Pseudogymnoascus destructans Transcriptional Response to Chronic Copper Stress
Abstract
:1. Introduction
2. Materials and Methods
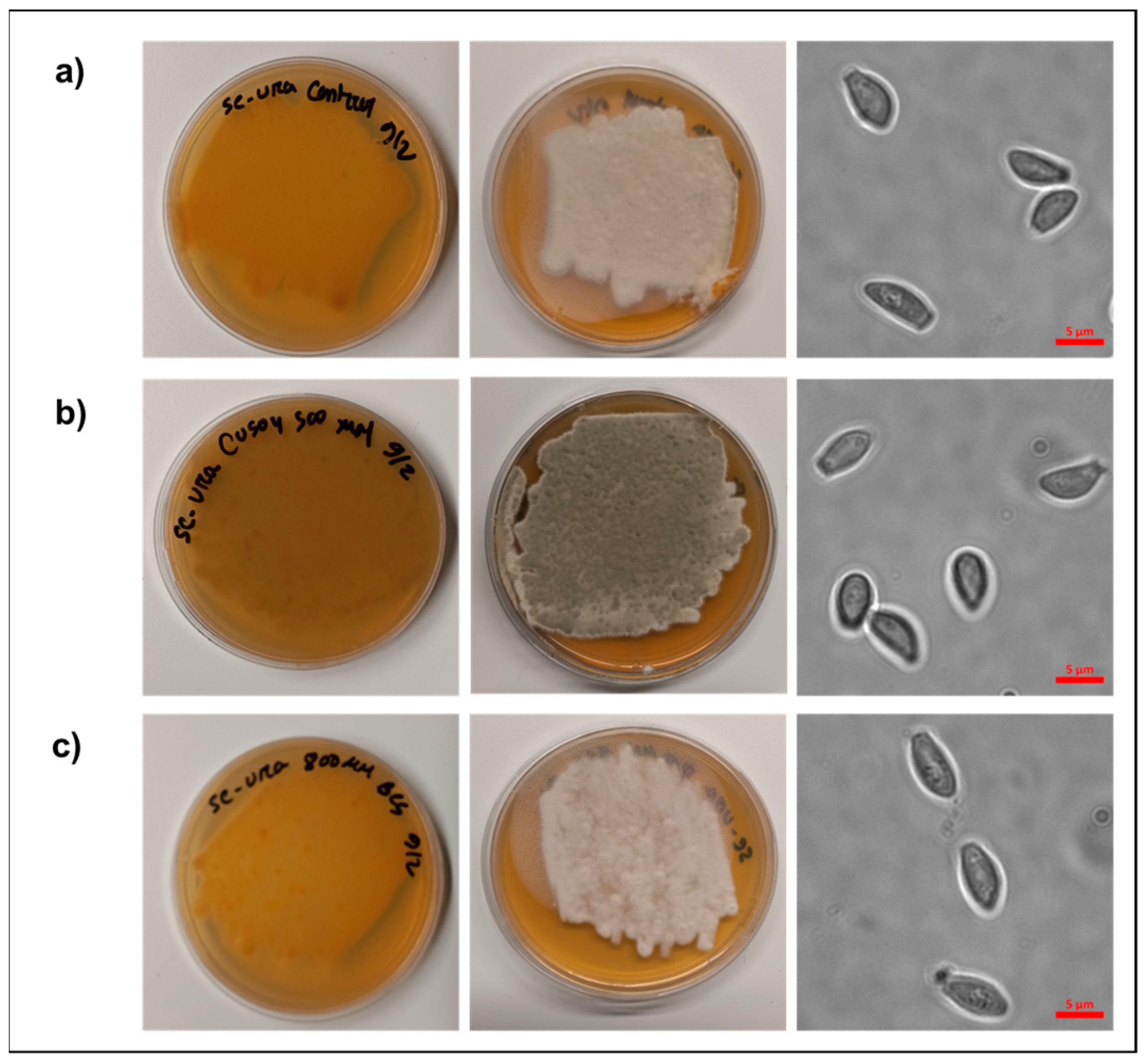
2.1. Media Preparation, Propagation, and Imaging of Pseudogymnoascus destructans
2.2. Pseudogymnoascus destructans RNA Extraction and Isolation
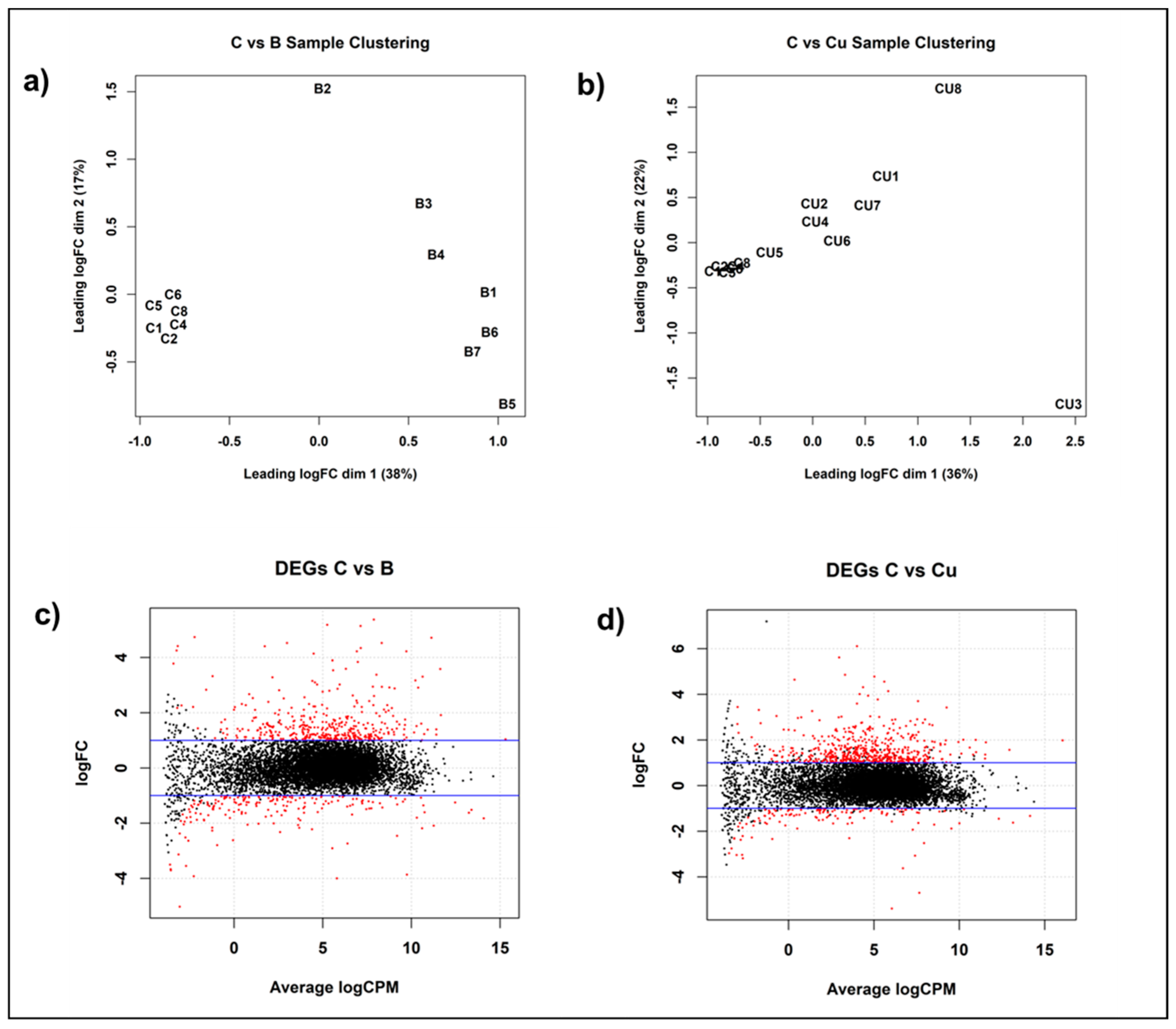
2.3. Differential Gene Expression Under Copper Stress
2.4. Gene Ontology (GO) and GO Enrichment Analyses
2.5. Identification of Pseudogymnoascus destructans Gene Homologs
2.6. RNA Sequencing Datasets
3. Results
3.1. Pseudogymnoascus destructans Growth Under Copper Stress
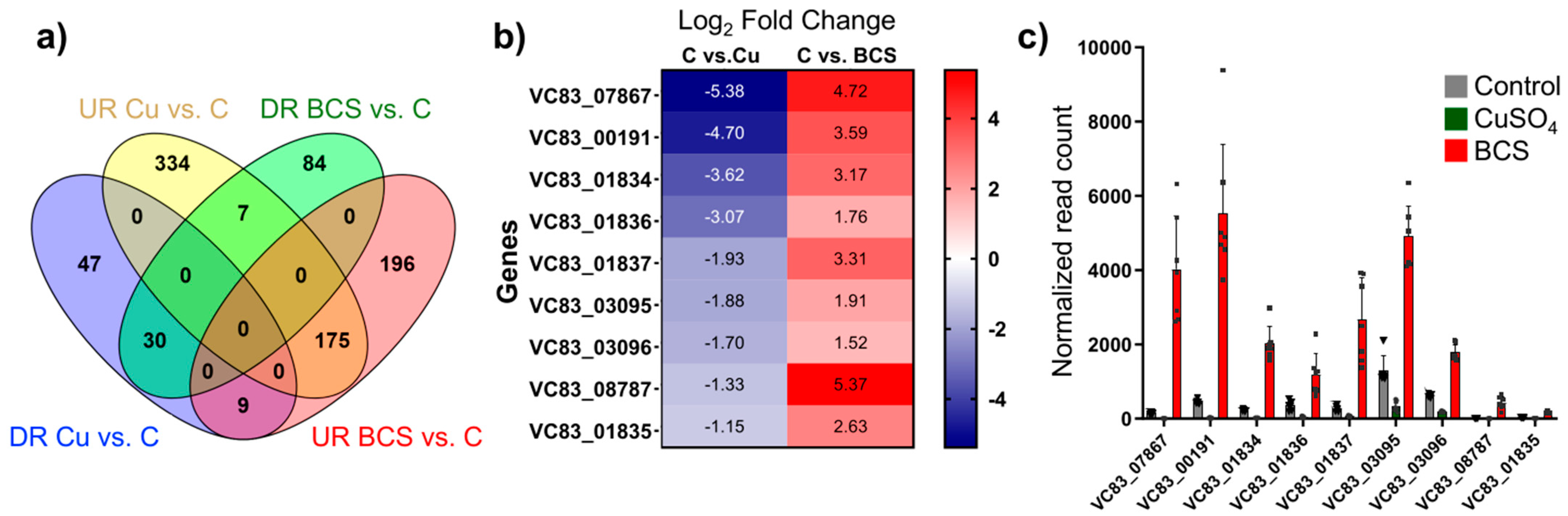
3.2. Differential Gene Expression in Response to Copper Stress
3.3. Comparative Gene Ontology Analysis of P. destructans Under Cu-Withholding and Cu-Overload Conditions
3.4. Putative Virulence Factors Controlled by Copper Stress
4. Discussion
4.1. Copper Stress Metabolic Adaptations
4.2. P. destructans Adaptation Under Cu-Withholding Stress Conditions
- Identification of a putative novel fungal high-affinity metal acquisition pathway in P. destructans
- Remodeling of P. destructans superoxide dismutase (SOD) enzymes under Cu-withholding stress
- Notable proteins involved in metal ion homeostasis and Cu-withholding stress adaptations
4.3. P. destructans Response to Copper Overload Conditions
4.4. Putative P. destructans Secreted Proteases and Virulence Factors Responsive to Cu Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Pd | Pseudogymnoascus destructans |
| BCS | Bathocuproinedisulfonic acid |
| GO | Gene ontology |
| FDR | False discovery rate |
| DEG | Differentially Expressed Gene |
| RNA | Ribonucleic acid |
| SOD | Superoxide dismutase |
| CTR | Copper transporter |
| BLP | Bim1-like protein |
| CRC | Copper-Responsive Gene Cluster |
| MFS | Major facilitator superfamily |
| HSP | Heat shock protein |
| SC-Ura | Synthetic Complete Medium minus Uracil |
| CPM | Counts Per Million |
| YPD | Yeast Extract Peptone Dextrose Medium |
References
- Festa, R.A.; Thiele, D.J. Copper: An essential metal in biology. Curr. Biol. 2011, 21, R877–R883. [Google Scholar] [CrossRef] [PubMed]
- Tsang, T.; Davis, C.I.; Brady, D.C. Copper biology. Curr. Biol. 2021, 31, R421–R427. [Google Scholar] [CrossRef] [PubMed]
- Solomon, E.I.; Heppner, D.E.; Johnston, E.M.; Ginsbach, J.W.; Cirera, J.; Qayyum, M.; Kieber-Emmons, M.T.; Kjaergaard, C.H.; Hadt, R.G.; Tian, L. Copper active sites in biology. Chem. Rev. 2014, 114, 3659–3853. [Google Scholar] [CrossRef]
- Liu, J.; Chakraborty, S.; Hosseinzadeh, P.; Yu, Y.; Tian, S.L.; Petrik, I.; Bhagi, A.; Lu, Y. Metalloproteins Containing Cytochrome, Iron-Sulfur, or Copper Redox Centers. Chem. Rev. 2014, 114, 4366–4469. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Abreu, I.A.; Cabelli, D.E.; Maroney, M.J.; Miller, A.F.; Teixeira, M.; Valentine, J.S. Superoxide dismutases and superoxide reductases. Chem. Rev. 2014, 114, 3854–3918. [Google Scholar] [CrossRef]
- Broxton, C.N.; Culotta, V.C. SOD Enzymes and Microbial Pathogens: Surviving the Oxidative Storm of Infection. PLoS Pathog. 2016, 12, 8–13. [Google Scholar] [CrossRef]
- Koo, C.W.; Rosenzweig, A.C. Biochemistry of aerobic biological methane oxidation. Chem. Soc. Rev. 2021, 50, 3424–3436. [Google Scholar] [CrossRef]
- Klinman, J.P. The copper-enzyme family of dopamine beta-monooxygenase and peptidylglycine alpha-hydroxylating monooxygenase: Resolving the chemical pathway for substrate hydroxylation. J. Biol. Chem. 2006, 281, 3013–3016. [Google Scholar] [CrossRef]
