Clinical Characterization of the Frequent Exacerbator Phenotype in Asthma
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Study Design and Setting
2.3. Evaluation of Clinical, Functional, and Blood Parameters
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics and Comorbidities
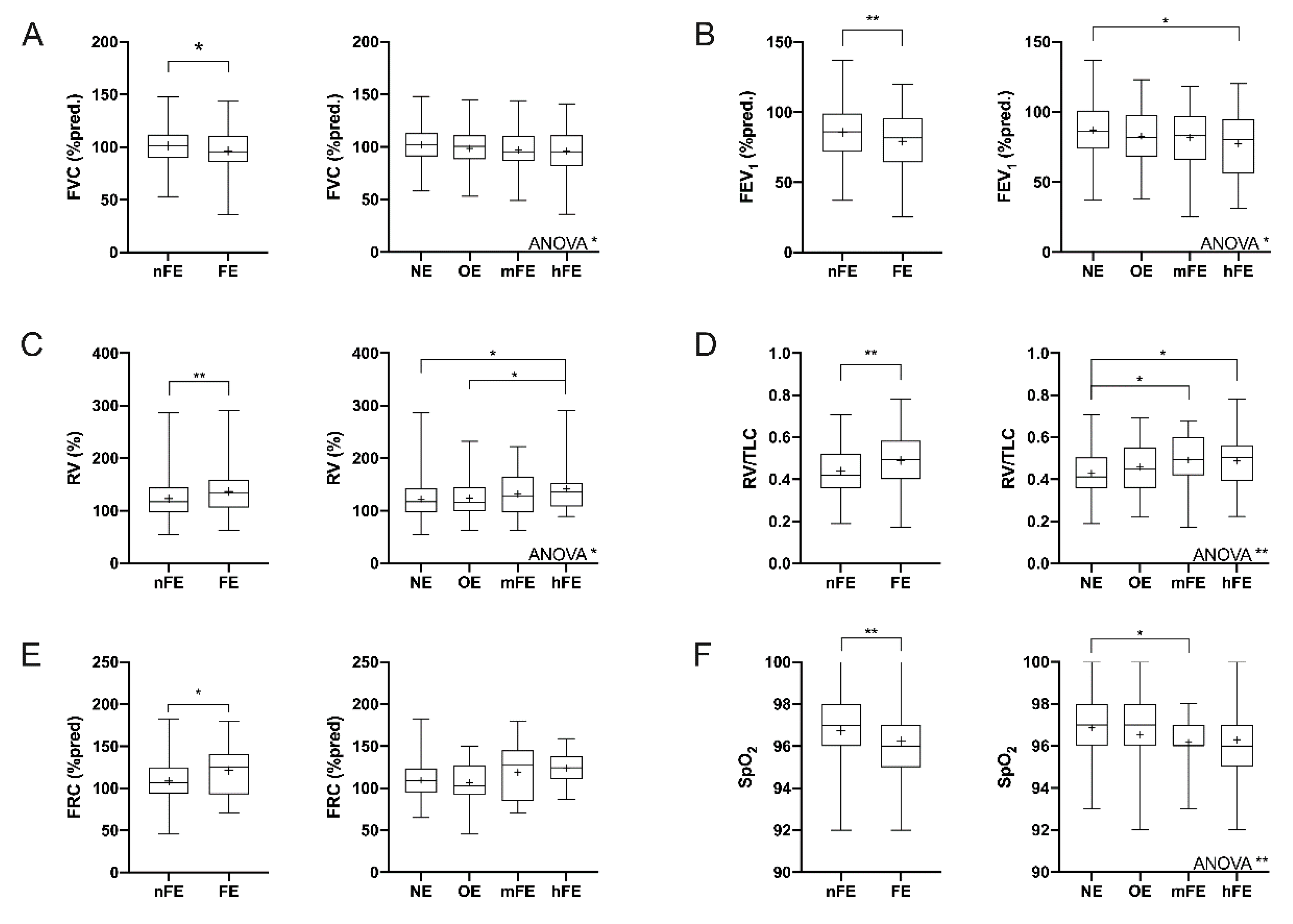
3.2. Functional Parameters
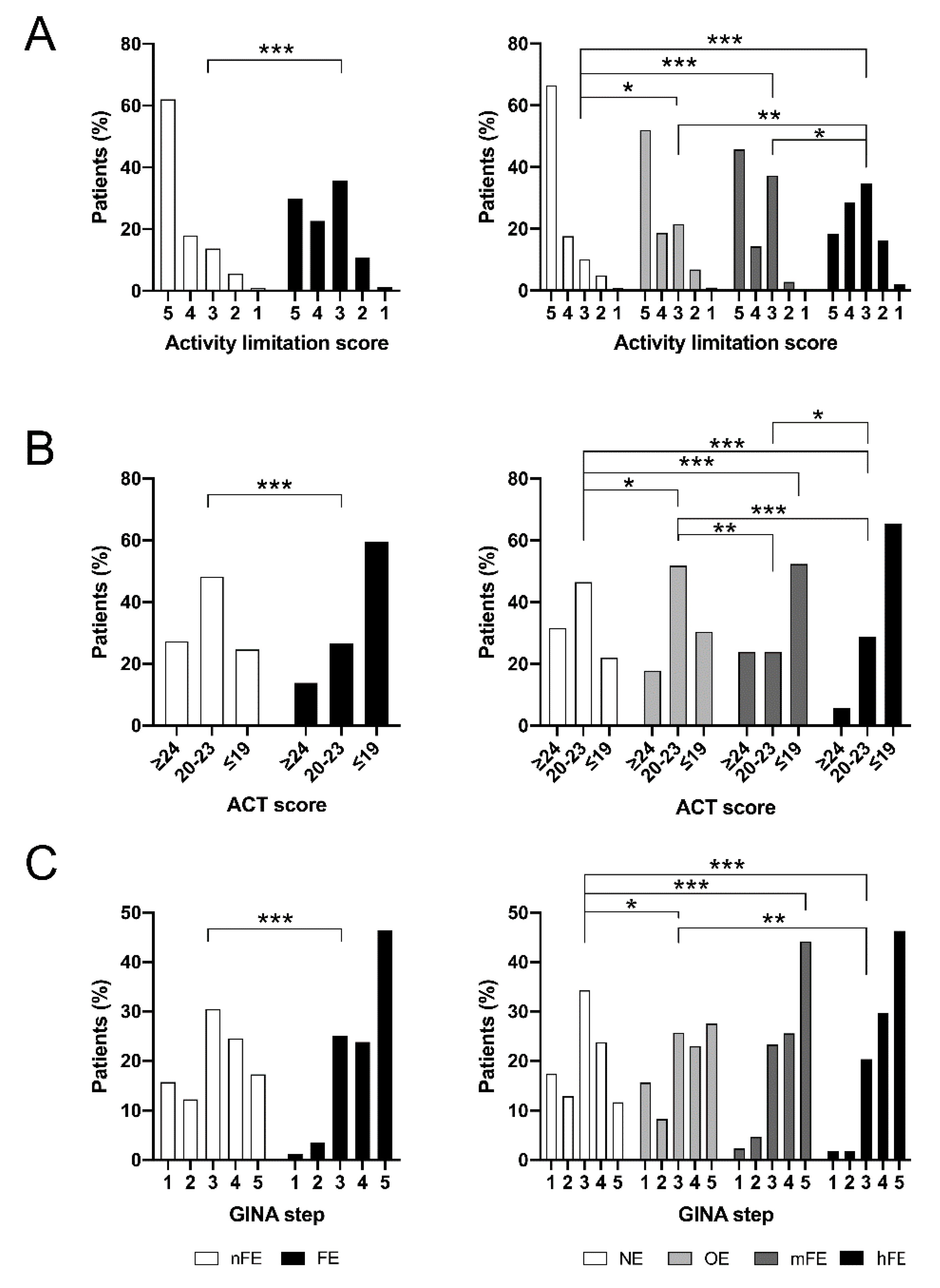
3.3. Asthma Control
3.4. Asthma Treatment
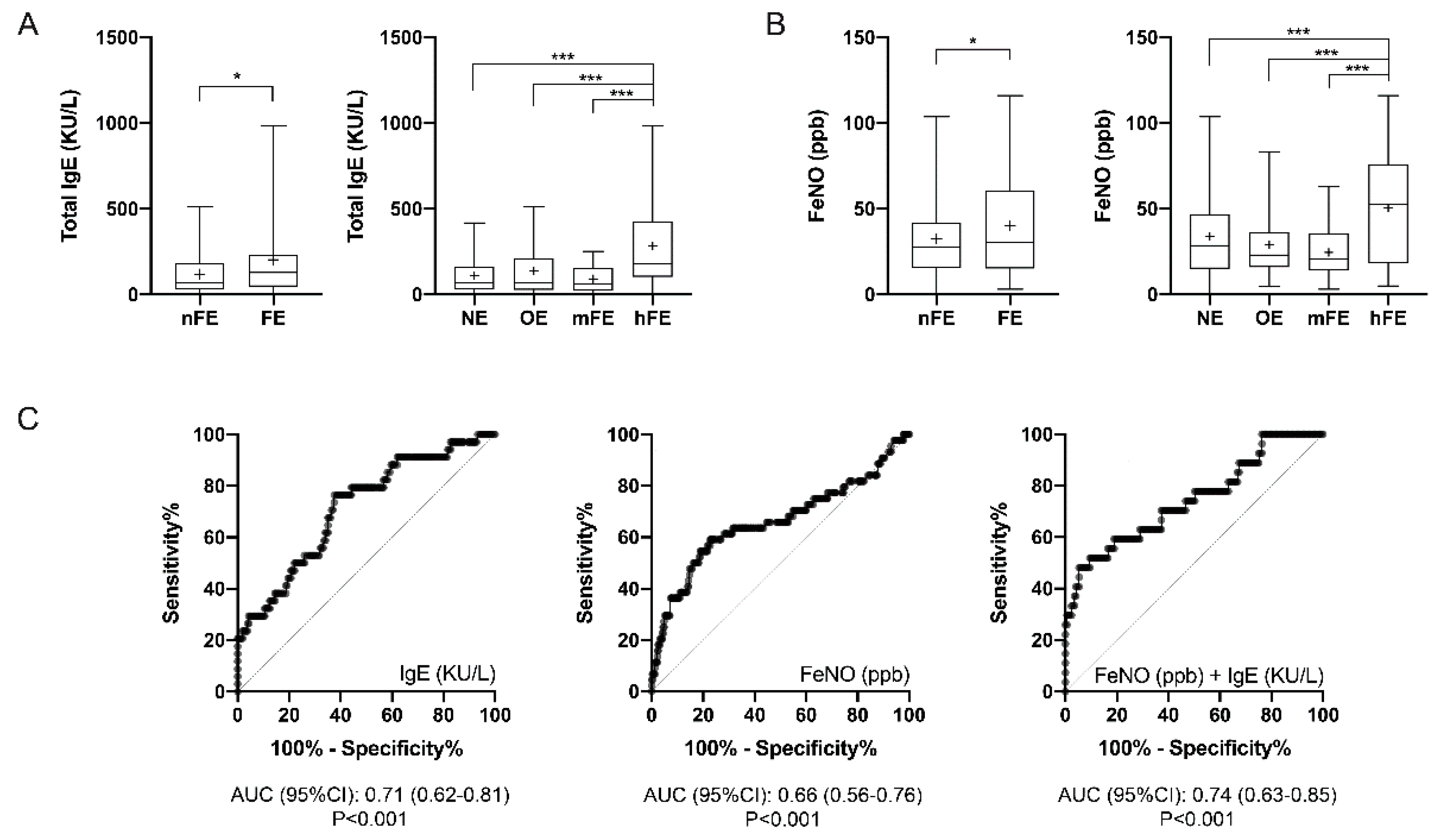
3.5. T2-High Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reddel, H.K.; Taylor, D.R.; Bateman, E.D.; Boulet, L.P.; Boushey, H.A.; Busse, W.W.; Casale, T.B.; Chanez, P.; Enright, P.L.; Gibson, P.G.; et al. An official American Thoracic Society/European Respiratory Society statement: Asthma control and exacerbations: Standardizing endpoints for clinical asthma trials and clinical practice. Am. J. Respir. Crit. Care Med. 2009, 180, 59–99. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F.; Wenzel, S.E.; Brozek, J.L.; Bush, A.; Castro, M.; Sterk, P.J.; Adcock, I.M.; Bateman, E.D.; Bel, E.H.; Bleecker, E.R.; et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2014, 43, 343–373. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.M.; Eid, N. The impact of asthma exacerbations and preventive strategies. Curr. Med. Res. Opin. 2015, 31, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Accordini, S.; Corsico, A.G.; Braggion, M.; Gerbase, M.W.; Gislason, D.; Gulsvik, A.; Heinrich, J.; Janson, C.; Jarvis, D.; Jogi, R.; et al. The cost of persistent asthma in Europe: An international population-based study in adults. Int. Arch. Allergy Immunol. 