Autologous and Heterologous Minor and Major Bone Regeneration with Platelet-Derived Growth Factors
Abstract
1. Introduction
2. Materials and Methods
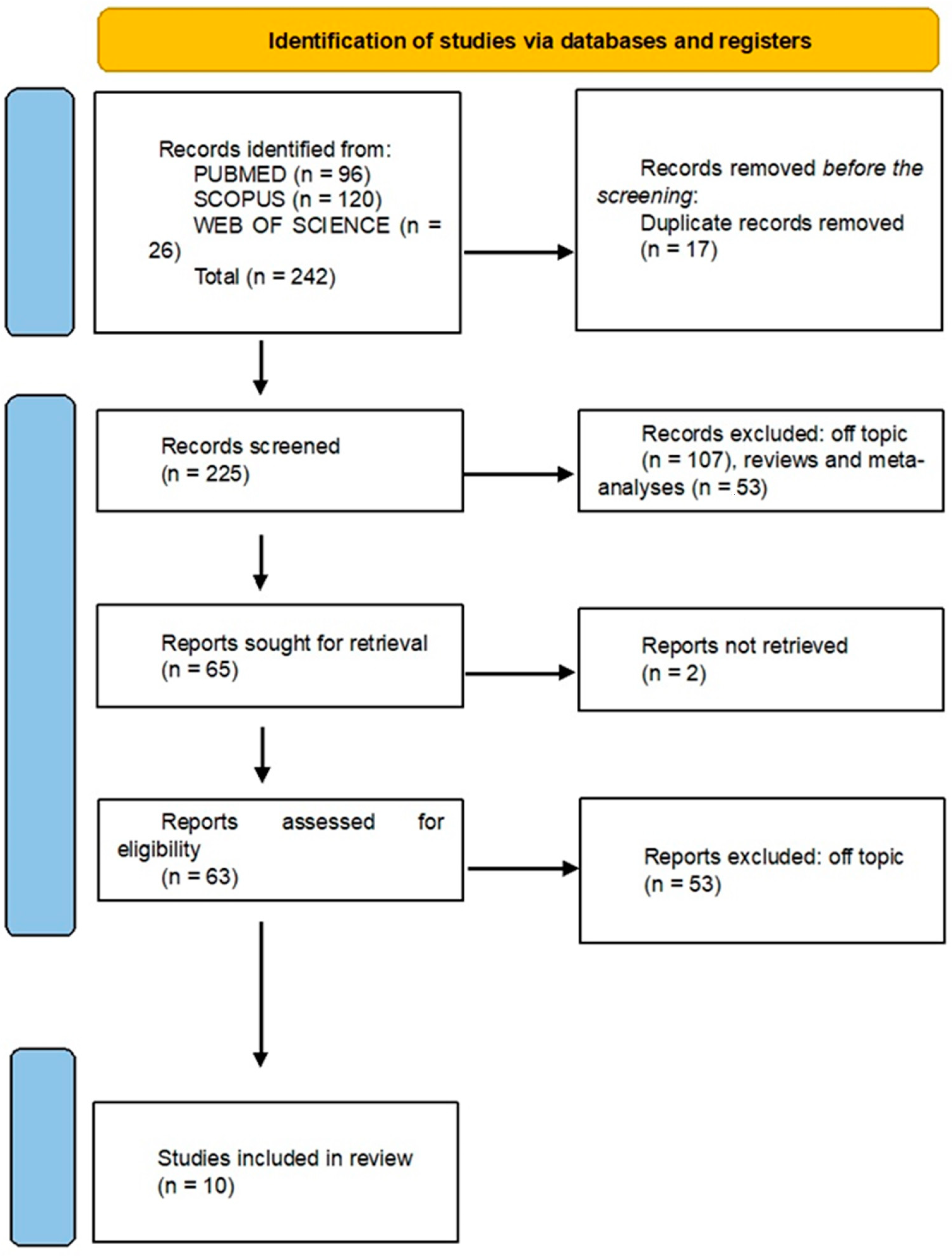
2.1. Processing Searches
2.2. Data Processing
2.3. Quality Assessment
3. Results
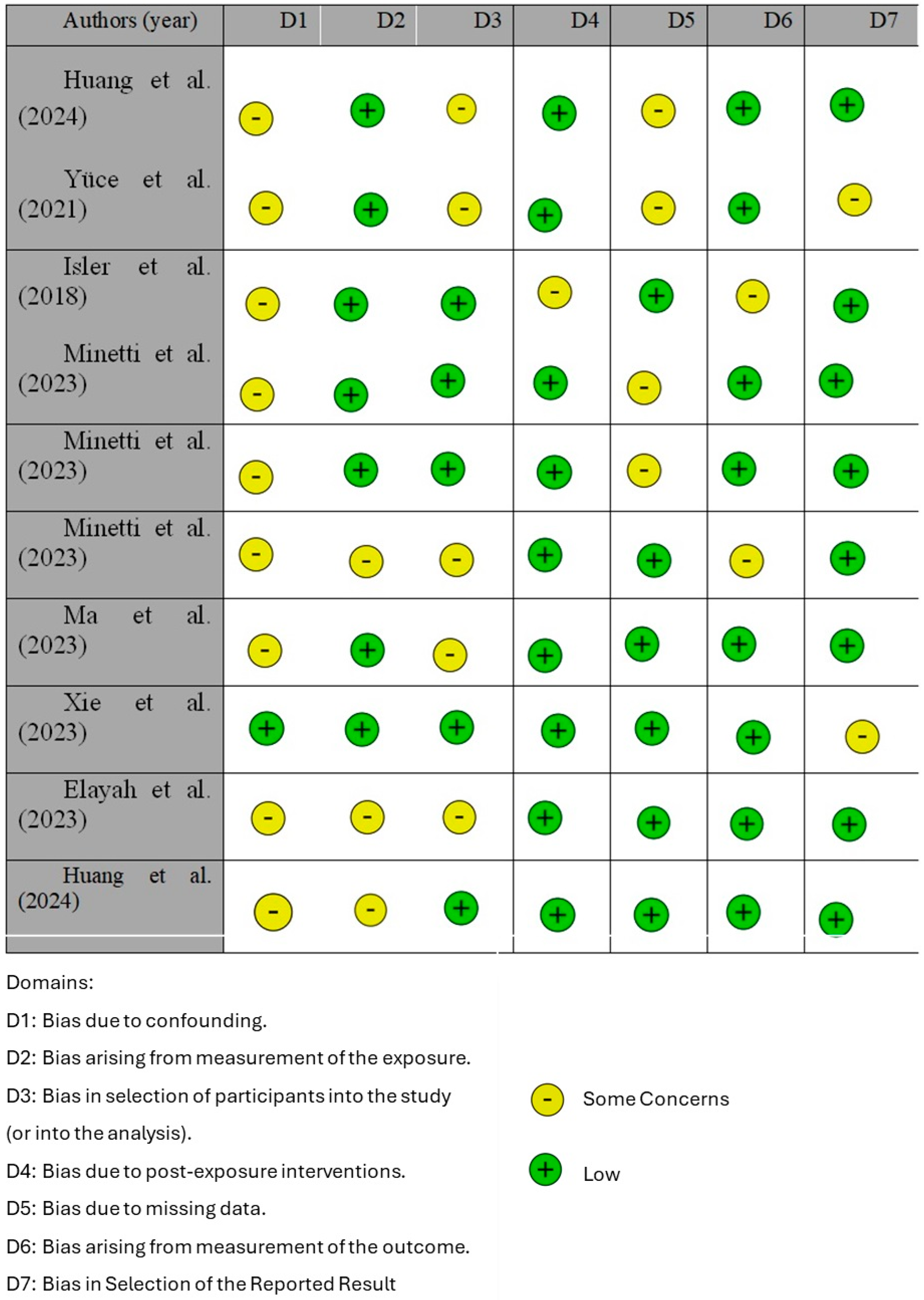
3.1. Quality Assessment and Risk of Bias of Included Articles
3.2. Results and Comparative Analysis
3.3. Clinical Significance and Limitations
4. Discussion
4.1. Effectiveness of CGFs
4.2. Bone Quality and Quantity
4.3. Autologous Materials
4.4. Biomolecular Mechanisms
4.5. Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CGF | concentrated growth factor |
| EGF | epidermal growth factor |
| MRONJ | medication-related osteonecrosis of the jaw |
| PDGF | platelet-derived growth factor |
| VEGF | vascular endothelial growth factor |
References
- Aghamohamadi, Z.; Kadkhodazadeh, M.; Torshabi, M.; Tabatabaei, F. A Compound of Concentrated Growth Factor and Periodontal Ligament Stem Cell-Derived Conditioned Medium. Tissue Cell 2020, 65, 101373. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Kawakita, A.; Ueda, N.; Funahara, R.; Tachibana, A.; Kobayashi, M.; Kondou, E.; Takeda, D.; Kojima, Y.; Sato, S.; et al. A Multicenter Retrospective Study of the Risk Factors Associated with Medication-Related Osteonecrosis of the Jaw after Tooth Extraction in Patients Receiving Oral Bisphosphonate Therapy: Can Primary Wound Closure and a Drug Holiday Really Prevent MRONJ? Osteoporos. Int. 2017, 28, 2465–2473. [Google Scholar] [CrossRef] [PubMed]
- Romasco, T.; Tumedei, M.; Inchingolo, F.; Pignatelli, P.; Montesani, L.; Iezzi, G.; Petrini, M.; Piattelli, A.; Di Pietro, N. A Narrative Review on the Effectiveness of Bone Regeneration Procedures with OsteoBiol® Collagenated Porcine Grafts: The Translational Research Experience over 20 Years. J. Funct. Biomater. 2022, 13, 121. [Google Scholar] [CrossRef]
- De Stavola, L.; Tunkel, J. A New Approach to Maintenance of Regenerated Autogenous Bone Volume: Delayed Relining with Xenograft and Resorbable Membrane. Int. J. Oral Maxillofac. Implant. 2013, 28, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hu, Y.; Fu, Y.; Zou, D.; Lu, J.; Lyu, C. Emerging Roles of Platelet Concentrates and Platelet-Derived Extracellular Vesicles in Regenerative Periodontology and Implant Dentistry. APL Bioeng. 2022, 6, 031503. [Google Scholar] [CrossRef]
- Misch, K.A.; Yi, E.S.; Sarment, D.P. Accuracy of Cone Beam Computed Tomography for Periodontal Defect Measurements. J. Periodontol. 2006, 77, 1261–1266. [Google Scholar] [CrossRef]
- Van der Weijden, F.; Dell’Acqua, F.; Slot, D.E. Alveolar Bone Dimensional Changes of Post-Extraction Sockets in Humans: A Systematic Review. J. Clin. Periodontol. 2009, 36, 1048–1058. [Google Scholar] [CrossRef]
- Elayah, S.A.; Younis, H.; Cui, H.; Liang, X.; Sakran, K.A.; Alkadasi, B.; Al-Moraissi, E.A.; Albadani, M.; Al-Okad, W.; Tu, J.; et al. Alveolar Ridge Preservation in Post-Extraction Sockets Using Concentrated Growth Factors: A Split-Mouth, Randomized, Controlled Clinical Trial. Front. Endocrinol. 2023, 14, 1163696. [Google Scholar] [CrossRef]
- Siritham, A.; Powcharoen, W.; Wanichsaithong, P.; Supanchart, C. Analgesics Effect of Local Diclofenac in Third Molar Surgery: A Randomized, Controlled Trial. Clin. Oral Investig. 2023, 27, 6073–6080. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Kim, S.-G.; Oh, J.-S.; Jin, S.-C.; Son, J.-S.; Kim, S.-Y.; Lim, S.-Y. Analysis of the Inorganic Component of Autogenous Tooth Bone Graft Material. J. Nanosci. Nanotechnol. 2011, 11, 7442–7445. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Inchingolo, A.M.; Latini, G.; Palmieri, G.; Di Pede, C.; Trilli, I.; Ferrante, L.; Inchingolo, A.D.; Palermo, A.; Lorusso, F.; et al. Application of Graphene Oxide in Oral Surgery: A Systematic Review. Materials 2023, 16, 6293. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Qin, Y.; Wei, M.; Niu, W. Application of Sticky Bone Combined with Concentrated Growth Factor (CGF) for Horizontal Alveolar Ridge Augmentation of Anterior Teeth: A Randomized Controlled Clinical Study. BMC Oral Health 2024, 24, 431. [Google Scholar] [CrossRef]
- Pang, K.-M.; Um, I.-W.; Kim, Y.-K.; Woo, J.-M.; Kim, S.-M.; Lee, J.-H. Autogenous Demineralized Dentin Matrix from Extracted Tooth for the Augmentation of Alveolar Bone Defect: A Prospective Randomized Clinical Trial in Comparison with Anorganic Bovine Bone. Clin. Oral Implant. Res. 2017, 28, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Kim, S.-G.; Yun, P.-Y.; Yeo, I.-S.; Jin, S.-C.; Oh, J.-S.; Kim, H.-J.; Yu, S.-K.; Lee, S.-Y.; Kim, J.-S.; et al. Autogenous Teeth Used for Bone Grafting: A Comparison with Traditional Grafting Materials. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, e39–e45. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, M.; Gallesio, G.; Mozzati, M. Autologous Platelet Concentrates for Bisphosphonate-Related Osteonecrosis of the Jaw Treatment and Prevention. A Systematic Review of the Literature. Eur. J. Cancer 2015, 51, 62–74. [Google Scholar] [CrossRef]
- Kapse, S.; Surana, S.; Satish, M.; Hussain, S.E.; Vyas, S.; Thakur, D. Autologous Platelet-Rich Fibrin: Can It Secure a Better Healing? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 8–18. [Google Scholar] [CrossRef]
- Minetti, E.; Palermo, A.; Inchingolo, A.D.; Patano, A.; Viapiano, F.; Ciocia, A.M.; de Ruvo, E.; Mancini, A.; Inchingolo, F.; Sauro, S.; et al. Autologous Tooth for Bone Regeneration: Dimensional Examination of Tooth Transformer® Granules. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 5421–5430. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.M.; Patano, A.; Di Pede, C.; Inchingolo, A.D.; Palmieri, G.; de Ruvo, E.; Campanelli, M.; Buongiorno, S.; Carpentiere, V.; Piras, F.; et al. Autologous Tooth Graft: Innovative Biomaterial for Bone Regeneration. Tooth Transformer® and the Role of Microbiota in Regenerative Dentistry. A Systematic Review. J. Funct. Biomater. 2023, 14, 132. [Google Scholar] [CrossRef] [PubMed]