- Taylor, A.B.; Stoj, C.S.; Ziegler, L.; Kosman, D.J.; Hart, P.J. The copper-iron connection in biology: Structure of the metallo-oxidase Fet3p. Proc. Natl. Acad. Sci. USA 2005, 102, 15459–15464. [Google Scholar] [CrossRef]
- Chen, L.; Min, J.; Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target. Ther. 2022, 7, 378. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Borghouts, C.; Osiewacz, H.D. GRISEA, a copper-modulated transcription factor from involved in senescence and morphogenesis, is an ortholog of MAC1 in Saccharomyces cerevisiae. Mol. Gen. Genet. 1998, 260, 492–502. [Google Scholar] [CrossRef]
- Labbé, S.; Zhu, Z.; Thiele, D.J. Copper-specific transcriptional repression of yeast genes encoding critical components in the copper transport pathway. J. Biol. Chem. 1997, 272, 15951–15958. [Google Scholar] [CrossRef]
- Thiele, D.J. Ace1 Regulates Expression of the Saccharomyces-Cerevisiae Metallothionein Gene. Mol. Cell. Biol. 1988, 8, 2745–2752. [Google Scholar] [CrossRef] [PubMed]
- Besold, A.N.; Culbertson, E.M.; Culotta, V.C. The Yin and Yang of copper during infection. J. Biol. Inorg. Chem. 2016, 21, 137–144. [Google Scholar] [CrossRef]
- García-Santamarina, S.; Thiele, D.J. Copper at the fungal pathogen-host axis. J. Biol. Chem. 2015, 290, 18945–18953. [Google Scholar] [CrossRef]
- Rosen, T.; Wang, K.K.A.; Nolan, E.M. Metal sequestration by S100 proteins in chemically diverse environments. Trends Microbiol. 2022, 30, 654–664. [Google Scholar] [CrossRef]
- Zackular, J.P.; Chazin, W.J.; Skaar, E.P. Nutritional immunity: S100 proteins at the host-pathogen interface. J. Biol. Chem. 2015, 290, 18991–18998. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, C.C.; Skaar, E.P. Nutritional immunity: The battle for nutrient metals at the host-pathogen interface. Nat. Rev. Microbiol. 2022, 20, 657–670. [Google Scholar] [CrossRef]
- White, C.; Lee, J.; Kambe, T.; Fritsche, K.; Petris, M.J. A Role for the ATP7A Copper-transporting ATPase in Macrophage Bactericidal Activity. J. Biol. Chem. 2009, 284, 33949–33956. [Google Scholar] [CrossRef]
- Bleackley, M.R.; Macgillivray, R.T. Transition metal homeostasis: From yeast to human disease. Biometals 2011, 24, 785–809. [Google Scholar] [CrossRef] [PubMed]
- Nevitt, T.; Öhrvik, H.; Thiele, D.J. Charting the travels of copper in eukaryotes from yeast to mammals. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2012, 1823, 1580–1593. [Google Scholar] [CrossRef]
- Li, C.X.; Gleason, J.E.; Zhang, S.X.; Bruno, V.M.; Cormack, B.P.; Culotta, V.C. Candida albicans adapts to host copper during infection by swapping metal cofactors for superoxide dismutase. Proc. Natl. Acad. Sci. USA 2015, 112, E5336–E5342. [Google Scholar] [CrossRef]
- Culbertson, E.M.; Bruno, V.M.; Cormack, B.P.; Culotta, V.C. Expanded role of the Cu-sensing transcription factor Mac1p in Candida albicans. Mol. Microbiol. 2020, 114, 1006–1018. [Google Scholar] [CrossRef]
- Raffa, N.; Won, T.H.; Sukowaty, A.; Candor, K.; Cui, C.S.; Halder, S.; Dai, M.J.; Landero-Figueroa, J.A.; Schroeder, F.C.; Keller, N.P. Dual-purpose isocyanides produced by contribute to cellular copper sufficiency and exhibit antimicrobial activity. Proc. Natl. Acad. Sci. USA 2021, 118, e2015224118. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Santamarina, S.; Probst, C.; Festa, R.A.; Ding, C.; Smith, A.D.; Conklin, S.E.; Brander, S.; Kinch, L.N.; Grishin, N.V.; Franz, K.J.; et al. A lytic polysaccharide monooxygenase-like protein functions in fungal copper import and meningitis. Nat. Chem. Biol. 2020, 16, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Garcia-Santamarina, S.; Ralle, M.; Loiselle, D.R.; Haystead, T.A.; Thiele, D.J. Transcription factor-driven alternative localization of Cryptococcus neoformans superoxide dismutase. J. Biol. Chem. 2021, 296, 100391. [Google Scholar] [CrossRef]
