A Phase 2 Clinical Trial on the Use of Cibinetide for the Treatment of Diabetic Macular Edema
Abstract
1. Introduction
2. Experimental Section
2.1. Eligibility Criteria
2.2. Definition of “Study Eye”
2.3. Primary Outcome
2.4. Secondary Outcomes
2.5. Exploratory Outcomes
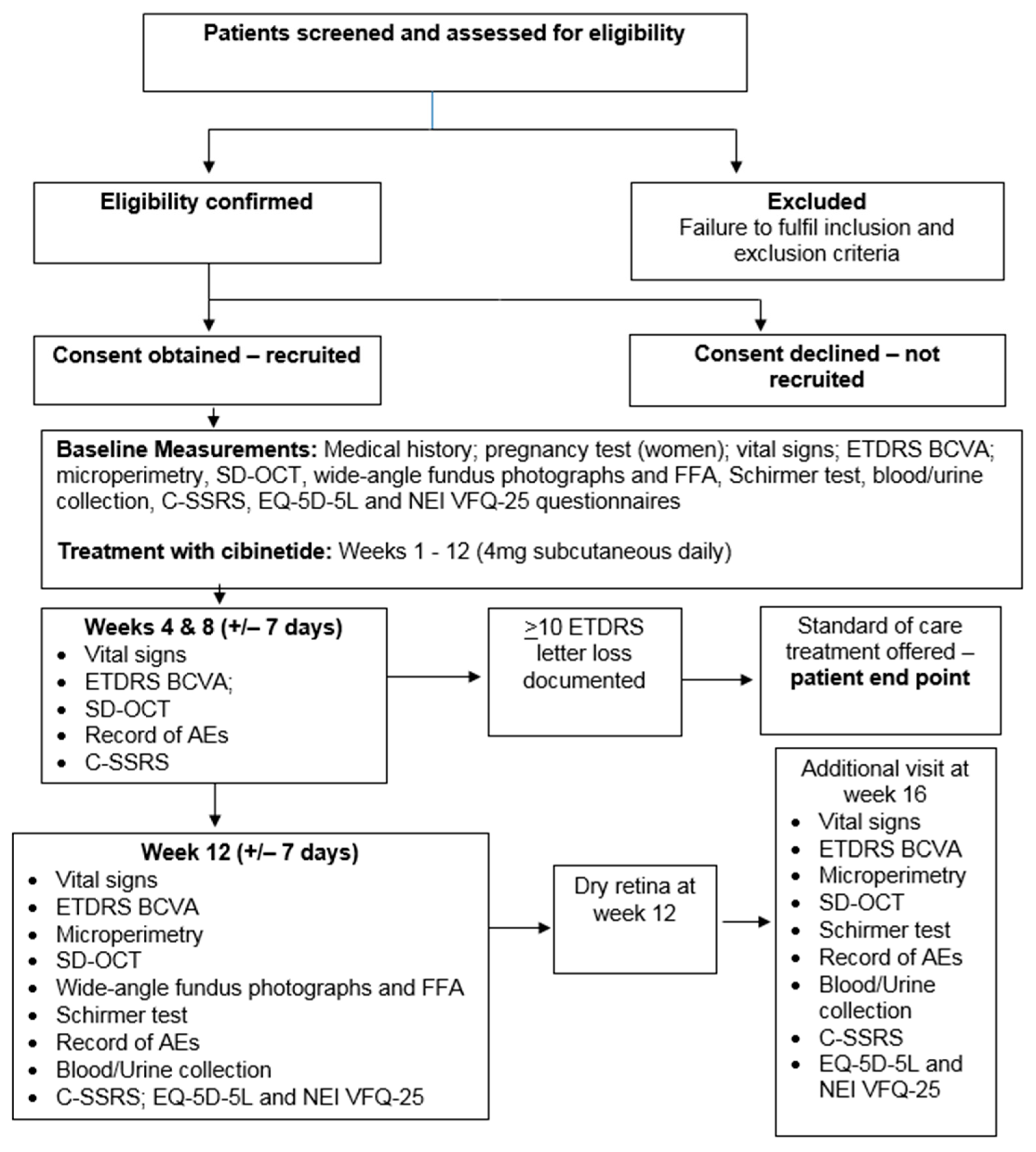
2.6. Clinical Evaluation and Time Points
- BCVA was obtained using ETDRS charts at 4 m. ETDRS total score was recorded.
- Mean central 10-degree macular sensitivity (expert test, 10-degree 45-stimulus grid) was determined with the MAIA microperimeter.
- CRT (average thickness in the central 1 mm) was obtained in both eyes by an experienced ophthalmic photographer using the SD-OCT Heidelberg Spectralis. The presence/absence of intraretinal, subretinal and sub-retinal pigment epithelium (RPE) fluid was noted.