- Borsani, E.; Bonazza, V.; Buffoli, B.; Nocini, P.F.; Albanese, M.; Zotti, F.; Inchingolo, F.; Rezzani, R.; Rodella, L.F. Beneficial Effects of Concentrated Growth Factors and Resveratrol on Human Osteoblasts In Vitro Treated with Bisphosphonates. Biomed. Res. Int. 2018, 2018, 4597321. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Malcangi, G.; Inchingolo, A.M.; Piras, F.; Settanni, V.; Garofoli, G.; Palmieri, G.; Ceci, S.; Patano, A.; De Leonardis, N.; et al. Benefits and Implications of Resveratrol Supplementation on Microbiota Modulations: A Systematic Review of the Literature. Int. J. Mol. Sci. 2022, 23, 4027. [Google Scholar] [CrossRef]
- Minetti, E.; Dipalma, G.; Palermo, A.; Patano, A.; Inchingolo, A.D.; Inchingolo, A.M.; Inchingolo, F. Biomolecular Mechanisms and Case Series Study of Socket Preservation with Tooth Grafts. J. Clin. Med. 2023, 12, 5611. [Google Scholar] [CrossRef] [PubMed]
- Kardos, D.; Hornyák, I.; Simon, M.; Hinsenkamp, A.; Marschall, B.; Várdai, R.; Kállay-Menyhárd, A.; Pinke, B.; Mészáros, L.; Kuten, O.; et al. Biological and Mechanical Properties of Platelet-Rich Fibrin Membranes after Thermal Manipulation and Preparation in a Single-Syringe Closed System. Int. J. Mol. Sci. 2018, 19, 3433. [Google Scholar] [CrossRef]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone Healing and Soft Tissue Contour Changes Following Single-Tooth Extraction: A Clinical and Radiographic 12-Month Prospective Study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–323. [Google Scholar]
- Meijndert, L.; Raghoebar, G.M.; Schüpbach, P.; Meijer, H.J.A.; Vissink, A. Bone Quality at the Implant Site after Reconstruction of a Local Defect of the Maxillary Anterior Ridge with Chin Bone or Deproteinised Cancellous Bovine Bone. Int. J. Oral Maxillofac. Surg. 2005, 34, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Leung, Y.Y.; Yeung, A.W.K.; Ismail, I.N.; Wong, N.S.M. Bone Regeneration at the Distal Aspect of the Adjacent Second Molar after Lower Third Molar Coronectomy: A Long-Term Analysis. Int. J. Oral Maxillofac. Surg. 2020, 49, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Sohn, D.-S.; Heo, J.-U.; Kwak, D.-H.; Kim, D.-E.; Kim, J.-M.; Moon, J.-W.; Lee, J.-H.; Park, I.-S. Bone Regeneration in the Maxillary Sinus Using an Autologous Fibrin-Rich Block with Concentrated Growth Factors Alone. Implant. Dent. 2011, 20, 389–395. [Google Scholar] [CrossRef]
- Huang, C.; Xu, Y. Can Concentrated Growth Factor Prevent Postoperative Complications of Impacted Third Molar Surgery? A Split-Mouth Randomized Double-Blind Trial. Clin. Oral Investig. 2024, 28, 234. [Google Scholar] [CrossRef] [PubMed]
- Micko, L.; Salma, I.; Skadins, I.; Egle, K.; Salms, G.; Dubnika, A. Can Our Blood Help Ensure Antimicrobial and Anti-Inflammatory Properties in Oral and Maxillofacial Surgery? Int. J. Mol. Sci. 2023, 24, 1073. [Google Scholar] [CrossRef]
- Alshujaa, B.; Talmac, A.C.; Altindal, D.; Alsafadi, A.; Ertugrul, A.S. Clinical and Radiographic Evaluation of the Use of PRF, CGF, and Autogenous Bone in the Treatment of Periodontal Intrabony Defects: Treatment of Periodontal Defect by Using Autologous Products. J. Periodontol. 2024, 95, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Matarasso, S.; Iorio Siciliano, V.; Aglietta, M.; Andreuccetti, G.; Salvi, G.E. Clinical and Radiographic Outcomes of a Combined Resective and Regenerative Approach in the Treatment of Peri-Implantitis: A Prospective Case Series. Clin. Oral Implant. Res. 2014, 25, 761–767. [Google Scholar] [CrossRef]
- Minamizato, T.; Koga, T.; I, T.; Nakatani, Y.; Umebayashi, M.; Sumita, Y.; Ikeda, T.; Asahina, I. Clinical Application of Autogenous Partially Demineralized Dentin Matrix Prepared Immediately after Extraction for Alveolar Bone Regeneration in Implant Dentistry: A Pilot Study. Int. J. Oral Maxillofac. Surg. 2018, 47, 125–132. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Jiang, C.; Guo, H.; Luo, G.; Huang, Y.; Yuan, C. Clinical Applications of Concentrated Growth Factors Membrane for Sealing the Socket in Alveolar Ridge Preservation: A Randomized Controlled Trial. Int. J. Implant. Dent. 2022, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Gharpure, A.S.; Bhatavadekar, N.B. Clinical Efficacy of Tooth-Bone Graft: A Systematic Review and Risk of Bias Analysis of Randomized Control Trials and Observational Studies. Implant. Dent. 2018, 27, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; John, G.; Schmucker, A.; Sahm, N.; Becker, J. Combined Surgical Therapy of Advanced Peri-Implantitis Evaluating Two Methods of Surface Decontamination: A 7-Year Follow-up Observation. J. Clin. Periodontol. 2017, 44, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Tanan Karaca, G.; Duygu, G.; Er, N.; Ozgun, E. Comparative Investigation of Anti-Inflammatory Effect of Platelet-Rich Fibrin after Mandibular Wisdom Tooth Surgery: A Randomized Controlled Study. J. Clin. Med. 2023, 12, 4250. [Google Scholar] [CrossRef]
- Park, S.-H.; Choi, H.; Han, J.-S.; Park, Y.-B. Comparative Study of Decalcification versus Nondecalcification for Histological Evaluation of One-Wall Periodontal Intrabony Defects in Dogs. Microsc. Res. Tech. 2015, 78, 94–104. [Google Scholar] [CrossRef]