- Nickles, G.R.; Oestereicher, B.; Keller, N.P.; Drott, M.T. Mining for a new class of fungal natural products: The evolution, diversity, and distribution of isocyanide synthase biosynthetic gene clusters. Nucleic Acids Res. 2023, 51, 7220–7235. [Google Scholar] [CrossRef]
- Gargas, A.; Trest, M.T.; Christensen, M.; Volk, T.J.; Blehert, D.S. Geomyces destructans sp. nov. associated with bat white-nose syndrome. Mycotaxon 2009, 108, 147–154. [Google Scholar] [CrossRef]
- Meteyer, C.U.; Buckles, E.L.; Blehert, D.S.; Hicks, A.C.; Green, D.E.; Shearn-Bochsler, V.; Thomas, N.J.; Gargas, A.; Behr, M.J. Histopathologic criteria to confirm white-nose syndrome in bats. J. Vet. Diagn. Investig. 2009, 21, 411–414. [Google Scholar] [CrossRef]
- Meteyer, C.U.; Barber, D.; Mandl, J.N. Pathology in euthermic bats with white nose syndrome suggests a natural manifestation of immune reconstitution inflammatory syndrome. Virulence 2012, 3, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Meteyer, C.U.; Dutheil, J.Y.; Keel, M.K.; Boyles, J.G.; Stukenbrock, E.H. Plant pathogens provide clues to the potential origin of bat white-nose syndrome. Virulence 2022, 13, 1020–1031. [Google Scholar] [CrossRef] [PubMed]
- Field, K.A.; Johnson, J.S.; Lilley, T.M.; Reeder, S.M.; Rogers, E.J.; Behr, M.J.; Reeder, D.A.M. The White-Nose Syndrome Transcriptome: Activation of Anti-fungal Host Responses in Wing Tissue of Hibernating Little Brown Myotis. PLoS Pathog. 2015, 11, e1005168. [Google Scholar] [CrossRef]
- Reeder, S.M.; Palmer, J.M.; Prokkola, J.M.; Lilley, T.M.; Reeder, D.A.M.; Field, K.A. Pseudogymnoascus destructans transcriptome changes during white-nose syndrome infections. Virulence 2017, 8, 1695–1707. [Google Scholar] [CrossRef] [PubMed]
- Lilley, T.M.; Prokkola, J.M.; Johnson, J.S.; Rogers, E.J.; Gronsky, S.; Kurta, A.; Reeder, D.M.; Field, K.A. Immune responses in hibernating little brown myotis (Myotis lucifugus) with white-nose syndrome. Proc. R. Soc. B 2017, 284, 20162232. [Google Scholar] [CrossRef]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Upadhyay, S.; Torres, G.; Lin, X.R. Laccases Involved in 1,8-Dihydroxynaphthalene Melanin Biosynthesis in Aspergillus fumigatus Are Regulated by Developmental Factors and Copper Homeostasis. Eukaryot. Cell 2013, 12, 1641–1652. [Google Scholar] [CrossRef]
- Smith, A.D.; Logeman, B.L.; Thiele, D.J. Copper Acquisition and Utilization in Fungi. Annu. Rev. Microbiol. 2017, 71, 597–623. [Google Scholar] [CrossRef]
- Laffont, C.; Arnoux, P. The ancient roots of nicotianamine: Diversity, role, regulation and evolution of nicotianamine-like metallophores. Metallomics 2020, 12, 1480–1493. [Google Scholar] [CrossRef]
- Cain, T.J.; Smith, A.T. Ferric iron reductases and their contribution to unicellular ferrous iron uptake. J. Inorg. Biochem. 2021, 218, 111407. [Google Scholar] [CrossRef] [PubMed]
- Dancis, A.; Yuan, D.S.; Haile, D.; Askwith, C.; Eide, D.; Moehle, C.; Kaplan, J.; Klausner, R.D. Molecular characterization of a copper transport protein in S. cerevisiae: An unexpected role for copper in iron transport. Cell 1994, 76, 393–402. [Google Scholar] [CrossRef]
- Marvin, M.E.; Williams, P.H.; Cashmore, A.M. The Candida albicans CTR1 gene encodes a functional copper transporter. Microbiology 2003, 149, 1461–1474. [Google Scholar] [CrossRef] [PubMed]
- Pena, M.M.O.; Puig, S.; Thiele, D.J. Characterization of the Saccharomyces cerevisiae high affinity copper transporter Ctr3. J. Biol. Chem. 2000, 275, 33244–33251. [Google Scholar] [CrossRef] [PubMed]
