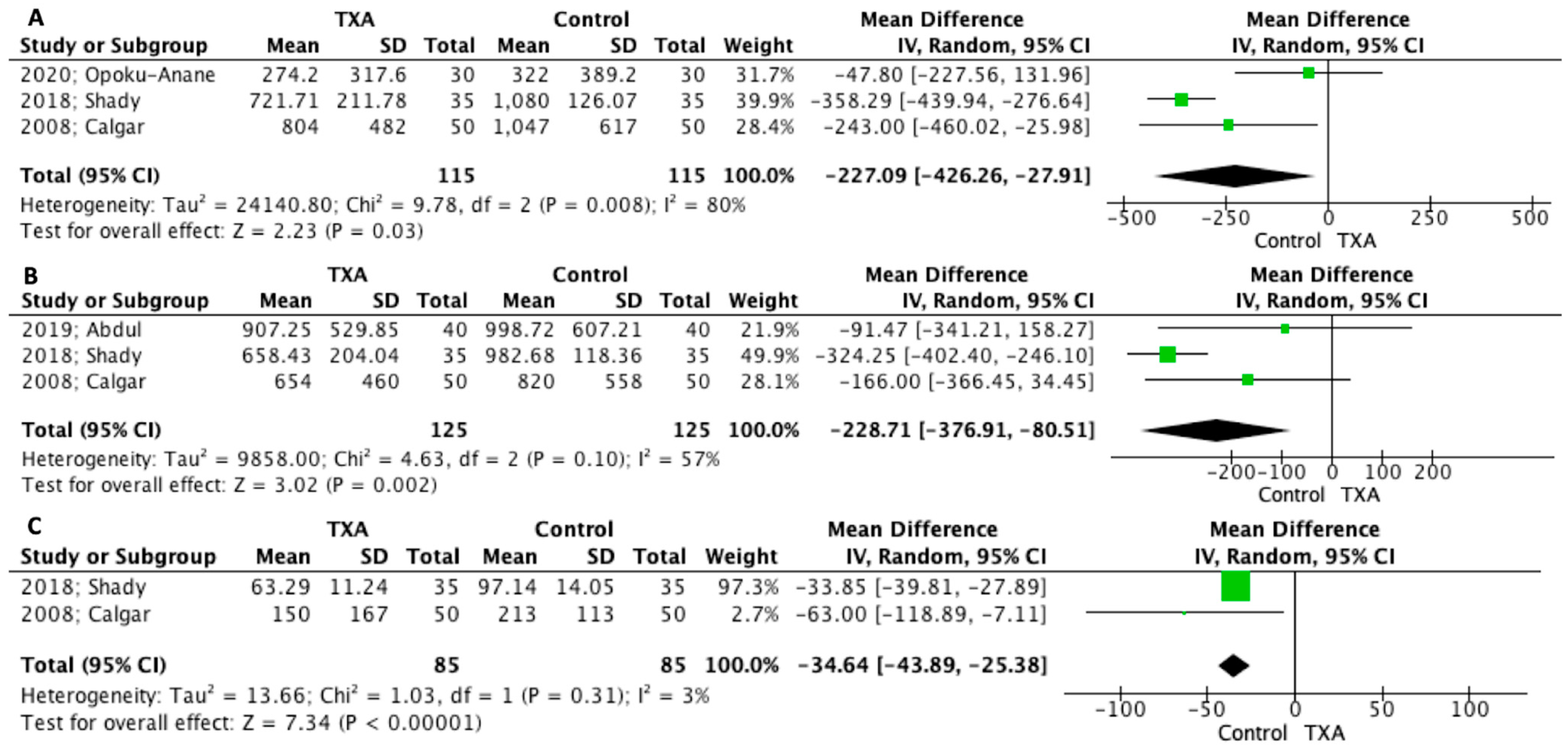
3.5. Main Outcomes
3.6. Secondary Outcomes
4. Discussion
4.1. Summary of Findings
4.2. Interpretation of Results and Clinical Implications
4.3. Comparison with Previous Meta-Analysis
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vilos, G.A.; Allaire, C.; Laberge, P.Y.; Leyland, N.; Special, C. The management of uterine leiomyomas. J. Obstet. Gynaecol. Can. 2015, 37, 157–178. [Google Scholar] [CrossRef]
- Conforti, A.; Mollo, A.; Alviggi, C.; Tsimpanakos, I.; Strina, I.; Magos, A.; De Placido, G. Techniques to reduce blood loss during open myomectomy: A qualitative review of literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 192, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Protopapas, A.; Giannoulis, G.; Chatzipapas, I.; Athanasiou, S.; Grigoriadis, T.; Kathopoulis, N.; Vlachos, D.-E.; Zaharakis, D.; Loutradis, D. Vasopressin during Laparoscopic Myomectomy: Does It Really Extend Its Limits? J. Minim. Invasive Gynecol. 2019, 26, 441–449. [Google Scholar] [CrossRef]
- Protopapas, A.; Kathopoulis, N.; Chatzipapas, I.; Athanasiou, S.; Grigoriadis, T.; Samartzis, K.; Kypriotis, K.; Vlachos, D.-E.; Zacharakis, D.; Loutradis, D. Misoprostol vs vasopressin as a single hemostatic agent in laparoscopic myomectomy: Comparable, or just better than nothing? J. Obstet. Gynaecol. Res. 2020, 46, 2356–2365. [Google Scholar] [CrossRef] [PubMed]
- Hobo, R.; Netsu, S.; Koyasu, Y.; Tsutsumi, O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet. Gynecol. 2009, 113 Pt 2, 484–486. [Google Scholar] [CrossRef]
- Alshryda, S.; Sarda, P.; Sukeik, M.; Nargol, A.; Blenkinsopp, J.; Mason, J.M. Tranexamic acid in total knee replacement: A systematic review and meta-analysis. J. Bone Jt. Surg. Br. 2011, 93, 1577–1585. [Google Scholar] [CrossRef]
- Karkouti, K.; Beattie, W.S.; Dattilo, K.M.; McCluskey, S.A.; Ghannam, M.; Hamdy, A.; Wijeysundera, D.N.; Fedorko, L.; Yau, T.M. A propensity score case-control comparison of aprotinin and tranexamic acid in high-transfusion-risk cardiac surgery. Transfusion 2006, 46, 327–338. [Google Scholar] [CrossRef]
- McCormack, P.L. Tranexamic acid: A review of its use in the treatment of hyperfibrinolysis. Drugs 2012, 72, 585–617. [Google Scholar] [CrossRef]
- Eder, S.; Baker, J.; Gersten, J.; Mabey, R.G.; Adomako, T.L. Efficacy and safety of oral tranexamic acid in women with heavy menstrual bleeding and fibroids. Womens Health 2013, 9, 397–403. [Google Scholar] [CrossRef]
- Lukes, A.S.; Moore, K.A.; Muse, K.N.; Gersten, J.K.; Hecht, B.R.; Edlund, M.; Richter, H.E.; Eder, S.E.; Attia, G.R.; Patrick, D.L.; et al. Tranexamic acid treatment for heavy menstrual bleeding: A randomized controlled trial. Obstet. Gynecol. 2010, 116, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Kacker, R. Random-effects model for meta-analysis of clinical trials: An update. Contemp. Clin. Trials 2007, 28, 105–114. [Google Scholar] [CrossRef]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Ngichabe, S.; Obura, T.; Stones, W. Intravenous tranexamic acid as an adjunct haemostat to ornipressin during open myomectomy. A randomized double blind placebo controlled trial. Ann. Surg. Innov. Res. 2015, 9, 10. [Google Scholar] [CrossRef]