- Huang, L.; Zou, R.; He, J.; Ouyang, K.; Piao, Z. Comparing Osteogenic Effects between Concentrated Growth Factors and the Acellular Dermal Matrix. Braz. Oral Res. 2018, 32, e29. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Kim, S.-H.; Sándor, G.K.; Kim, Y.-D. Comparison of Platelet-Rich Plasma (PRP), Platelet-Rich Fibrin (PRF), and Concentrated Growth Factor (CGF) in Rabbit-Skull Defect Healing. Arch. Oral Biol. 2014, 59, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Shetty, L.; Gangwani, K.; Londhe, U.; Bharadwaj, S.; Bakri, M.M.H.; Alamoudi, A.; Reda, R.; Bhandi, S.; Raj, A.T.; Patil, S.; et al. Comparison of the C-Reactive Protein Level and Visual Analog Scale Scores between Piezosurgery and Rotatory Osteotomy in Mandibular Impacted Third Molar Extraction. Life 2022, 12, 923. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt Doğan, Ş.; Öngöz Dede, F.; Ballı, U.; Atalay, E.N.; Durmuşlar, M.C. Concentrated Growth Factor in the Treatment of Adjacent Multiple Gingival Recessions: A Split-Mouth Randomized Clinical Trial. J. Clin. Periodontol. 2015, 42, 868–875. [Google Scholar] [CrossRef]
- Qin, J.; Wang, L.; Zheng, L.; Zhou, X.; Zhang, Y.; Yang, T.; Zhou, Y. Concentrated Growth Factor Promotes Schwann Cell Migration Partly through the Integrin Β1-Mediated Activation of the Focal Adhesion Kinase Pathway. Int. J. Mol. Med. 2016, 37, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Rochira, A.; Siculella, L.; Damiano, F.; Palermo, A.; Ferrante, F.; Carluccio, M.A.; Calabriso, N.; Giannotti, L.; Stanca, E. Concentrated Growth Factors (CGF) Induce Osteogenic Differentiation in Human Bone Marrow Stem Cells. Biology 2020, 9, 370. [Google Scholar] [CrossRef]
- Mauceri, R.; Panzarella, V.; Maniscalco, L.; Bedogni, A.; Licata, M.E.; Albanese, A.; Toia, F.; Cumbo, E.M.G.; Mazzola, G.; Di Fede, O.; et al. Conservative Surgical Treatment of Bisphosphonate-Related Osteonecrosis of the Jaw with Er,Cr:YSGG Laser and Platelet-Rich Plasma: A Longitudinal Study. Biomed. Res. Int. 2018, 2018, 3982540. [Google Scholar] [CrossRef] [PubMed]
- Miroshnychenko, A.; Azab, M.; Ibrahim, S.; Roldan, Y.; Diaz Martinez, J.P.; Tamilselvan, D.; He, L.; Urquhart, O.; Verdugo-Paiva, F.; Tampi, M.; et al. Corticosteroids for Managing Acute Pain Subsequent to Surgical Extraction of Mandibular Third Molars: A Systematic Review and Meta-Analysis. J. Am. Dent. Assoc. 2023, 154, 727–741.e10. [Google Scholar] [CrossRef]
- Bono, N.; Tarsini, P.; Candiani, G. Demineralized Dentin and Enamel Matrices as Suitable Substrates for Bone Regeneration. J. Appl. Biomater. Funct. Mater. 2017, 15, e236–e243. [Google Scholar] [CrossRef] [PubMed]
- de Faria Vasconcelos, K.; Evangelista, K.M.; Rodrigues, C.D.; Estrela, C.; de Sousa, T.O.; Silva, M.a.G. Detection of Periodontal Bone Loss Using Cone Beam CT and Intraoral Radiography. Dentomaxillofac Radiol. 2012, 41, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Minetti, E.; Palermo, A.; Malcangi, G.; Inchingolo, A.D.; Mancini, A.; Dipalma, G.; Inchingolo, F.; Patano, A.; Inchingolo, A.M. Dentin, Dentin Graft, and Bone Graft: Microscopic and Spectroscopic Analysis. J. Funct. Biomater. 2023, 14, 272. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Tsujino, T.; Yamaguchi, S.; Isobe, K.; Watanabe, T.; Kitamura, Y.; Okuda, K.; Nakata, K.; Kawase, T. Distribution of Platelets, Transforming Growth Factor-Β1, Platelet-Derived Growth Factor-BB, Vascular Endothelial Growth Factor and Matrix Metalloprotease-9 in Advanced Platelet-Rich Fibrin and Concentrated Growth Factor Matrices. J. Investig. Clin. Dent. 2019, 10, e12458. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Kim, J.-W.; Kim, S.-J. Does the Addition of Bone Morphogenetic Protein 2 to Platelet-Rich Fibrin Improve Healing After Treatment for Medication-Related Osteonecrosis of the Jaw? J. Oral Maxillofac. Surg. 2017, 75, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Elayah, S.A.; Liang, X.; Sakran, K.A.; Xie, L.; Younis, H.; Alajami, A.E.; Tu, J.; Na, S. Effect of Concentrated Growth Factor (CGF) on Postoperative Sequel of Completely Impacted Lower Third Molar Extraction: A Randomized Controlled Clinical Study. BMC Oral Health 2022, 22, 368. [Google Scholar] [CrossRef] [PubMed]
- Özveri Koyuncu, B.; Işık, G.; Özden Yüce, M.; Günbay, S.; Günbay, T. Effect of Concentrated Growth Factor (CGF) on Short-Term Clinical Outcomes after Partially Impacted Mandibular Third Molar Surgery: A Split-Mouth Randomized Clinical Study. J. Stomatol. Oral Maxillofac. Surg. 2020, 121, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Yüce, M.O.; Adalı, E.; Işık, G. The Effect of Concentrated Growth Factor (CGF) in the Surgical Treatment of Medication-Related Osteonecrosis of the Jaw (MRONJ) in Osteoporosis Patients: A Randomized Controlled Study. Clin. Oral Investig. 2021, 25, 4529–4541. [Google Scholar] [CrossRef] [PubMed]
- Isler, S.C.; Soysal, F.; Ceyhanlı, T.; Bakırarar, B.; Unsal, B. Regenerative Surgical Treatment of Peri-Implantitis Using Either a Collagen Membrane or Concentrated Growth Factor: A 12-Month Randomized Clinical Trial. Clin. Implant. Dent. Relat. Res. 2018, 20, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Minetti, E.; Palermo, A.; Savadori, P.; Patano, A.; Inchingolo, A.D.; Rapone, B.; Malcangi, G.; Inchingolo, F.; Dipalma, G.; Tartaglia, F.C.; et al. Socket Preservation Using Dentin Mixed with Xenograft Materials: A Pilot Study. Materials 2023, 16, 4945. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Lin, Y.; Sun, F.; Jiang, X.; Wei, T. The Impact of Autologous Concentrated Growth Factors on the Alveolar Ridge Preservation after Posterior Tooth Extraction: A Prospective, Randomized Controlled Clinical Trial. Clin. Implant. Dent. Relat. Res. 2021, 23, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Krausz, A.A.; Machtei, E.E.; Peled, M. Effects of Lower Third Molar Extraction on Attachment Level and Alveolar Bone Height of the Adjacent Second Molar. Int. J. Oral Maxillofac. Surg. 2005, 34, 756–760. [Google Scholar] [CrossRef]