- Georgatsou, E.; Alexandraki, D. Regulated expression of the Saccharomyces cerevisiae Fre1p/Fre2p Fe/Cu reductase related genes. Yeast 1999, 15, 573–584. [Google Scholar] [CrossRef]
- Dancis, A.; Roman, D.G.; Anderson, G.J.; Hinnebusch, A.G.; Klausner, R.D. Ferric reductase of Saccharomyces cerevisiae: Molecular characterization, role in iron uptake, and transcriptional control by iron. Proc. Natl. Acad. Sci. USA 1992, 89, 3869–3873. [Google Scholar] [CrossRef]
- Probst, C.; Garcia-Santamarina, S.; Brooks, J.T.; Van der Kloet, I.; Baars, O.; Ralle, M.; Thiele, D.J.; Alspaugh, J.A. Interactions between copper homeostasis and the fungal cell wall affect copper stress resistance. PLoS Pathog. 2022, 18, e1010195. [Google Scholar] [CrossRef]
- McFarlane, J.S.; Davis, C.L.; Lamb, A.L. Staphylopine, pseudopaline, and yersinopine dehydrogenases: A structural and kinetic analysis of a new functional class of opine dehydrogenase. J. Biol. Chem. 2018, 293, 8009–8019. [Google Scholar] [CrossRef]
- McFarlane, J.S.; Lamb, A.L. Biosynthesis of an Opine Metallophore by Pseudomonas aeruginosa. Biochemistry 2017, 56, 5967–5971. [Google Scholar] [CrossRef]
- Ghssein, G.; Brutesco, C.; Ouerdane, L.; Fojcik, C.; Izaute, A.; Wang, S.L.; Hajjar, C.; Lobinski, R.; Lemaire, D.; Richaud, P.; et al. Biosynthesis of a broad-spectrum nicotianamine-like metallophore in Staphylococcus aureus. Science 2016, 352, 1105–1109. [Google Scholar] [CrossRef]
- Beaudoin, J.; Labbe, S. The fission yeast copper-sensing transcription factor Cuf1 regulates the copper transporter gene expression through an Ace1/Amt1-like recognition sequence. J. Biol. Chem. 2001, 276, 15472–15480. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Yin, J.; Tovar, E.M.; Fitzpatrick, D.A.; Higgins, D.G.; Thiele, D.J. The copper regulon of the human fungal pathogen Cryptococcus neoformans H99. Mol. Microbiol. 2011, 81, 1560–1576. [Google Scholar] [CrossRef]
- Raja, M.R.; Waterman, S.R.; Qiu, J.; Bleher, R.; Williamson, P.R.; O’Halloran, T.V. A copper hyperaccumulation phenotype correlates with pathogenesis in Cryptococcus neoformans. Metallomics 2013, 5, 363–371. [Google Scholar] [CrossRef]
- Brander, S.; Horvath, I.; Ipsen, J.; Peciulyte, A.; Olsson, L.; Hernández-Rollán, C.; Nørholm, M.H.H.; Mossin, S.; Leggio, L.L.; Probst, C.; et al. Biochemical evidence of both copper chelation and oxygenase activity at the histidine brace. Sci. Rep. 2020, 10, 16369. [Google Scholar] [CrossRef]
- Anne, S.; Friudenberg, A.D.; Peterson, R.L. Characterization of a High-Affinity Copper Transporter CTR1a in the White-Nose Syndrome Causing Fungal Pathogen Pseudogymnoascus destructans. J. Fungi 2024, 10, 729. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.W.; Bauler, M.; Moore, R.E.; Klebba, P.E.; Philpott, C.C. The role of the FRE family of plasma membrane reductases in the uptake of siderophore-iron in Saccharomyces cerevisiae. J. Biol. Chem. 2001, 276, 10218–10223. [Google Scholar] [CrossRef]
- Georgatsou, E.; Mavrogiannis, L.A.; Fragiadakis, G.S.; Alexandraki, D. The yeast Fre1p/Fre2p cupric reductases facilitate copper uptake and are regulated by the copper-modulated Mac1p activator. J. Biol. Chem. 1997, 272, 13786–13792. [Google Scholar] [CrossRef] [PubMed]
- Woodacre, A.; Mason, R.P.; Jeeves, R.E.; Cashmore, A.M. Copper-dependent transcriptional regulation by Candida albicans Mac1p. Microbiology 2008, 154, 1502–1512. [Google Scholar] [CrossRef]
- Garcia-Santamarina, S.; Festa, R.A.; Smith, A.D.; Yu, C.H.; Probst, C.; Ding, C.; Homer, C.M.; Yin, J.; Noonan, J.P.; Madhani, H.; et al. Genome-wide analysis of the regulation of Cu metabolism in Cryptococcus neoformans. Mol. Microbiol. 2018, 108, 473–494. [Google Scholar] [CrossRef]