- Retinal perfusion was assessed in both eyes by wide angle fundus fluorescein angiography (WA-FFA), undertaken by an experienced ophthalmic photographer and evaluated qualitatively by an experienced clinician (NL) for the presence/absence/extension of areas of retinal ischaemia at baseline and at week 12.
- Basal tear production was determined in the study eye using Schirmer’s test, undertaken by a research nurse after a drop of topical anaesthetic was applied. The extent of moisture in mm on the filter paper was recorded.
- Patient-reported outcomes (PROs) were evaluated by means of the EuroQol 5 Dimensions (EQ-5D-5L) [16] and the National Eye Institute Visual Function Questionnaire (NEI VFQ-25) [17]. Given that one individual with a pre-existing history of depression out of over 200 treated had suicidal ideation while using cibinetide [18], the potential for suicidal ideation was evaluated in this trial using the Columbia Suicide Severity Rating Scale (C-SSRS) [19].
- Cibinetide antibodies were evaluated in blood samples.
- Adverse Events (AEs) and Serious Adverse Events (SAEs) were assessed by the Chief Investigator (CI) for causality, seriousness and severity. All SAEs, if there had been any, would have been assessed for expectedness based on the cibinetide Investigator Brochure (IB).
2.7. Intervention
2.8. Sample Size and Statistical Analysis
3. Results
Safety
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef]
- Egan, C.; Zhu, H.; Lee, A.; Sim, D.; Mitry, D.; Bailey, C.; Johnston, R.; Chakravarthy, U.; Denniston, A.; Tufail, A.; et al. The United Kingdom Diabetic Retinopathy Electronic Medical Record Users Group, Report 1: baseline characteristics and visual acuity outcomes in eyes treated with intravitreal injections of ranibizumab for diabetic macular oedema. Br. J. Ophthalmol. 2017, 101, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Elman, M.J.; Ayala, A.; Bressler, N.M.; Browning, D.; Flaxel, C.J.; Glassman, A.R.; Jampol, L.M.; Stone, T.W.; Diabetic Retinopathy Clinical Research, Network. Intravitreal Ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology 2015, 122, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.A.; Glassman, A.R.; Ayala, A.R.; Jampol, L.M.; Bressler, N.M.; Bressler, S.B.; Brucker, A.J.; Ferris, F.L.; Hampton, G.R.; Jhaveri, C.; et al. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema: Two-Year Results from a Comparative Effectiveness Randomized Clinical Trial. Ophthalmology 2016, 123, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.P.; Simo, R.; et al. The progress in understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res. 2016, 51, 156–186. [Google Scholar] [CrossRef]
- Chibber, R.; Ben-Mahmud, B.M.; Chibber, S.; Kohner, E.M. Leukocytes in diabetic retinopathy. Curr. Diabetes Rev. 2007, 3, 3–14. [Google Scholar] [CrossRef]
- Bradshaw, E.M.; Raddassi, K.; Elyaman, W.; Orban, T.; Gottlieb, P.A.; Kent, S.C.; Hafler, D.A. Monocytes from patients with type 1 diabetes spontaneously secrete proinflammatory cytokines inducing Th17 cells. J. Immunol. 2009, 183, 4432–4439. [Google Scholar] [CrossRef]
- Collino, M.; Thiemermann, C.; Cerami, A.; Brines, M. Flipping the molecular switch for innate protection and repair of tissues: Long-lasting effects of a non-erythropoietic small peptide engineered from erythropoietin. Pharmacol. Ther. 2015, 151, 32–40. [Google Scholar] [CrossRef]
- Ahmet, I.; Tae, H.J.; Juhaszova, M.; Riordon, D.R.; Boheler, K.R.; Sollott, S.J.; Brines, M.; Cerami, A.; Lakatta, E.G.; Talan, M.I. A small nonerythropoietic helix B surface peptide based upon erythropoietin structure is cardioprotective against ischemic myocardial damage. Mol. Med. 2011, 17, 194–200. [Google Scholar] [CrossRef]
- Brines, M.; Patel, N.S.; Villa, P.; Brines, C.; Mennini, T.; De Paola, M.; Erbayraktar, Z.; Erbayraktar, S.; Sepodes, B.; Thiemermann, C. Nonerythropoietic, tissue-protective peptides derived from the tertiary structure of erythropoietin. Proc. Natl. Acad. Sci. USA 2008, 105, 10925–10930. [Google Scholar] [CrossRef]
- McVicar, C.M.; Hamilton, R.; Colhoun, L.M.; Gardiner, T.A.; Brines, M.; Cerami, A.; Stitt, A.W. Intervention with an erythropoietin-derived peptide protects against neuroglial and vascular degeneration during diabetic retinopathy. Diabetes 2011, 60, 2995–3005. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Lois, N. Erythropoietin in diabetic retinopathy. Vision Res. 2017, 139, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Collino, M.; Benetti, E.; Rogazzo, M.; Chiazza, F.; Mastrocola, R.; Nigro, D.; Cutrin, J.C.; Aragno, M.; Fantozzi, R.; Minetto, M.A.; et al. A non-erythropoietic peptide derivative of erythropoietin decreases susceptibility to diet-induced insulin resistance in mice. Br. J. Pharmacol. 2014, 171, 5802–5815. [Google Scholar] [CrossRef]
- Muller, C.; Yassin, K.; Li, L.S.; Palmblad, M.; Efendic, S.; Berggren, P.O.; Cerami, A.; Brines, M.; Östenson, C.G. ARA290 improves insulin release and glucose tolerance in type 2 diabetic GK rats. Mol. Med. 2016, 21, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Brines, M.; Dunne, A.N.; Van Velzen, M.; Proto, P.L.; Ostenson, C.G.; Kirk, R.I.; Petropoulos, I.N.; Javed, S.; Malik, R.A.; Cerami, A.; et al. ARA 290, a nonerythropoietic peptide engineered from erythropoietin, improves metabolic control and neuropathic symptoms in patients with type 2 diabetes. Mol. Med. 2015, 20, 658–666. [Google Scholar] [CrossRef] [PubMed]
- EQ-5D-5L. Available online: https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/ (accessed on 25 May 2020).