- Norton, M.R.; Odell, E.W.; Thompson, I.D.; Cook, R.J. Efficacy of Bovine Bone Mineral for Alveolar Augmentation: A Human Histologic Study. Clin. Oral Implant. Res. 2003, 14, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Avantario, P.; Azzollini, D.; Buongiorno, S.; Viapiano, F.; Campanelli, M.; Ciocia, A.M.; De Leonardis, N.; et al. Effects of Resveratrol, Curcumin and Quercetin Supplementation on Bone Metabolism-A Systematic Review. Nutrients 2022, 14, 3519. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cheng, J.; Cai, Y.; Zhang, J.; Yin, X.; Luan, Q. Efficacy of Concentrated Growth Factor (CGF) in the Surgical Treatment of Oral Diseases: A Systematic Review and Meta-Analysis. BMC Oral Health 2023, 23, 712. [Google Scholar] [CrossRef] [PubMed]
- Baslarli, O.; Tumer, C.; Ugur, O.; Vatankulu, B. Evaluation of Osteoblastic Activity in Extraction Sockets Treated with Platelet-Rich Fibrin. Med. Oral Patol. Oral Cir. Bucal 2015, 20, e111–e116. [Google Scholar] [CrossRef]
- Bilginaylar, K.; Uyanik, L.O. Evaluation of the Effects of Platelet-Rich Fibrin and Piezosurgery on Outcomes after Removal of Impacted Mandibular Third Molars. Br. J. Oral Maxillofac. Surg. 2016, 54, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Kim, Y.-K.; Park, Y.-H.; Park, J.-C.; Ku, J.-K.; Um, I.-W.; Kim, J.-Y. Evaluation of the Healing Potential of Demineralized Dentin Matrix Fixed with Recombinant Human Bone Morphogenetic Protein-2 in Bone Grafts. Materials 2017, 10, 1049. [Google Scholar] [CrossRef] [PubMed]
- Passarelli, P.C.; Romeo, A.; Lopez, M.A.; De Angelis, P.; Desantis, V.; Piccirillo, G.B.; Papa, R.; Papi, P.; Pompa, G.; Moffa, A.; et al. Evaluation of the Periodontal Healing of the Second Mandibular Molar Distal Site Following Insertion of PRF in the Third Molar Post Extraction Alveolus. J. Biol. Regul. Homeost. Agents 2020, 34, 111–118. [Google Scholar]
- Kumar, N.; Prasad, K.; Ramanujam, L.; K, R.; Dexith, J.; Chauhan, A. Evaluation of Treatment Outcome after Impacted Mandibular Third Molar Surgery with the Use of Autologous Platelet-Rich Fibrin: A Randomized Controlled Clinical Study. J. Oral Maxillofac. Surg. 2015, 73, 1042–1049. [Google Scholar] [CrossRef]
- Detsch, R.; Mayr, H.; Ziegler, G. Formation of Osteoclast-like Cells on HA and TCP Ceramics. Acta Biomater. 2008, 4, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Umebayashi, M.; Ohba, S.; Kurogi, T.; Noda, S.; Asahina, I. Full Regeneration of Maxillary Alveolar Bone Using Autogenous Partially Demineralized Dentin Matrix and Particulate Cancellous Bone and Marrow for Implant-Supported Full Arch Rehabilitation. J. Oral Implantol. 2020, 46, 122–127. [Google Scholar] [CrossRef]
- Masuki, H.; Okudera, T.; Watanebe, T.; Suzuki, M.; Nishiyama, K.; Okudera, H.; Nakata, K.; Uematsu, K.; Su, C.-Y.; Kawase, T. Growth Factor and Pro-Inflammatory Cytokine Contents in Platelet-Rich Plasma (PRP), Plasma Rich in Growth Factors (PRGF), Advanced Platelet-Rich Fibrin (A-PRF), and Concentrated Growth Factors (CGF). Int. J. Implant. Dent. 2016, 2, 19. [Google Scholar] [CrossRef]
- Rodella, L.F.; Favero, G.; Boninsegna, R.; Buffoli, B.; Labanca, M.; Scarì, G.; Sacco, L.; Batani, T.; Rezzani, R. Growth Factors, CD34 Positive Cells, and Fibrin Network Analysis in Concentrated Growth Factors Fraction. Microsc. Res. Tech. 2011, 74, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided Bone Regeneration: Materials and Biological Mechanisms Revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- MacBeth, N.; Trullenque-Eriksson, A.; Donos, N.; Mardas, N. Hard and Soft Tissue Changes Following Alveolar Ridge Preservation: A Systematic Review. Clin. Oral Implant. Res. 2017, 28, 982–1004. [Google Scholar] [CrossRef]
- Dahlin, C.; Linde, A.; Gottlow, J.; Nyman, S. Healing of Bone Defects by Guided Tissue Regeneration. Plast. Reconstr. Surg. 1988, 81, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Carmagnola, D.; Adriaens, P.; Berglundh, T. Healing of Human Extraction Sockets Filled with Bio-Oss. Clin. Oral Implant. Res. 2003, 14, 137–143. [Google Scholar] [CrossRef]
- Yifat, M.; Hila, E.; Avraham, H.; Inchingolo, F.; Mortellaro, C.; Peleg, O.; Mijiritsky, E. Histologic and Radiographic Characteristics of Bone Filler Under Bisphosphonates. J. Craniofac. Surg. 2019, 30, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Brugnami, F.; Then, P.R.; Moroi, H.; Leone, C.W. Histologic Evaluation of Human Extraction Sockets Treated with Demineralized Freeze-Dried Bone Allograft (DFDBA) and Cell Occlusive Membrane. J. Periodontol. 1996, 67, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Persson, R.E.; Hollender, L.G.; Laurell, L.; Persson, G.R. Horizontal Alveolar Bone Loss and Vertical Bone Defects in an Adult Patient Population. J. Periodontol. 1998, 69, 348–356. [Google Scholar] [CrossRef]
- Urban, I.A.; Nagursky, H.; Lozada, J.L.; Nagy, K. Horizontal Ridge Augmentation with a Collagen Membrane and a Combination of Particulated Autogenous Bone and Anorganic Bovine Bone-Derived Mineral: A Prospective Case Series in 25 Patients. Int. J. Periodontics Restor. Dent. 2013, 33, 299–307. [Google Scholar] [CrossRef]
- Bessho, K.; Tanaka, N.; Matsumoto, J.; Tagawa, T.; Murata, M. Human Dentin-Matrix-Derived Bone Morphogenetic Protein. J. Dent. Res. 1991, 70, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Rijal, G.; Shin, H.-I. Human Tooth-Derived Biomaterial as a Graft Substitute for Hard Tissue Regeneration. Regen. Med. 2017, 12, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fok, M.R.; Pelekos, G.; Jin, L.; Tonetti, M.S. In Vitro and Ex Vivo Kinetic Release Profile of Growth Factors and Cytokines from Leucocyte- and Platelet-Rich Fibrin (L-PRF) Preparations. Cells 2022, 11, 2089. [Google Scholar] [CrossRef]
- Tabatabaei, F.; Aghamohammadi, Z.; Tayebi, L. In Vitro and in Vivo Effects of Concentrated Growth Factor on Cells and Tissues. J. Biomed. Mater. Res. A 2020, 108, 1338–1350. [Google Scholar] [CrossRef]