- Schalk, I.J. Bacterial siderophores: Diversity, uptake pathways and applications. Nat. Rev. Microbiol. 2025, 23, 24–40. [Google Scholar] [CrossRef]
- Reyes, R.M.; Rosenzweig, A.C. Methanobactins: Structures, Biosynthesis, and Microbial Diversity. Annu. Rev. Microbiol. 2024, 78, 383–401. [Google Scholar] [CrossRef]
- Citiulo, F.; Jacobsen, I.D.; Miramón, P.; Schild, L.; Brunke, S.; Zipfel, P.; Brock, M.; Hube, B.; Wilson, D. Scavenges Host Zinc via Pra1 during Endothelial Invasion. PLoS Pathog. 2012, 8, e1002777. [Google Scholar] [CrossRef] [PubMed]
- Mastropasqua, M.C.; D’Orazio, M.; Cerasi, M.; Pacello, F.; Gismondi, A.; Canini, A.; Canuti, L.; Consalvo, A.; Ciavardelli, D.; Chirullo, B.; et al. Growth of Pseudomonas aeruginosa in zinc poor environments is promoted by a nicotianamine-related metallophore. Mol. Microbiol. 2017, 106, 543–561. [Google Scholar] [CrossRef]
- Grim, K.P.; Francisco, B.S.; Radin, J.N.; Brazel, E.B.; Kelliher, J.L.; Solórzano, P.K.P.; Kim, P.C.; McDevitt, C.A.; Kehl-Fie, T.E. The Metallophore Staphylopine Enables To Compete with the Host for Zinc and Overcome Nutritional Immunity. mBio 2017, 8, e01281-17. [Google Scholar] [CrossRef] [PubMed]
- Bellotti, D.; Rowinska-Zyrek, M.; Remelli, M. How Zinc-Binding Systems, Expressed by Human Pathogens, Acquire Zinc from the Colonized Host Environment: A Critical Review on Zincophores. Curr. Med. Chem. 2021, 28, 7312–7338. [Google Scholar] [CrossRef]
- Laffont, C.; Brutesco, C.; Hajjar, C.; Cullia, G.; Fanelli, R.; Ouerdane, L.; Cavelier, F.; Arnoux, P. Simple rules govern the diversity of bacterial nicotianamine-like metallophores. Biochem. J. 2019, 476, 2221–2233. [Google Scholar] [CrossRef] [PubMed]
- Flieger, M.; Bandouchova, H.; Cerny, J.; Chudiéková, M.; Kolarik, M.; Kovacova, V.; Martínková, N.; Novák, P.; Šebesta, O.; Stodùlková, E.; et al. Vitamin B2 as a virulence factor in Pseudogymnoascus destructans skin infection. Sci. Rep. 2016, 6, 33200. [Google Scholar] [CrossRef]
- Mascuch, S.J.; Moree, W.J.; Hsu, C.C.; Turner, G.G.; Cheng, T.L.; Blehert, D.S.; Kilpatrick, A.M.; Frick, W.F.; Meehan, M.J.; Dorrestein, P.C.; et al. Direct detection of fungal siderophores on bats with white-nose syndrome via fluorescence microscopy-guided ambient ionization mass spectrometry. PLoS ONE 2015, 10, e0119668. [Google Scholar] [CrossRef]
- Culotta, V.C.; Joh, H.D.; Lin, S.J.; Slekar, K.H.; Strain, J. A physiological role for Saccharomyces cerevisiae copper/zinc superoxide dismutase in copper buffering. J. Biol. Chem. 1995, 270, 29991–29997. [Google Scholar] [CrossRef]
- Askwith, C.; Eide, D.; Van Ho, A.; Bernard, P.S.; Li, L.; Davis-Kaplan, S.; Sipe, D.M.; Kaplan, J. The FET3 gene of S. cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. Cell 1994, 76, 403–410. [Google Scholar] [CrossRef]
- Nett, J.E.; Lepak, A.J.; Marchillo, K.; Andes, D.R. Time course global gene expression analysis of an in vivo Candida biofilm. J. Infect. Dis. 2009, 200, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Frohner, I.E.; Bourgeois, C.; Yatsyk, K.; Majer, O.; Kuchler, K. Candida albicans cell surface superoxide dismutases degrade host-derived reactive oxygen species to escape innate immune surveillance. Mol. Microbiol. 2009, 71, 240–252. [Google Scholar] [CrossRef]
- Schatzman, S.S.; Peterson, R.L.; Teka, M.; He, B.; Cabelli, D.E.; Cormack, B.P.; Culotta, V.C. Copper-only superoxide dismutase enzymes and iron starvation stress in Candida fungal pathogens. J. Biol. Chem. 2020, 295, 570–583. [Google Scholar] [CrossRef]