- Rasheedy, R.; Makled, A.; Abou-Gamrah, A.; Giuma, H. Intrauterine Instillation of Tranexamic Acid in Hysteroscopic Myomectomy: A Double-Blind, Placebo-Controlled, Parallel-Group Randomized Clinical Trial. J. Minim. Invasive Gynecol. 2020, 27, 1264–1272.e2. [Google Scholar] [CrossRef]
- Mousa, S.A.; Yassen, A.M.; Alhadary, H.S.; Sadek, E.E.S.; Abdel-Hady, E.-S. Hematological profile and transfusion requirement during hysteroscopic myomectomy: A comparative study between oxytocin and tranexamic acid infusion. Egypt. J. Anaesth. 2012, 28, 125–132. [Google Scholar] [CrossRef]
- Shaaban, M.M.; Ahmed, M.R.; Farhan, R.E.; Dardeer, H.H. Efficacy of Tranexamic Acid on Myomectomy-Associated Blood Loss in Patients With Multiple Myomas: A Randomized Controlled Clinical Trial. Reprod. Sci. 2016, 23, 908–912. [Google Scholar] [CrossRef]
- Abdul, I.F.; Amadu, M.B.; Adesina, K.T.; Olarinoye, A.O.; Omokanye, L.O. Adjunctive use of tranexamic acid to tourniquet in reducing haemorrhage during abdominal myomectomy—A randomized controlled trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 242, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Caglar, G.S.; Tasci, Y.; Kayikcioglu, F.; Haberal, A. Intravenous tranexamic acid use in myomectomy: A prospective randomized double-blind placebo controlled study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 137, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Opoku-Anane, J.; Vargas, M.V.; Marfori, C.Q.; Moawad, G.; Maasen, M.S.; Robinson, J.K. Intraoperative tranexamic acid to decrease blood loss during myomectomy: A randomized, double-blind, placebo-controlled trial. Am. J. Obstet. Gynecol. 2020, 223, 413.e1–413.e7. [Google Scholar] [CrossRef] [PubMed]
- Shady, N.W.; Sallam, H.F.; Fahmy, H. Reducing blood loss during open myomectomy with intravenous versus topical tranexamic acid: A double-blinded randomized placebo-controlled trial. Middle East Fertil. Soc. J. 2018, 23, 225–231. [Google Scholar] [CrossRef]
- Topsoee, M.F.; Settnes, A.; Ottesen, B.; Bergholt, T. A systematic review and meta-analysis of the effect of prophylactic tranexamic acid treatment in major benign uterine surgery. Int. J. Gynaecol. Obstet. 2017, 136, 120–127. [Google Scholar] [CrossRef]
- Fusca, L.; Perelman, I.; Fergusson, D.; Boutet, M.; Chen, I. The Effectiveness of Tranexamic Acid at Reducing Blood Loss and Transfusion Requirement for Women Undergoing Myomectomy: A Systematic Review and Meta-analysis. J. Obstet. Gynaecol. Can. 2019, 41, 1185–1192.e1. [Google Scholar] [CrossRef]