2013, 160, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Bloom, C.I.; Nissen, F.; Douglas, I.J.; Smeeth, L.; Cullinan, P.; Quint, J.K. Exacerbation risk and characterisation of the UK’s asthma population from infants to old age. Thorax 2018, 73, 313–320. [Google Scholar] [CrossRef]
- Holguin, F.; Cardet, J.C.; Chung, K.F.; Diver, S.; Ferreira, D.S.; Fitzpatrick, A.; Gaga, M.; Kellermeyer, L.; Khurana, S.; Knight, S.; et al. Management of severe asthma: A European Respiratory Society/American Thoracic Society guideline. Eur. Respir. J. 2020, 55, 1900588. [Google Scholar] [CrossRef]
- Agache, I.; Akdis, C.; Jutel, M.; Virchow, J.C. Untangling asthma phenotypes and endotypes. Allergy 2012, 67, 835–846. [Google Scholar] [CrossRef]
- Bel, E.H.; Sousa, A.; Fleming, L.; Bush, A.; Chung, K.F.; Versnel, J.; Wagener, A.H.; Wagers, S.S.; Sterk, P.J.; Compton, C.H.; et al. Diagnosis and definition of severe refractory asthma: An international consensus statement from the Innovative Medicine Initiative (IMI). Thorax 2011, 66, 910–917. [Google Scholar] [CrossRef]
- Zeiger, R.S.; Schatz, M.; Li, Q.; Chen, W.; Khatry, D.B.; Gossage, D.; Tran, T.N. High blood eosinophil count is a risk factor for future asthma exacerbations in adult persistent asthma. J. Allergy Clin. Immunol. Pract. 2014, 2, 741–750. [Google Scholar] [CrossRef]