- Thywissen, A.; Heinekamp, T.; Dahse, H.M.; Schmaler-Ripcke, J.; Nietzsche, S.; Zipfel, P.F.; Brakhage, A.A. Conidial Dihydroxynaphthalene Melanin of the Human Pathogenic Fungus Aspergillus fumigatus Interferes with the Host Endocytosis Pathway. Front. Microbiol. 2011, 2, 96. [Google Scholar] [CrossRef] [PubMed]
- Isidoro-Ayza, M.; Klein, B.S. Pathogenic strategies of Pseudogymnoascus destructans during torpor and arousal of hibernating bats. Science 2024, 385, 194–200. [Google Scholar] [CrossRef]
- Gralla, E.B.; Thiele, D.J.; Silar, P.; Valentine, J.S. ACE1, a copper-dependent transcription factor, activates expression of the yeast copper, zinc superoxide dismutase gene. Proc. Natl. Acad. Sci. USA 1991, 88, 8558–8562. [Google Scholar] [CrossRef]
- Culotta, V.C.; Howard, W.R.; Liu, X.F. CRS5 encodes a metallothionein-like protein in Saccharomyces cerevisiae. J. Biol. Chem. 1994, 269, 25295–25302. [Google Scholar] [CrossRef] [PubMed]
- Peña, M.M.O.; Koch, K.A.; Thiele, D.J. Dynamic Regulation of Copper Uptake and Detoxification Genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 1998, 18, 2514–2523. [Google Scholar] [CrossRef]
- Calzada, A.; Hodgson, B.; Kanemaki, M.; Bueno, A.; Labib, K. Molecular anatomy and regulation of a stable replisome at a paused eukaryotic DNA replication fork. Genes. Dev. 2005, 19, 1905–1919. [Google Scholar] [CrossRef]
- Bando, M.; Katou, Y.; Komata, M.; Tanaka, H.; Itoh, T.; Sutani, T.; Shirahige, K. Csm3, Tof1, and Mrc1 form a heterotrimeric mediator complex that associates with DNA replication forks. J. Biol. Chem. 2009, 284, 34355–34365. [Google Scholar] [CrossRef]
- Elledge, S.J.; Davis, R.W. Two genes differentially regulated in the cell cycle and by DNA-damaging agents encode alternative regulatory subunits of ribonucleotide reductase. Genes. Dev. 1990, 4, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Robbins, N.; Cowen, L.E. Roles of Hsp90 in Candida albicans morphogenesis and virulence. Curr. Opin. Microbiol. 2023, 75, 102351. [Google Scholar] [CrossRef] [PubMed]
- Cleare, L.G.; Zamith-Miranda, D.; Nosanchuk, J.D. Heat Shock Proteins in Histoplasma and Paracoccidioides. Clin. Vaccine Immunol. 2017, 24, e00221-17. [Google Scholar] [CrossRef] [PubMed]
- Nagao, J.; Cho, T.; Uno, J.; Ueno, K.; Imayoshi, R.; Nakayama, H.; Chibana, H.; Kaminishi, H. Candida albicans Msi3p, a homolog of the Saccharomyces cerevisiae Sse1p of the Hsp70 family, is involved in cell growth and fluconazole tolerance. FEMS Yeast Res. 2012, 12, 728–737. [Google Scholar] [CrossRef]
- O’Donoghue, A.J.; Knudsen, G.M.; Beekman, C.; Perry, J.A.; Johnson, A.D.; DeRisi, J.L.; Craik, C.S.; Bennett, R.J. Destructin-1 is a collagen-degrading endopeptidase secreted by Pseudogymnoascus destructans, the causative agent of white-nose syndrome. Proc. Natl. Acad. Sci. USA 2015, 112, 7478–7483. [Google Scholar] [CrossRef]


| GotermID | goACC | Biological Process | GOsize 1 | Fold Enrichment 2 | p-Value 3 | DEG 4 |
|---|---|---|---|---|---|---|
| 4666 | GO:0006536 | Glutamate metabolic process | 1 | 16.50 | 0 | 1 |
| 4712 | GO:0006582 | Melanin metabolic process | 1 | 16.50 | 0 | 1 |
| 9015 | GO:0015703 | Chromate transport | 1 | 16.50 | 0 | 1 |
| 17658 | GO:0042128 | Nitrate assimilation | 1 | 16.50 | 0 | 1 |
| 9010 | GO:0015698 | Inorganic anion transport | 1 | 16.50 | 0 | 1 |
| 6710 | GO:0009102 | Biotin biosynthetic process | 1 | 16.50 | 0 | 1 |
| 19608 | GO:0045132 | Meiotic chromosome segregation | 1 | 16.50 | 0 | 1 |
| 4918 | GO:0006801 | Superoxide metabolic process | 4 | 12.37 | 1.34 × 10−5 | 3 |