- Baradwan, S.; Hafidh, B.; Latifah, H.M.; Gari, A.; Sabban, H.; Abduljabbar, H.H.; Tawfiq, A.; Hakeem, G.F.; Alkaff, A.; AlSghan, R.; et al. Prophylactic tranexamic acid during myomectomy: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 276, 82–91. [Google Scholar] [CrossRef]


| Year; Author | Country | Type of Study | Inclusion Criteria | Surgical Approach | Compared Groups |
|---|---|---|---|---|---|
| 2019; Abdul | Nigeria | DB-RCT | No pregnancy; symptomatic fibroid scheduled for abdominal myomectomy, Uterine size <= 28 weeks, women without pre-operative Anemia (i.e., Hemoglobin concentration ≥ 10 g/dL); no current therapy with gonadotropin-releasing hormone analogues, mefenamic acid and other hormones, no previous abdominal surgery, possibility to use hemostatic tourniquet, no chronic liver diseases, no nephropathies, no bleeding disorders and past thromboembolic disorders, no hypersensitivity to TXA, agree to consent | Abdominal myomectomy | TXA (10 mg/kg) 10–15 min before incision + tourniquet tying (cervico-isthmic junction IO) vs. placebo (water for injection) + tourniquet |
| 2020; Opoku-Anane | USA | DB-RCT | Age between 18 & 50 years, fibroids ≥ 10 cm, intramural or broad ligament fibroid ≥6 cm ≥5 fibroids, symptomatic fibroids, uterine sparing surgery, no contraindication to TXA (thromboembolic disease, ischemic heart disease, malignancy, hematuria, liver disease, chronic kidney disease, subarachnoid hemorrhage, no pregnancy, no hypersensitivity to TXA, no use of factor IX complex concentrates, anti- inhibitor coagulant concentrates, and all-trans retinoic acid—within 2 weeks of the planned surgery | Laparoscopic, Robotic or abdominal myomectomy | TXA 15 mg/kg 20 min before incision vs. placebo (normal saline iv bolus 20 min before incision |
| 2018; Shady | Egypt | DB-RCT | Symptomatic leiomyomas, scheduled for abdominal myomectomy with myoma staging from (3 to 6), no vaginal or laparoscopic myomectomy, no preoperative embolization or gonadotrophin releasing hormone analogue, no cervical and broad ligament myoma, no cardiac, hepatic, renal or thromboembolic disease, no allergy to TXA | Abdominal myomectomy | TXA 1 gr TXA (2 amp kapron 500 mg 5 mL iv before skin incision + topical application of normal saline on myoma bed vs. placebo (110 mL normal saline iv before incision) + topical application of normal saline on myoma bed |
| 2008; Calgar | Turkey | DB-RCT | No malignancy, no history of thromboembolic disease, no ischemic heart disease, no sub-arachnoidal bleeding, no hematuria, BMI ≤ 30 | Abdominal myomectomy | TXA 10 mg/kg (max 1 g) for 10 min 15 min before incision + continuous infusion of 1 mg/kg/h in 1 L saline for 10 h vs. placebo saline bolus in 15 min before incision + continuous 1 L saline during 10 h |