- Green, R.H.; Brightling, C.E.; McKenna, S.; Hargadon, B.; Parker, D.; Bradding, P.; Wardlaw, A.J.; Pavord, I.D. Asthma exacerbations and sputum eosinophil counts: A randomised controlled trial. Lancet 2002, 360, 1715–1721. [Google Scholar] [CrossRef]
- Kupczyk, M.; ten Brinke, A.; Sterk, P.J.; Bel, E.H.; Papi, A.; Chanez, P.; Nizankowska-Mogilnicka, E.; Gjomarkaj, M.; Gaga, M.; Brusselle, G.; et al. Frequent exacerbators--a distinct phenotype of severe asthma. Clin. Exp. Allergy. 2014, 44, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Price, D.B.; Rigazio, A.; Campbell, J.D.; Bleecker, E.R.; Corrigan, C.J.; Thomas, M.; Wenzel, S.E.; Wilson, A.M.; Small, M.B.; Gopalan, G.; et al. Blood eosinophil count and prospective annual asthma disease burden: A UK cohort study. Lancet Respir. Med. 2015, 3, 849–858. [Google Scholar] [CrossRef]
- Edris, A.; De Feyter, S.; Maes, T.; Joos, G.; Lahousse, L. Monoclonal antibodies in type 2 asthma: A systematic review and network meta-analysis. Respir. Res. 2019, 20, 179. [Google Scholar] [CrossRef] [PubMed]
- Denlinger, L.C.; Phillips, B.R.; Ramratnam, S.; Ross, K.; Bhakta, N.R.; Cardet, J.C.; Castro, M.; Peters, S.P.; Phipatanakul, W.; Aujla, S.; et al. Inflammatory and Comorbid Features of Patients with Severe Asthma and Frequent Exacerbations. Am. J. Respir. Crit. Care Med. 2017, 195, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Norzila, M.Z.; Fakes, K.; Henry, R.L.; Simpson, J.; Gibson, P.G. Interleukin-8 secretion and neutrophil recruitment accompanies induced sputum eosinophil activation in children with acute asthma. Am. J. Respir. Crit. Care Med. 2000, 161 Pt 1, 769–774. [Google Scholar] [CrossRef]
- Vedel-Krogh, S.; Fallgaard Nielsen, S.; Lange, P.; Vestbo, J.; Nordestgaard, B.G. Association of Blood Eosinophil and Blood Neutrophil Counts with Asthma Exacerbations in the Copenhagen General Population Study. Clin. Chem. 2017, 63, 823–832. [Google Scholar] [CrossRef]
- Irvin, C.; Zafar, I.; Good, J.; Rollins, D.; Christianson, C.; Gorska, M.M.; Martin, R.J.; Alam, R. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J. Allergy Clin. Immunol. 2014, 134, 1175–1186. [Google Scholar] [CrossRef]
- Bullone, M.; Carriero, V.; Bertolini, F.; Folino, A.; Mannelli, A.; Di Stefano, A.; Gnemmi, I.; Torchio, R.; Ricciardolo, F.L.M. Elevated serum IgE, oral corticosteroid dependence and IL-17/22 expression in highly neutrophilic asthma. Eur. Respir. J. 2019, 54, 1900068. [Google Scholar] [CrossRef]
- Castillo, J.R.; Peters, S.P.; Busse, W.W. Asthma Exacerbations: Pathogenesis, Prevention, and Treatment. J. Allergy Clin. Immunol. Pract. 2017, 5, 918–927. [Google Scholar] [CrossRef]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Available online: www.ginasthma.org (accessed on 8 December 2019).