- Mol, A.; Balasundaram, A. In Vitro Cone Beam Computed Tomography Imaging of Periodontal Bone. Dentomaxillofac. Radiol. 2008, 37, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Wenisch, S.; Stahl, J.-P.; Horas, U.; Heiss, C.; Kilian, O.; Trinkaus, K.; Hild, A.; Schnettler, R. In Vivo Mechanisms of Hydroxyapatite Ceramic Degradation by Osteoclasts: Fine Structural Microscopy. J. Biomed. Mater. Res. A 2003, 67, 713–718. [Google Scholar] [CrossRef]
- Choi, Y.S.; Kim, Y.C.; Ji, S.; Choi, Y. Increased Bacterial Invasion and Differential Expression of Tight-Junction Proteins, Growth Factors, and Growth Factor Receptors in Periodontal Lesions. J. Periodontol. 2014, 85, e313–e322. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, Z.; Zheng, D.; Lin, P.; Cai, Y.; Hong, S.; Lai, Y.; Wu, D. Inlay Osteotome Sinus Floor Elevation with Concentrated Growth Factor Application and Simultaneous Short Implant Placement in Severely Atrophic Maxilla. Sci. Rep. 2016, 6, 27348. [Google Scholar] [CrossRef] [PubMed]
- Comuzzi, L.; Tumedei, M.; Romasco, T.; Petrini, M.; Afrashtehfar, K.I.; Inchingolo, F.; Piattelli, A.; Di Pietro, N. Insertion Torque, Removal Torque, and Resonance Frequency Analysis Values of Ultrashort, Short, and Standard Dental Implants: An In Vitro Study on Polyurethane Foam Sheets. J. Funct. Biomater. 2022, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Tatullo, M.; Marrelli, M.; Inchingolo, A.M.; Scacco, S.; Inchingolo, A.D.; Dipalma, G.; Vermesan, D.; Abbinante, A.; Cagiano, R. Trial with Platelet-Rich Fibrin and Bio-Oss Used as Grafting Materials in the Treatment of the Severe Maxillar Bone Atrophy: Clinical and Radiological Evaluations. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 1075–1084. [Google Scholar] [PubMed]
- Atieh, M.A.; Alsabeeha, N.H.M.; Payne, A.G.T.; Duncan, W.; Faggion, C.M.; Esposito, M. Interventions for Replacing Missing Teeth: Alveolar Ridge Preservation Techniques for Dental Implant Site Development. Cochrane Database Syst. Rev. 2015, 2015, CD010176. [Google Scholar] [CrossRef]
- Esposito, M.; Grusovin, M.G.; Worthington, H.V. Interventions for Replacing Missing Teeth: Treatment of Peri-Implantitis. Cochrane Database Syst. Rev. 2012, 1, CD004970. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, J.; Huang, Y.; Pan, Q.; Nie, M. Local Application of Platelet-Rich Fibrin During Lower Third Molar Extraction Improves Treatment Outcomes. J. Oral Maxillofac. Surg. 2017, 75, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Daly, B.J.; Sharif, M.O.; Jones, K.; Worthington, H.V.; Beattie, A. Local Interventions for the Management of Alveolar Osteitis (Dry Socket). Cochrane Database Syst. Rev. 2022, 9, CD006968. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.; Gleason, D.M.; Murray, R.; Tchekmedyian, S.; Venner, P.; Lacombe, L.; Chin, J.L.; Vinholes, J.J.; Goas, J.A.; Zheng, M.; et al. Long-Term Efficacy of Zoledronic Acid for the Prevention of Skeletal Complications in Patients with Metastatic Hormone-Refractory Prostate Cancer. J. Natl. Cancer Inst. 2004, 96, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Khoshkam, V.; Suárez-López Del Amo, F.; Monje, A.; Lin, G.-H.; Chan, H.-L.; Wang, H.-L. Long-Term Radiographic and Clinical Outcomes of Regenerative Approach for Treating Peri-Implantitis: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2016, 31, 1303–1310. [Google Scholar] [CrossRef]
- Peng, K.Y.; Tseng, Y.C.; Shen, E.C.; Chiu, S.C.; Fu, E.; Huang, Y.W. Mandibular Second Molar Periodontal Status after Third Molar Extraction. J. Periodontol. 2001, 72, 1647–1651. [Google Scholar] [CrossRef]
- Sánchez Jorge, M.I.; Ocaña, R.A.; Valle Rodríguez, C.; Peyró Fernández-Montes, B.; Rico-Romano, C.; Bazal-Bonelli, S.; Sánchez-Labrador, L.; Cortés-Bretón Brinkmann, J. Mandibular Third Molar Extraction: Perceived Surgical Difficulty in Relation to Professional Training. BMC Oral Health 2023, 23, 485. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Q.; Wang, F.; Wang, Z. Maxillary Sinus Floor Augmentation and Dental Implant Placement Using Dentin Matrix Protein-1 Gene-Modified Bone Marrow Stromal Cells Mixed with Deproteinized Boving Bone: A Comparative Study in Beagles. Arch. Oral Biol. 2016, 64, 102–108. [Google Scholar] [CrossRef]
- Mavrokokki, T.; Cheng, A.; Stein, B.; Goss, A. Nature and Frequency of Bisphosphonate-Associated Osteonecrosis of the Jaws in Australia. J. Oral Maxillofac. Surg. 2007, 65, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, N.; Pierozzi, E.; Inchingolo, A.M.; Pahwa, B.; De Carlo, A.; Palermo, A.; Scarola, R.; Dipalma, G.; Corsalini, M.; Inchingolo, A.D.; et al. New Biograft Solution, Growth Factors and Bone Regenerative Approaches in Neurosurgery, Dentistry, and Orthopedics: A Review. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 7653–7664. [Google Scholar] [CrossRef] [PubMed]
- Firoozi, P.; Moreira Falci, S.G.; Kim, S.-G.; Assael, L.A. Nonpharmacological Complementary Interventions for the Management of Pain after Third Molar Surgery: An Umbrella Review of Current Meta-Analyses. Pain. Res. Manag. 2022, 2022, 1816748. [Google Scholar] [CrossRef]
- Xu, Y.; Qiu, J.; Sun, Q.; Yan, S.; Wang, W.; Yang, P.; Song, A. One-Year Results Evaluating the Effects of Concentrated Growth Factors on the Healing of Intrabony Defects Treated with or without Bone Substitute in Chronic Periodontitis. Med. Sci. Monit. 2019, 25, 4384–4389. [Google Scholar] [CrossRef] [PubMed]
- Assael, L.A. Oral Bisphosphonates as a Cause of Bisphosphonate-Related Osteonecrosis of the Jaws: Clinical Findings, Assessment of Risks, and Preventive Strategies. J. Oral Maxillofac. Surg. 2009, 67, 35–43. [Google Scholar] [CrossRef]
- Robinson, A.; Scully, C. Pharmacology: New Therapies and Challenges. Br. Dent. J. 2014, 217, 258–259. [Google Scholar] [CrossRef]