| Year; Author | Patient No | Operative Time (min) | IO Blood Loss (mL) | PO Blood Loss (mL) | PrO/PO Hemoglobin (g/dL) | Number of Fibroids/Fibroid Weight/Size | PrO/PO Hematocrit | Blood Transfusion N (%) | Hospital Stay (Days) |
|---|---|---|---|---|---|---|---|---|---|
| 2019; Abdul | 40 vs. 40 | 157.63 ± 47.66 vs. 158.43 ± 68.24 | 907.25 ± 529.85 vs. 998.72 ± 607.21 | N/A | 11.40 ± 0.94 vs. 11.34 ± 1.28/10.17 ± 0.73 vs. 10.06 ± 1.18 | 14.88 ± 11.8 vs. 14.73 ± 13.31/(vol-mls) 941.75 ± 673.59 vs. 778.25 ± 609.3/1027.4 ± 750.45 vs. 845.72 ± 684.33 (g) | 34.3 ± 2.4 vs. 34.18 ± 3.47/30.58 ± 2.11 vs. 30.15 ± 3.29 | 12(30) vs. 18 (30) UNITS 0.75 ± 1.28 vs. 1.13 ± 1.64 | 4.8 ± 0.61 vs. 5.55 ± 0.71 |
| 2020; Opoku-Anane | 30 vs. 30 | 173.56 ± 82.51 vs. 187.53 ± 98.1 | 200 (100–508) vs. 240 (105–605) (IQR) TOTAL 274.152 ± 317.5954 vs. 321.995 vs. 389.21 | N/A | 13.39 ± 1.95 vs. 11.11 ± 2.02 | 6.07 ± 5.45 vs. 6.71 ± 6.23 /324 (178–562) vs. 398 (143–647) 356.8 ± 298.91 vs. 395.86 ± 392.32/8.6 (6–10) vs. 8.5 (7–10) 8.17 ± 3.11 vs. 8.5 ± 2.34 | 37.8 ± 4.20 vs. 36.78 ± 5.22 | None: 30 vs. 26 1 unit: 0 vs. 2 units: 0 vs. 1 4 units: 0 vs. 1 | N/A |
| 2018; Shady | 35 vs. 35 | 68.6 ± 10.77 vs. 97.66 ± 8.8 | 658.43 ± 204.04 vs. 982.68 ± 118.36 721.71 ± 211.78 vs. 1080 ± 126.07 (TOTAL) | 63.29 ± 11.24 vs. 97.14 ± 14.05 | 10.57 ± 0.81 vs. 10.56 ± 0.77/10.02 ± 0.81 vs. 9.83 ± 0.63 | 4(1–8) vs. 4 (1–8) 4 ± 1.75 vs. 4 ± 1.75/N/A/12.77 ± 4.12 vs. 12.66 ± 3.96 | N/A | 6 (17.1) vs. 19 (54.3) | 3.54 ± 0.85 vs. 3.66 ± 0.84 |
| 2008; Calgar | 50 vs. 50 | 73 ± 22 vs. 84 ± 29 | 654 ± 460 vs. 820 ± 558 804 ± 482 vs. 1047 ± 617 (TOTAL) | 150 ± 167 vs. 213 ± 113 | 11.4 ± 2 vs. 12 ± 1.6/9.97 ± 1.5 vs. 9.76 ± 1.4 | Vol (cm3) 457 ± 669 vs. 286 ± 259 | 36 ± 5 vs. 37 ± 4/31.7 ± 3.9 vs. 30.7 ± 3.4 | 15(30) vs. 10 (20) UNITS 0.3 ± 0.8 vs. 0.3 ± 0.7 | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kathopoulis, N.; Prodromidou, A.; Zacharakis, D.; Chatzipapas, I.; Diakosavvas, M.; Kypriotis, K.; Grigoriadis, T.; Protopapas, A. The Effect of Intravenous Tranexamic Acid on Myomectomy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Pers. Med. 2022, 12, 1492. https://doi.org/10.3390/jpm12091492
Kathopoulis N, Prodromidou A, Zacharakis D, Chatzipapas I, Diakosavvas M, Kypriotis K, Grigoriadis T, Protopapas A. The Effect of Intravenous Tranexamic Acid on Myomectomy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Personalized Medicine. 2022; 12(9):1492. https://doi.org/10.3390/jpm12091492
Chicago/Turabian StyleKathopoulis, Nikolaos, Anastasia Prodromidou, Dimitrios Zacharakis, Ioannis Chatzipapas, Michail Diakosavvas, Konstantinos Kypriotis, Themos Grigoriadis, and Athanasios Protopapas. 2022. "The Effect of Intravenous Tranexamic Acid on Myomectomy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Journal of Personalized Medicine 12, no. 9: 1492. https://doi.org/10.3390/jpm12091492