- Carriero, V.; Bertolini, F.; Sprio, A.E.; Bullone, M.; Ciprandi, G.; Ricciardolo, F.L.M. High levels of plasma fibrinogen could predict frequent asthma exacerbations. J. Allergy Clin. Immunol. Pract. 2020, 8, 2392–2395. [Google Scholar] [CrossRef]
- Meltzer, E.O.; Schatz, M.; Nathan, R.; Garris, C.; Stanford, R.H.; Kosinski, M. Reliability, validity, and responsiveness of the Rhinitis Control Assessment Test in patients with rhinitis. J. Allergy Clin. Immunol. 2013, 131, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Nathan, R.A.; Sorkness, C.A.; Kosinski, M.; Schatz, M.; Li, J.T.; Marcus, P.; Murray, J.J.; Pendergraft, T.B. Development of the asthma control test: A survey for assessing asthma control. J. Allergy Clin. Immunol. 2004, 113, 59–65. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Mullol, J.; Bachert, C.; Alobid, I.; Baroody, F.; Cohen, N.; Cervin, A.; Douglas, R.; Gevaert, P.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol. Suppl. 2012, 23, 1–298. [Google Scholar]
- Muraro, A.; Roberts, G.; Halken, S.; Agache, I.; Angier, E.; Fernandez-Rivas, M.; Gerth van Wijk, R.; Jutel, M.; Lau, S.; Pajno, G.; et al. EAACI guidelines on allergen immunotherapy: Executive statement. Allergy 2018, 73, 739–743. [Google Scholar] [CrossRef]
- Hellings, P.W.; Fokkens, W.J.; Bachert, C.; Akdis, C.A.; Bieber, T.; Agache, I.; Bernal-Sprekelsen, M.; Canonica, G.W.; Gevaert, P.; Joos, G.; et al. Positioning the principles of precision medicine in care pathways for allergic rhinitis and chronic rhinosinusitis—A EUFOREA-ARIA-EPOS-AIRWAYS ICP statement. Allergy 2017, 72, 1297–1305. [Google Scholar] [CrossRef]
- Motulsky, H.J.; Brown, R.E. Detecting outliers when fitting data with nonlinear regression—A new method based on robust nonlinear regression and the false discovery rate. BMC Bioinform. 2006, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.R.; Song, H.J.; Nam, J.H.; Hong, S.H.; Yang, S.Y.; Ju, S.; Lee, S.W.; Kim, T.B.; Kim, H.L.; Lee, E.K. Risk factors of asthma exacerbation based on asthma severity: A nationwide population-based observational study in South Korea. BMJ Open 2018, 8, e020825. [Google Scholar] [CrossRef]
- Ohta, K.; Tanaka, H.; Tohda, Y.; Kohrogi, H.; Chihara, J.; Sakakibara, H.; Adachi, M.; Tamura, G. Asthma exacerbations in patients with asthma and rhinitis: Factors associated with asthma exacerbation and its effect on QOL in patients with asthma and rhinitis. Allergol. Int. 2019, 68, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Fuseini, H.; Newcomb, D.C. Mechanisms Driving Gender Differences in Asthma. Curr. Allergy Asthma Rep. 2017, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Zein, J.G.; Erzurum, S.C. Asthma is Different in Women. Curr. Allergy Asthma Rep. 2015, 15, 28. [Google Scholar] [CrossRef]
- Vermeulen, F.; Chirumberro, A.; Rummens, P.; Bruyneel, M.; Ninane, V. Relationship between the sensation of activity limitation and the results of functional assessment in asthma patients. J. Asthma 2017, 54, 570–577. [Google Scholar] [CrossRef]