- Blair, P.; Flaumenhaft, R. Platelet Alpha-Granules: Basic Biology and Clinical Correlates. Blood Rev. 2009, 23, 177–189. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, B. Platelet-Rich Fibrin (PRF): A Second-Generation Platelet Concentrate. Part II: Platelet-Related Biologic Features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e45–e50. [Google Scholar] [CrossRef]
- Egierska, D.; Perszke, M.; Mazur, M.; Duś-Ilnicka, I. Platelet-Rich Plasma and Platelet-Rich Fibrin in Oral Surgery: A Narrative Review. Dent. Med. Probl. 2023, 60, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Coviello, V.; Peluso, F.; Dehkhargani, S.Z.; Verdugo, F.; Raffaelli, L.; Manicone, P.F.; D’ Addona, A. Platelet-Rich Plasma Improves Wound Healing in Multiple Myeloma Bisphosphonate-Associated Osteonecrosis of the Jaw Patients. J. Biol. Regul. Homeost. Agents 2012, 26, 151–155. [Google Scholar] [PubMed]
- Lee, J.W.; Kwon, O.H.; Kim, T.K.; Cho, Y.K.; Choi, K.Y.; Chung, H.Y.; Cho, B.C.; Yang, J.D.; Shin, J.H. Platelet-Rich Plasma: Quantitative Assessment of Growth Factor Levels and Comparative Analysis of Activated and Inactivated Groups. Arch. Plast. Surg. 2013, 40, 530–535. [Google Scholar] [CrossRef]
- Varghese, M.P.; Manuel, S.; Kumar L K, S. Potential for Osseous Regeneration of Platelet-Rich Fibrin-A Comparative Study in Mandibular Third Molar Impaction Sockets. J. Oral Maxillofac. Surg. 2017, 75, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Hounsome, J.; Pilkington, G.; Mahon, J.; Boland, A.; Beale, S.; Kotas, E.; Renton, T.; Dickson, R. Prophylactic Removal of Impacted Mandibular Third Molars: A Systematic Review and Economic Evaluation. Health Technol. Assess. 2020, 24, 1–116. [Google Scholar] [CrossRef] [PubMed]
- Miksad, R.A.; Lai, K.-C.; Dodson, T.B.; Woo, S.-B.; Treister, N.S.; Akinyemi, O.; Bihrle, M.; Maytal, G.; August, M.; Gazelle, G.S.; et al. Quality of Life Implications of Bisphosphonate-Associated Osteonecrosis of the Jaw. Oncologist 2011, 16, 121–132. [Google Scholar] [CrossRef]
- Lei, L.; Yu, Y.; Han, J.; Shi, D.; Sun, W.; Zhang, D.; Chen, L. Quantification of Growth Factors in Advanced Platelet-Rich Fibrin and Concentrated Growth Factors and Their Clinical Efficacy as Adjunctive to the GTR Procedure in Periodontal Intrabony Defects. J. Periodontol. 2020, 91, 462–472. [Google Scholar] [CrossRef]
- Qiao, J.; An, N.; Ouyang, X. Quantification of Growth Factors in Different Platelet Concentrates. Platelets 2017, 28, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Ritto, F.G.; Pimentel, T.; Canellas, J.V.S.; Junger, B.; Cruz, M.; Medeiros, P.J. Randomized Double-Blind Clinical Trial Evaluation of Bone Healing after Third Molar Surgery with the Use of Leukocyte- and Platelet-Rich Fibrin. Int. J. Oral Maxillofac. Surg. 2019, 48, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.; Matsumoto, T. Recent Advances in the Management of Osteoporosis. F1000Res 2017, 6, 625. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Decker, A.M.; Nibali, L.; Pilipchuk, S.P.; Berglundh, T.; Giannobile, W.V. Regenerative Medicine for Periodontal and Peri-Implant Diseases. J. Dent. Res. 2016, 95, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Tatullo, M.; Marrelli, M.; Inchingolo, A.M.; Inchingolo, A.D.; Dipalma, G.; Flace, P.; Girolamo, F.; Tarullo, A.; Laino, L.; et al. Regenerative Surgery Performed with Platelet-Rich Plasma Used in Sinus Lift Elevation before Dental Implant Surgery: An Useful Aid in Healing and Regeneration of Bone Tissue. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1222–1226. [Google Scholar]
- Epstein, M.S.; Ephros, H.D.; Epstein, J.B. Review of Current Literature and Implications of RANKL Inhibitors for Oral Health Care Providers. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, e437–e442. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, J.; Cecchinato, D.; Donati, M.; Tomasi, C.; Liljenberg, B. Ridge Preservation with the Use of Deproteinized Bovine Bone Mineral. Clin. Oral Implant. Res. 2014, 25, 786–790. [Google Scholar] [CrossRef]
- Cardaropoli, D.; Tamagnone, L.; Roffredo, A.; Gaveglio, L.; Cardaropoli, G. Socket Preservation Using Bovine Bone Mineral and Collagen Membrane: A Randomized Controlled Clinical Trial with Histologic Analysis. Int. J. Periodontics Restor. Dent. 2012, 32, 421–430. [Google Scholar]
- Ramaglia, L.; Guida, A.; Iorio-Siciliano, V.; Cuozzo, A.; Blasi, A.; Sculean, A. Stage-Specific Therapeutic Strategies of Medication-Related Osteonecrosis of the Jaws: A Systematic Review and Meta-Analysis of the Drug Suspension Protocol. Clin. Oral Investig. 2018, 22, 597–615. [Google Scholar] [CrossRef]
- Inchingolo, A.M.; Inchingolo, A.D.; Nardelli, P.; Latini, G.; Trilli, I.; Ferrante, L.; Malcangi, G.; Palermo, A.; Inchingolo, F.; Dipalma, G. Stem Cells: Present Understanding and Prospects for Regenerative Dentistry. J. Funct. Biomater. 2024, 15, 308. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Kuraguchi, J.; Kogashiwa, Y.; Yokoi, H.; Satomi, T.; Kohno, N. Successful Treatment of Bisphosphonate-Related Osteonecrosis of the Jaw (BRONJ) Patients with Sitafloxacin: New Strategies for the Treatment of BRONJ. Bone 2015, 73, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Daugela, P.; Cicciù, M.; Saulacic, N. Surgical Regenerative Treatments for Peri-Implantitis: Meta-Analysis of Recent Findings in a Systematic Literature Review. J. Oral Maxillofac. Res. 2016, 7, e15. [Google Scholar] [CrossRef] [PubMed]
- Vittorini Orgeas, G.; Clementini, M.; De Risi, V.; de Sanctis, M. Surgical Techniques for Alveolar Socket Preservation: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2013, 28, 1049–1061. [Google Scholar] [CrossRef]
- Bailey, E.; Kashbour, W.; Shah, N.; Worthington, H.V.; Renton, T.F.; Coulthard, P. Surgical Techniques for the Removal of Mandibular Wisdom Teeth. Cochrane Database Syst. Rev. 2020, 7, CD004345. [Google Scholar] [CrossRef]