| 4283 | GO:0006118 | Electron transport | 104 | 2.37 | 0.0004 | 15 |
| 6276 | GO:0008643 | Carbohydrate transport | 10 | 4.95 | 0.0020 | 3 |
| 4851 | GO:0006725 | Aromatic compound metabolic process | 10 | 4.95 | 0.0020 | 3 |
| 6724 | GO:0009116 | Nucleoside metabolic process | 2 | 8.25 | 0.0036 | 1 |
| 4970 | GO:0006857 | Oligopeptide transport | 2 | 8.25 | 0.0036 | 1 |
| 24525 | GO:0051276 | Chromosome organization and Biogenesis | 2 | 8.25 | 0.0036 | 1 |
| 5077 | GO:0006979 | Response to oxidative stress | 6 | 5.50 | 0.0038 | 2 |
| 4977 | GO:0006865 | Amino acid transport | 8 | 4.12 | 0.0098 | 2 |
| 4940 | GO:0006825 | Copper ion transport | 3 | 5.50 | 0.0105 | 1 |
| 24507 | GO:0051258 | Protein polymerization | 3 | 5.50 | 0.0105 | 1 |
| 5112 | GO:0007017 | Microtubule-based process | 3 | 5.50 | 0.0105 | 1 |
| 9784 | GO:0016575 | Histone deacetylation | 3 | 5.50 | 0.0105 | 1 |
| 6791 | GO:0009186 | Deoxyribonucleoside diphosphate Metabolic process | 3 | 5.50 | 0.0105 | 1 |
| 9582 | GO:0016311 | Dephosphorylation | 9 | 3.66 | 0.0141 | 2 |
| 5113 | GO:0007018 | Microtubule-based movement | 10 | 3.30 | 0.0192 | 2 |
| 24310 | GO:0051056 | Regulation of small GTPase mediated signal transduction | 4 | 4.12 | 0.0202 | 1 |
| GotermID | goACC | Biological Process | GOsize 1 | Fold Enrichment 2 | p-Value 3 | DEG 4 |
|---|---|---|---|---|---|---|
| 5073 | GO:0006974 | Response to DNA damage stimulus | 1 | 14.42 | 0 | 1 |
| 22806 | GO:0048478 | Replication fork protection | 1 | 14.42 | 0 | 1 |
| 4601 | GO:0006467 | Protein thiol-disulfide exchange | 1 | 14.42 | 0 | 1 |
| 5240 | GO:0007155 | Cell adhesion | 1 | 14.42 | 0 | 1 |
| 4712 | GO:0006582 | Melanin metabolic process | 1 | 14.42 | 0 | 1 |
| 267 | GO:0000272 | Polysaccharide catabolic process | 1 | 14.42 | 0 | 1 |
| 4599 | GO:0006465 | Signal peptide processing | 2 | 14.42 | 0 | 2 |
| 92 | GO:0000087 | M phase of mitotic cell cycle | 1 | 14.42 | 0 | 1 |
| 5135 | GO:0007040 | Lysosome organization and biogenesis | 1 | 14.42 | 0 | 1 |
| 4792 | GO:0006665 | Sphingolipid metabolic process | 1 | 14.42 | 0 | 1 |
| 5022 | GO:0006916 | Anti-apoptosis | 1 | 14.42 | 0 | 1 |
| 4199 | GO:0006031 | Chitin biosynthetic process | 1 | 14.42 | 0 | 1 |
| 4689 | GO:0006559 | L-phenylalanine catabolic process | 1 | 14.42 | 0 | 1 |
| 4700 | GO:0006570 | Tyrosine metabolic process | 1 | 14.42 | 0 | 1 |
| 17658 | GO:0042128 | Nitrate assimilation | 1 | 14.42 | 0 | 1 |
| 9010 | GO:0015698 | Inorganic anion transport | 1 | 14.42 | 0 | 1 |
| 4392 | GO:0006231 | dTMP biosynthetic process | 1 | 14.42 | 0 | 1 |
| 24016 | GO:0050757 | Thymidylate synthase biosynthetic process | 1 | 14.42 | 0 | 1 |
| 6681 | GO:0009072 | Aromatic amino acid family metabolic process | 1 | 14.42 | 0 | 1 |
| 19608 | GO:0045132 | Meiotic chromosome segregation | 1 | 14.42 | 0 | 1 |
| 4429 | GO:0006270 | DNA replication initiation | 6 | 7.21 | 0.0003 | 3 |
| 4283 | GO:0006118 | Electron transport | 104 | 2.21 | 0.0007 | 16 |
| 6276 | GO:0008643 | Carbohydrate transport | 10 | 4.32 | 0.0034 | 3 |
| 4421 | GO:0006260 | DNA replication | 16 | 3.60 | 0.0036 | 4 |
| 5143 | GO:0007049 | Cell cycle | 2 | 7.21 | 0.0048 | 1 |
| 4144 | GO:0005976 | Polysaccharide metabolic process | 2 | 7.21 | 0.0048 | 1 |
| 4970 | GO:0006857 | Oligopeptide transport | 2 | 7.21 | 0.0048 | 1 |
| 4928 | GO:0006811 | Ion transport | 2 | 7.21 | 0.0048 | 1 |
| 24525 | GO:0051276 | Chromosome organization and biogenesis | 2 | 7.21 | 0.0048 | 1 |
| 4484 | GO:0006334 | Nucleosome assembly | 13 | 3.32 | 0.0098 | 3 |
| 4940 | GO:0006825 | Copper ion transport | 3 | 4.81 | 0.0137 | 1 |
| 5112 | GO:0007017 | Microtubule-based process | 3 | 4.81 | 0.0137 | 1 |