- Albers, F.C.; Mullerova, H.; Gunsoy, N.B.; Shin, J.Y.; Nelsen, L.M.; Bradford, E.S.; Cockle, S.M.; Suruki, R.Y. Biologic treatment eligibility for real-world patients with severe asthma: The IDEAL study. J. Asthma 2018, 55, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Cowan, D.C.; Cowan, J.O.; Palmay, R.; Williamson, A.; Taylor, D.R. Effects of steroid therapy on inflammatory cell subtypes in asthma. Thorax 2010, 65, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Denlinger, L.C.; Heymann, P.; Lutter, R.; Gern, J.E. Exacerbation-Prone Asthma. J. Allergy Clin. Immunol. Pract. 2019, 8, 474–482. [Google Scholar] [CrossRef]
- Silvestri, M.; Bontempelli, M.; Giacomelli, M.; Malerba, M.; Rossi, G.A.; Di Stefano, A.; Rossi, A.; Ricciardolo, F.L. High serum levels of tumour necrosis factor-alpha and interleukin-8 in severe asthma: Markers of systemic inflammation? Clin. Exp. Allergy 2006, 36, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Ortega, H.; Chupp, G.; Bardin, P.; Bourdin, A.; Garcia, G.; Hartley, B.; Yancey, S.; Humbert, M. The role of mepolizumab in atopic and nonatopic severe asthma with persistent eosinophilia. Eur. Respir. J. 2014, 44, 239–241. [Google Scholar] [CrossRef]
- Pavord, I.D.; Korn, S.; Howarth, P.; Bleecker, E.R.; Buhl, R.; Keene, O.N.; Ortega, H.; Chanez, P. Mepolizumab for severe eosinophilic asthma (DREAM): A multicentre, double-blind, placebo-controlled trial. Lancet 2012, 380, 651–659. [Google Scholar] [CrossRef]
- Ricciardolo, F.L.M.; Silkoff, P.E. Perspectives on exhaled nitric oxide. J. Breath Res. 2017, 11, 47104. [Google Scholar] [CrossRef]
- Gandhi, N.A.; Pirozzi, G.; Graham, N.M.H. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev. Clin. Immunol. 2017, 13, 425–437. [Google Scholar] [CrossRef]
- Zervas, E.; Samitas, K.; Papaioannou, A.I.; Bakakos, P.; Loukides, S.; Gaga, M. An algorithmic approach for the treatment of severe uncontrolled asthma. ERJ Open Res. 2018, 4, 00125–2017. [Google Scholar] [CrossRef]
- Ricciardolo, F.L. Revisiting the role of exhaled nitric oxide in asthma. Curr. Opin. Pulm. Med. 2014, 20, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Ricciardolo, F.L.M.; Sorbello, V.; Folino, A.; Gallo, F.; Massaglia, G.M.; Favata, G.; Conticello, S.; Vallese, D.; Gani, F.; Malerba, M.; et al. Identification of IL-17F/frequent exacerbator endotype in asthma. J. Allergy Clin. Immunol. 2017, 140, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Sorbello, V.; Ciprandi, G.; Di Stefano, A.; Massaglia, G.M.; Favata, G.; Conticello, S.; Malerba, M.; Folkerts, G.; Profita, M.; Rolla, G.; et al. Nasal IL-17F is related to bronchial IL-17F/neutrophilia and exacerbations in stable atopic severe asthma. Allergy 2015, 70, 236–240. [Google Scholar] [CrossRef] [PubMed]
| Demographic and Clinical Parameters | nFE (n = 367) | FE (n = 97) | NE (n = 248) | OE (n = 119) | mFE (n = 43) | hFE (n = 54) |
|---|---|---|---|---|---|---|
| Age (years) | 58.2 ± 15.6 | 59.9 ± 13.9 | 57.7 ± 15.6 | 59.2 ± 15.6 | 60.4 ± 14.1 | 59.5 ± 13.8 |
| Gender (F) | 219/367 (59.7%) | 71/97 (73.2%) * | 145/248 (58.5%) | 74/119 (62.2%) | 35/43 (81.4%) ## § | 36/54 (66.7%) |
| BMI (kg/m2) | 27.1 ± 5.8 | 26.4 ± 4.8 | 27.2 ± 5.9 | 26.9 ± 5.5 | 25.7 ± 4.3 | 26.9 ± 5.2 |
| Current smokers (≥10 PY) | 27/367 (7.3%) | 1/97 (1.0%) * | 19/248 (7.7%) | 8/119 (6.7%) | 1/43 (2.3%) | 0/54 (0.0%) |
| Former smokers (≥10 PY) | 81/367 (22.1%) | 18/97 (18.6%) | 55/248 (22.2%) | 26/119 (21.8%) | 7/43 (16.3) | 11/54 (20.4%) |