- Nørholt, S.E.; Hartlev, J. Surgical Treatment of Osteonecrosis of the Jaw with the Use of Platelet-Rich Fibrin: A Prospective Study of 15 Patients. Int. J. Oral Maxillofac. Surg. 2016, 45, 1256–1260. [Google Scholar] [CrossRef]
- Roccuzzo, M.; Pittoni, D.; Roccuzzo, A.; Charrier, L.; Dalmasso, P. Surgical Treatment of Peri-Implantitis Intrabony Lesions by Means of Deproteinized Bovine Bone Mineral with 10% Collagen: 7-Year-Results. Clin. Oral Implant. Res. 2017, 28, 1577–1583. [Google Scholar] [CrossRef]
- Roos-Jansåker, A.-M.; Persson, G.R.; Lindahl, C.; Renvert, S. Surgical Treatment of Peri-Implantitis Using a Bone Substitute with or without a Resorbable Membrane: A 5-Year Follow-Up. J. Clin. Periodontol. 2014, 41, 1108–1114. [Google Scholar] [CrossRef]
- Carcuac, O.; Derks, J.; Abrahamsson, I.; Wennström, J.L.; Petzold, M.; Berglundh, T. Surgical Treatment of Peri-Implantitis: 3-Year Results from a Randomized Controlled Clinical Trial. J. Clin. Periodontol. 2017, 44, 1294–1303. [Google Scholar] [CrossRef]
- Graziani, F.; D’Aiuto, F.; Gennai, S.; Petrini, M.; Nisi, M.; Cirigliano, N.; Landini, L.; Bruno, R.M.; Taddei, S.; Ghiadoni, L. Systemic Inflammation after Third Molar Removal: A Case-Control Study. J. Dent. Res. 2017, 96, 1505–1512. [Google Scholar] [CrossRef]
- Qiao, J.; Duan, J.; Zhang, Y.; Chu, Y.; Sun, C. The Effect of Concentrated Growth Factors in the Treatment of Periodontal Intrabony Defects. Future Sci. OA 2016, 2, FS136. [Google Scholar] [CrossRef]
- Campana, M.D.; Aliberti, A.; Acerra, A.; Sammartino, P.; Dolce, P.; Sammartino, G.; Gasparro, R. The Effectiveness and Safety of Autologous Platelet Concentrates as Hemostatic Agents after Tooth Extraction in Patients on Anticoagulant Therapy: A Systematic Review of Randomized, Controlled Trials. J. Clin. Med. 2023, 12, 5342. [Google Scholar] [CrossRef] [PubMed]
- Hazballa, D.; Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Santacroce, L.; Minetti, E.; Di Venere, D.; Limongelli, L.; Bordea, I.R.; Scarano, A.; et al. The Effectiveness of Autologous Demineralized Tooth Graft for the Bone Ridge Preservation: A Systematic Review of the Literature. J. Biol. Regul. Homeost. Agents 2021, 35, 283–294. [Google Scholar] [CrossRef]
- Zhang, E.; Miramini, S.; Patel, M.; Richardson, M.; Ebeling, P.; Zhang, L. The Effects of Mechanical Instability on PDGF Mediated Inflammatory Response at Early Stage of Fracture Healing under Diabetic Condition. Comput. Methods Programs Biomed. 2023, 229, 107319. [Google Scholar] [CrossRef]
- Inchingolo, A.M.; Gargiulo Isacco, C.; Inchingolo, A.D.; Nguyen, K.C.D.; Cantore, S.; Santacroce, L.; Scacco, S.; Cirulli, N.; Corriero, A.; Puntillo, F.; et al. The Human Microbiota Key Role in the Bone Metabolism Activity. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 2659–2670. [Google Scholar] [CrossRef] [PubMed]
- Wilde, F.; Heufelder, M.; Winter, K.; Hendricks, J.; Frerich, B.; Schramm, A.; Hemprich, A. The Role of Surgical Therapy in the Management of Intravenous Bisphosphonates-Related Osteonecrosis of the Jaw. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 111, 153–163. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.A.; Mombelli, A. The Therapy of Peri-Implantitis: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2014, 29, 325–345. [Google Scholar] [CrossRef] [PubMed]
- Wushou, A.; Zheng, Y.; Han, Y.; Yang, Z.-C.; Han, F.-K. The Use of Autogenous Tooth Bone Graft Powder in the Treatment of Osseous Defects after Impacted Mandibular Third Molar Extraction: A Prospective Split-Mouth Clinical Pilot Study. BMC Oral Health 2022, 22, 433. [Google Scholar] [CrossRef]
- Temmerman, A.; Vandessel, J.; Castro, A.; Jacobs, R.; Teughels, W.; Pinto, N.; Quirynen, M. The Use of Leucocyte and Platelet-Rich Fibrin in Socket Management and Ridge Preservation: A Split-Mouth, Randomized, Controlled Clinical Trial. J. Clin. Periodontol. 2016, 43, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-L.; Wu, S.-L.; Tsai, C.-C.; Ko, S.-Y.; Chiang, W.-F.; Yang, J.-W. The Use of Solid-Phase Concentrated Growth Factors for Surgical Defects in the Treatment of Dysplastic Lesions of the Oral Mucosa. J. Oral Maxillofac. Surg. 2016, 74, 2549–2556. [Google Scholar] [CrossRef]
- DeLoach, L.J.; Higgins, M.S.; Caplan, A.B.; Stiff, J.L. The Visual Analog Scale in the Immediate Postoperative Period: Intrasubject Variability and Correlation with a Numeric Scale. Anesth. Analg. 1998, 86, 102–106. [Google Scholar] [CrossRef]
- Lohmann, C.H.; Andreacchio, D.; Köster, G.; Carnes, D.L.; Cochran, D.L.; Dean, D.D.; Boyan, B.D.; Schwartz, Z. Tissue Response and Osteoinduction of Human Bone Grafts in Vivo. Arch. Orthop. Trauma. Surg. 2001, 121, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Mozzati, M.; Arata, V.; Gallesio, G. Tooth Extraction in Patients on Zoledronic Acid Therapy. Oral Oncol. 2012, 48, 817–821. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Lee, J.; Um, I.-W.; Kim, K.-W.; Murata, M.; Akazawa, T.; Mitsugi, M. Tooth-Derived Bone Graft Material. J. Korean Assoc. Oral Maxillofac. Surg. 2013, 39, 103–111. [Google Scholar] [CrossRef]
- Tsai, L.-L.; Huang, Y.-F.; Chang, Y.-C. Treatment of Bisphosphonate-Related Osteonecrosis of the Jaw with Platelet-Rich Fibrin. J. Formos. Med. Assoc. 2016, 115, 585–586. [Google Scholar] [CrossRef]
| Authors | Type of the Study | Aim of the Study | Materials and Methods | Results |
|---|---|---|---|---|
| Huang et al. (2018) [37] | Split-mouth randomized double-blind clinical trial | To evaluate the effectiveness of CGF in reducing postoperative complications after impacted third molar extraction. | A total of 25 patients with bilaterally impacted third molars. CGF was applied on one side, while the other side served as a control. Pain, swelling, and bone healing were assessed using CBCT. | Significant reduction in pain on the 3rd and 7th postoperative days in CGF sites compared to controls. No significant differences in swelling or bone healing between groups. |