| 24507 | GO:0051258 | Protein polymerization | 3 | 4.81 | 0.0137 | 1 |
| 9350 | GO:0016051 | Carbohydrate biosynthetic process | 3 | 4.81 | 0.0137 | 1 |
| 4977 | GO:0006865 | Amino acid transport | 8 | 3.61 | 0.0142 | 2 |
| Control vs. BCS | |||||
|---|---|---|---|---|---|
| Gene ID | Conserved Domains | Log2FC | logCPM | p-Value | FDR |
| VC83_02553 | Alpha-crystallin domain (HSP) | 4.52 | 8.32 | 1.16 × 10−8 | 4.36 × 10−7 |
| VC83_00970 | Heat shock protein 78, mitochondrial | 1.14 | 7.74 | 3.75 × 10−5 | 0.0004443 |
| VC83_01046 | Heat shock protein 78, mitochondrial | 1.21 | 11.40 | 4.61 × 10−5 | 0.0005284 |
| VC83_08187 | Heat shock protein 78, mitochondrial | 1.60 | 10.55 | 5.41× 10−6 | 8.85 × 10−5 |
| VC83_06435 | Hsp90 | 1.39 | 8.27 | 1.37× 10−5 | 0.0001892 |
| VC83_01360 | Zip; ZIP Zinc transporter | 1.48 | 5.58 | 3.15 × 10−6 | 5.59 × 10−5 |
| VC83_00191 | Ctr; Ctr copper transporter family | 3.58 | 11.62 | 1.54 × 10−57 | 3.70 × 10−54 |
| VC83_04094 | ATX1;HMA; Heavy-metal-associated domain (HMA) | 1.17 | 7.67 | 1.22 × 10−7 | 3.53 × 10−6 |
| VC83_07867 | BLP3 | 4.71 | 11.12 | 2.13 × 10−68 | 1.03 × 10−64 |
| VC83_00261 | Endo Mannanase, GH76 Family | 1.31 | 6.56 | 0.0013 | 0.0087237 |
| VC83_05292 | N/A | 1.02 | 9.59 | 1.38 × 10−5 | 0.0001895 |
| VC83_06039 | Ascorbate peroxidases and cytochrome C peroxidases | 1.42 | 5.80 | 4.94 × 10−10 | 2.73 × 10−8 |
| VC83_01624 | ABC-type multidrug transport system | 1.70 | 6.78 | 3.02 × 10−15 | 5.10 × 10−13 |
| VC83_09074 | Destructin-3 | 1.46 | 4.99 | 0.0003 | 0.0030929 |
| VC83_02181 | Peptidases_S53 | 2.51 | 2.79 | 2.53 × 10−7 | 6.44 × 10−6 |
| Control vs. Cu | |||||
| VC83_02553 | Alpha-crystallin domain (HSP) | 3.70 | 7.57 | 1.59 × 10−6 | 0.0001101 |
| VC83_01046 | Hsp70 chaperone | 1.35 | 11.51 | 0.0002 | 0.0054754 |
| VC83_08187 | Alpha-crystallin domain (HSP) | 1.66 | 10.60 | 2.65 × 10−5 | 0.0009256 |
| VC83_06435 | STI1 Hsp90 cochaperone | 1.24 | 8.16 | 8.33 × 10−5 | 0.0022095 |
| VC83_00191 | Ctr; Ctr copper transporter family | −4.69 | 7.65 | 1.20 × 10−45 | 3.84 × 10−42 |
| VC83_04094 | ATX1; HMA; Heavy-metal-associated domain (HMA) | 1.53 | 7.94 | 3.65 × 10−11 | 1.30 × 10−8 |
| VC83_07867 | BLP3 | −5.38 | 6.04 | 1.65 × 10−145 | 1.58 × 10−141 |
| VC83_09076 | Pectate_lyase_ | 1.60 | 6.45 | 1.98 × 10−10 | 6.13 × 10−8 |
| VC83_06039 | Ascorbate peroxidases and cytochrome C peroxidases | 1.59 | 5.94 | 0.0012 | 0.0148732 |
| VC83_08771 | MFS_1; Major Facilitator Superfamily | −1.05 | 6.82 | 4.13 × 10−8 | 5.45 × 10−6 |
| VC83_09074 | Destructin-3 | 2.90 | 6.24 | 1.35 × 10−13 | 9.26 × 10−11 |
| VC83_06062 | Destructin-1 | 2.01 | 10.95 | 0.0022 | 0.0227017 |
| VC83_02181 | Peptidases_S53 | 2.43 | 2.74 | 0.0007 | 0.0111273 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anne, S.; McDonald, M.R.; Lu, Y.; Peterson, R.L. Pseudogymnoascus destructans Transcriptional Response to Chronic Copper Stress. J. Fungi 2025, 11, 372. https://doi.org/10.3390/jof11050372
Anne S, McDonald MR, Lu Y, Peterson RL. Pseudogymnoascus destructans Transcriptional Response to Chronic Copper Stress. Journal of Fungi. 2025; 11(5):372. https://doi.org/10.3390/jof11050372
Chicago/Turabian StyleAnne, Saika, Maranda R. McDonald, Yuan Lu, and Ryan L. Peterson. 2025. "Pseudogymnoascus destructans Transcriptional Response to Chronic Copper Stress" Journal of Fungi 11, no. 5: 372. https://doi.org/10.3390/jof11050372
APA StyleAnne, S., McDonald, M. R., Lu, Y., & Peterson, R. L. (2025). Pseudogymnoascus destructans Transcriptional Response to Chronic Copper Stress. Journal of Fungi, 11(5), 372. https://doi.org/10.3390/jof11050372