| Allergy | 193/367 (52.6%) | 55/97 (56.7%) | 134/248 (54.0%) | 59/119 (49.6%) | 24/43 (55.8%) | 31/54 (57.4%) |
| Polysensitization | 165/193 (85.5%) | 43/55 (78.2%) | 117/134 (87.3%) | 48/59 (81.4%) | 19/24 (79.2%) | 24/31 (77.4%) |
| Seasonal allergen sensitization | 161/193 (83.4%) | 47/55 (85.4%) | 114/134 (85.1%) | 47/59 (79.7%) | 22/24 (91.7%) | 25/31 (80.6%) |
| Perennial allergen sensitization | 144/193 (74.6%) | 38/55 (69.1%) | 102/134 (76.1%) | 42/59 (71.2%) | 16/24 (66.7%) | 22/31 (71.0%) |
| Mild asthma (GINA steps 1–2) | 101/367 (27.5%) | 5/97 (5.1%) *** | 75/248 (30.2%) | 26/119 (21.9%) | 3/43 (7.0%) ## | 2/54 (3.7%) ### §§ |
| Moderate asthma (GINA steps 3–4) | 207/367 (56.4%) | 48/97 (49.5%) | 144/248 (58.1%) | 63/119 (52.9%) | 21/43 (48.8%) | 27/54 (50.0%) |
| Severe asthma (GINA step 5) | 59/367 (16.1%) | 44/97 (45.4%) *** | 29/248 (11.7%) | 30/119 (25.2%) ## | 19/43 (44.2%) ### § | 25/54 (46.3%) ### § |
| Age at asthma onset | 37.5 ± 19.3 | 36.5 ± 17.1 | 37.7 ± 19.5 | 37.2 ± 19.0 | 37.2 ± 16.8 | 35.9 ± 17.5 |
| Asthma onset (<18 years) | 76/367 (20.7%) | 21/97 (21.6%) | 54/248 (21.8%) | 22/119 (18.5%) | 9/43 (20.9%) | 12/54 (22.2%) |
| Asthma duration (years) | 22.2 ± 17.0 | 24.9 ± 16.5 | 21.0 ± 16.4 | 22.2 ± 16.6 | 23.5 ± 13.2 | 24.4 ± 16.9 |
| Serious asthma exacerbation history | 54/367 (14.7%) | 31/97 (32.0%) *** | 30/248 (12.1%) | 24/119 (20.2%) | 12/43 (27.9%) # | 19/54 (35.2%) ### |
| Near-fatal asthma exacerbation history | 3/54 (5.6%) | 2/31 (6.4%) | 0/30 (0.0%) | 3/24 (12.5%) | 1/12 (8.3%) | 1/19 (5.3%) |
| T2-high phenotype | 293/366 (80.0%) | 84/97 (86.6%) | 203/247 (82.2%) | 90/119 (75.6%) | 35/43 (81.4%) | 49/54 (90.7%) |
| White blood cells (cells/mm2) | 7233 ± 2091 | 7565 ± 1770 * | 7149 ± 1816 | 7412 ± 2580 | 7286 ± 1642 | 7788 ± 1851 |
| Blood eosinophils (cells/mm2) | 311.4 ± 239.5 | 325.8 ± 282.5 | 315.4 ± 245.5 | 302.8 ± 226.7 | 290.5 ± 239.5 | 353.9 ± 312.1 |
| Blood neutrophils (cells/mm2) | 4239 ± 1802 | 4252 ± 1390 | 4116 ± 1446 | 4486 ± 2362 | 4100 ± 1144 | 4368 ± 1556 |
| Comorbidities | nFE (n = 367) | FE (n = 97) | NE (n = 248) | OE (n = 119) | mFE (n = 43) | hFE (n = 54) |
|---|---|---|---|---|---|---|
| Rhinitis | 246/367 (67.0%) | 69/97 (71.1%) | 173/248 (69.8%) | 73/119 (61.3%) | 31/43 (72.1%) | 38/54 (70.4%) |
| CRSsNP | 67/367 (18.2%) | 19/97 (19.6%) | 43/248 (17.3%) | 24/119 (20.2%) | 11/43 (25.6%) | 8/54 (14.8%) |
| CRSwNP | 41/367 (11.2%) | 18/97 (18.6%) | 30/248 (12.1%) | 11/119 (9.2%) | 5/43 (11.6%) | 13/54 (24.1%) #§ |
| CRSwNP-AERD | 13/367 (3.5%) | 7/97 (7.2%) | 11/248 (4.4%) | 2/119 (1.7%) | 1/43 (2.3%) | 6/54 (11.1%) § |
| CRSwNP + CRSwNP-AERD | 54/367 (14.7%) | 25/97 (25.8%) * | 41/248 (16.5%) | 13/119 (10.9%) | 6/43 (14.0%) | 19/54 (35.2%) ## §§§ † |
| Bronchiectasis | 23/367 (6.3%) | 11/97 (11.3%) | 15/248 (6.0%) | 8/119 (6.7%) | 5/43 (11.6%) | 6/54 (11.1%) |
| Pneumonia history | 39/367 (10.6%) | 13/97 (13.4%) | 33/248 (13.3%) | 6/119 (5.0%) | 6/43 (14.0%) | 7/54 (13.0%) |
| Obstructive sleep apnea syndrome | 20/367 (5.4%) | 4/97 (4.1%) | 14/248 (5.6%) | 6/119 (5.0%) | 2/43 (4.6%) | 2/54 (3.7%) |
| Gastroesophageal reflux disease | 85/367 (23.2%) | 26/97 (26.8%) | 62/248 (25.0%) | 23/119 (19.3%) | 13/43 (30.2%) | 13/54 (24.1%) |
| Obesity | 89/367 (24.2%) | 21/97 (21.6%) | 57/248 (23.0%) | 32/119 (26.9%) | 6/43 (14.0%) | 15/54 (27.8%) |