| Yüce et al. (2021) [52] | Randomized Controlled Trial | To evaluate the effectiveness of concentrated growth factor (CGF) in the healing process of osteoporotic patients with MRONJ | A total of 28 elderly women with osteoporosis and MRONJ, divided into two groups: one treated with CGF and primary closure, the other with primary closure only. Postoperative analysis conducted over 6 months. | Complete healing in 19 out of 28 patients. The CGF group showed less bone exposure and infections, but results were not statistically significant. |
| Isler et al. (2018) [53] | A 12-month randomized clinical trial | To evaluate the clinical and radiographic outcomes of regenerative surgical treatment for peri-implantitis using CGF or collagen membranes. | A total of 52 patients with peri-implantitis were treated using bone substitutes combined with either collagen membranes or concentrated growth factors. Clinical and radiographic evaluations were conducted at baseline, 6, and 12 months. | Both treatment methods led to significant improvements in clinical and radiographic outcomes. At 12 months, collagen membranes showed better results in probing depth and clinical attachment level. |
| Minetti et al. (2023) [21] | Case Series Study | To assess the effectiveness of socket preservation using autologous tooth grafts. | A total of 20 socket preservation procedures with 18-month follow-up. Histological evaluation during implant placement. | Significant bone regeneration with uniform structure and no inflammation. Histomorphometric analysis shows promising results; further research needed for long-term outcomes. |
| Minetti et al. (2023) [54] | Pilot Study | To analyze mixed graft materials (50% dentin + 50% xenograft) for socket preservation. | Seven socket preservation surgeries with histological analysis at 4 and 8 months. | New bone formation at 29.03% (4 months) and 34.11% (8 months). Different absorption rates: dentin 71–90%; xenograft 6–26%. Dentin resorption increases new bone formation. |
| Minetti et al. (2023) [17] | Observational Study | To evaluate the granule size of bone graft materials from Tooth Transformer® for osteogenesis. | Laser analysis of granules produced by Tooth Transformer® device. | A total of 85% of granules were 100–1000 μm, aligning with literature recommendations for osteogenesis and bone regeneration. |
| Ma et al. (2023) [55] | Randomized Controlled Trial | To evaluate the impact of CGF on alveolar ridge preservation post-extraction. | A total of 50 patients randomized to CGF or control groups; healing scores, CBCT, and computerized microtomography analyses were performed. | CGF improves healing scores, reduces vertical and horizontal bone resorption, and enhances new bone formation compared to controls. |
| Xie et al. (2023) [12] | Randomized Controlled Trial | To evaluate sticky bone combined with CGF for anterior alveolar ridge augmentation. | A total of 28 patients randomized to sticky bone with CGF or saline-mixed bone powders; CBCT analysis and VAS scores. | Sticky bone with CGF improves bone augmentation (72% vs. 57% volume conversion) and reduces pain (lower VAS scores). |
| Elayah et al. (2023) [8] | Randomized Controlled Trial | To assess the efficacy of CGF in ridge preservation following lower third molar extraction. | A total of 60 sites in 30 patients compared CGF-treated sockets to controls; CBCT and histological analysis. | CGF-treated sockets show greater bone height, width, and density. Improved periodontal pocket reduction and bone preservation. |
| Huang et al. (2024) [27] | Randomized, Double-Blind, Split-Mouth Trial | To evaluate the effect of concentrated growth factor (CGF) in reducing postoperative complications after mandibular third molar extractions. | A total of 25 patients with bilaterally impacted third molars (50 extraction sites) were included. Each patient acted as their own control. CGF was placed in one extraction socket, while the other was sutured without CGF. Pain, swelling, and bone healing were assessed postoperatively. | Significant pain reduction was observed on the 3rd and 7th postoperative days in the CGF group. No significant differences were found in facial swelling or bone healing between the CGF and control groups. No adverse effects were reported. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dipalma, G.; Inchingolo, A.M.; Colonna, V.; Marotti, P.; Carone, C.; Ferrante, L.; Inchingolo, F.; Palermo, A.; Inchingolo, A.D. Autologous and Heterologous Minor and Major Bone Regeneration with Platelet-Derived Growth Factors. J. Funct. Biomater. 2025, 16, 16. https://doi.org/10.3390/jfb16010016
Dipalma G, Inchingolo AM, Colonna V, Marotti P, Carone C, Ferrante L, Inchingolo F, Palermo A, Inchingolo AD. Autologous and Heterologous Minor and Major Bone Regeneration with Platelet-Derived Growth Factors. Journal of Functional Biomaterials. 2025; 16(1):16. https://doi.org/10.3390/jfb16010016
Chicago/Turabian StyleDipalma, Gianna, Angelo Michele Inchingolo, Valeria Colonna, Pierluigi Marotti, Claudio Carone, Laura Ferrante, Francesco Inchingolo, Andrea Palermo, and Alessio Danilo Inchingolo. 2025. "Autologous and Heterologous Minor and Major Bone Regeneration with Platelet-Derived Growth Factors" Journal of Functional Biomaterials 16, no. 1: 16. https://doi.org/10.3390/jfb16010016
APA StyleDipalma, G., Inchingolo, A. M., Colonna, V., Marotti, P., Carone, C., Ferrante, L., Inchingolo, F., Palermo, A., & Inchingolo, A. D. (2025). Autologous and Heterologous Minor and Major Bone Regeneration with Platelet-Derived Growth Factors. Journal of Functional Biomaterials, 16(1), 16. https://doi.org/10.3390/jfb16010016