| Diabetes | 23/367 (6.3%) | 7/97 (7.2%) | 13/248 (5.2%) | 10/119 (8.4%) | 2/43 (4.6%) | 5/54 (9.3%) |
| Hypertension | 119/367 (32.4%) | 30/97 (30.9%) | 85/248 (34.3%) | 34/119 (28.6%) | 14/43 (32.6%) | 16/54 (29.6%) |
| Heart failure | 6/367 (1.6%) | 2/97 (2.1%) | 5/248 (2.0%) | 1/119 (0.8%) | 1/43 (2.3%) | 1/53 (1.8%) |
| Acute myocardial infarction | 20/367 (5.4%) | 3/97 (3.1%) | 11/248 (4.4%) | 9/119 (7.6%) | 3/43 (7.0%) | 0/54 (0.0%) |
| Arrhythmia | 27/367 (7.4%) | 7/97 (7.2%) | 17/248 (6.8%) | 10/119 (8.4%) | 3/43 (7.0%) | 4/54 (7.4%) |
| Anxiety depression syndrome | 52/367 (14.2%) | 14/97 (14.4%) | 35/248 (14.1%) | 17/119 (14.3%) | 8/43 (18.6%) | 6/54 (11.1%) |
| Osteoporosis | 31/397 (8.4%) | 6/97 (6.2%) | 20/248 (8.1%) | 11/119 (9.2%) | 2/43 (4.6%) | 4/54 (7.4%) |
| Arthropathy | 31/367 (8.4%) | 9/97 (9.3%) | 18/248 (7.3%) | 13/119 (10.9%) | 2/43 (4.6%) | 7/54 (13.0%) |
| Chronic pain | 23/367 (5.9%) | 7/97 (7.2%) | 13/248 (5.2%) | 10/119 (8.4%) | 3/43 (7.0%) | 4/54 (7.4%) |
| Treatment | nFE (n = 367) | FE (n = 97) | NE (n = 248) | OE (n = 119) | mFE (n = 43) | hFE (n = 54) |
|---|---|---|---|---|---|---|
| ICS | 307/367 (83.6%) | 95/97 (97.9%) *** | 205/248 (82.7%) | 102/119 (85.7%) | 42/43 (97.7%) # | 53/54 (98.1%) ## § |
| ICS/day (μg BDP-HFA) | 263.5 ± 201 | 446.4 ± 237.6 *** | 239.1 ± 176 | 314.3 ± 238.0## | 423.3 ± 235.9 ### § | 464.8 ± 239.6 ### §§§ |
| LABA | 265/397 (72.2%) | 92/97 (94.8%) *** | 172/248 (69.4%) | 93/119 (78.2%) | 40/43 (93.0%) ## | 52/54 (96.3%) ### §§ |
| LAMA | 56/367 (15.3%) | 25/97 (25.8%) * | 37/248 (14.9%) | 19/119 (16.0%) | 9/43 (20.9%) | 16/54 (29.6%) # |
| OCS (≥6 months/year) | 9/367 (2.4%) | 7/97 (7.2%) | 4/248 (1.6%) | 5/119 (4.2%) | 0/43 (0.0%) | 7/54 (13.0%) ### † |
| Omalizumab | 18/367 (4.9%) | 9/88 (9.3%) | 12/248 (4.8%) | 6/119 (5.0%) | 6/43 (14.0%) | 3/54 (5.6%) |
| Mepolizumab | 7/367 (1.9%) | 13/97 (13.4%) *** | 3/248 (1.2%) | 4/119 (3.4%) | 3/43 (7.0%) | 10/54 (18.5%) ### §§ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sprio, A.E.; Carriero, V.; Levra, S.; Botto, C.; Bertolini, F.; Di Stefano, A.; Maniscalco, M.; Ciprandi, G.; Ricciardolo, F.L.M. Clinical Characterization of the Frequent Exacerbator Phenotype in Asthma. J. Clin. Med. 2020, 9, 2226. https://doi.org/10.3390/jcm9072226
Sprio AE, Carriero V, Levra S, Botto C, Bertolini F, Di Stefano A, Maniscalco M, Ciprandi G, Ricciardolo FLM. Clinical Characterization of the Frequent Exacerbator Phenotype in Asthma. Journal of Clinical Medicine. 2020; 9(7):2226. https://doi.org/10.3390/jcm9072226
Chicago/Turabian StyleSprio, Andrea Elio, Vitina Carriero, Stefano Levra, Carlotta Botto, Francesca Bertolini, Antonino Di Stefano, Mauro Maniscalco, Giorgio Ciprandi, and Fabio Luigi Massimo Ricciardolo. 2020. "Clinical Characterization of the Frequent Exacerbator Phenotype in Asthma" Journal of Clinical Medicine 9, no. 7: 2226. https://doi.org/10.3390/jcm9072226
APA StyleSprio, A. E., Carriero, V., Levra, S., Botto, C., Bertolini, F., Di Stefano, A., Maniscalco, M., Ciprandi, G., & Ricciardolo, F. L. M. (2020). Clinical Characterization of the Frequent Exacerbator Phenotype in Asthma. Journal of Clinical Medicine, 9(7), 2226. https://doi.org/10.3390/jcm9072226