- National Eye Institute Visual Function Questionnaire 25. Available online: https://www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/outreach-materials/visual-function-questionnaire-25 (accessed on 25 May 2020).
- Culver, D.A.; Dahan, A.; Bajorunas, D.; Jeziorska, M.; Van Velzen, M.; Aarts, L.P.; Tavee, J.; Tannemaat, M.R.; Dunne, A.N.; Kirk, R.I.; et al. Cibinetide Improves Corneal Nerve Fiber Abundance in Patients With Sarcoidosis-Associated Small Nerve Fiber Loss and Neuropathic Pain. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO52–BIO60. [Google Scholar] [CrossRef] [PubMed]
- Columbia-suicide severity rating scale. Available online: https://cssrs.columbia.edu (accessed on 25 May 2020).
- Chen, C.W.; Drechsler, C.; Suntharalingam, P.; Karumanchi, S.A.; Wanner, C.; Berg, A.H. High Glycated Albumin and Mortality in Persons with Diabetes Mellitus on Hemodialysis. Clin. Chem. 2017, 63, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.H.; Drechsler, C.; Wenger, J.; Buccafusca, R.; Hod, T.; Kalim, S.; Ramma, W.; Parikh, S.M.; Steen, H.; Friedman, D.J. Carbamylation of serum albumin as a risk factor for mortality in patients with kidney failure. Sci. Transl. Med. 2013, 5, 175ra129. [Google Scholar] [CrossRef]
- ARA290 Trial Documents. Available online: http://www.nictu.hscni.net/ara290-trial-documents (accessed on 13 July 2020).
- Olink Target 96 Inflammation. Available online: www.olink.com/products/inflammation (accessed on 13 July 2020).
- Fiore, T.; Androudi, S.; Iaccheri, B.; Lupidi, M.; Fabrizio, G.; Fruttini, D.; Biondi, L.; Cagini, C. Repeatability and reproducibility of retinal thickness measurements in diabetic patients with spectral domain optical coherence tomography. Curr. Eye Res. 2013, 38, 674–679. [Google Scholar] [CrossRef]
- Polito, A.; Del Borrello, M.; Polini, G.; Furlan, F.; Isola, M.; Bandello, F. Diurnal variation in clinically significant diabetic macular edema measured by the Stratus OCT. Retina 2006, 26, 14–20. [Google Scholar] [CrossRef]
- Lois, N. Treatment for diabetic macular oedema: Looking further into the evidence. Ann. Eye Sci. 2018, 3, 2. [Google Scholar] [CrossRef]
- Robertson, C.S.; Cherian, L.; Shah, M.; Garcia, R.; Navarro, J.C.; Grill, R.J.; Hand, C.C.; Tian, T.S.; Hannay, H.J. Neuroprotection with an erythropoietin mimetic peptide (pHBSP) in a model of mild traumatic brain injury complicated by hemorrhagic shock. J. Neurotrauma. 2012, 29, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.S.; Garcia, R.; Gaddam, S.S.K.; Grill, R.J.; Cerami Hand, C.; Tian, T.S.; Hannay, H.J. Treatment of mild traumatic brain injury with an erythropoietin-mimetic peptide. J. Neurotrauma. 2013, 30, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.; Simó, R. Erythropoietin produced by the retina: its role in physiology and diabetic retinopathy. Endocrine 2012, 41, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wang, W.; Gu, Q.; Xu, X. Erythropoietin protects retinal neurons and glial cells in early-stage streptozotocin-induced diabetic rats. Exp. Eye Res. 2008, 86, 375–382. [Google Scholar] [CrossRef]
- Bretz, C.A.; Simmons, A.B.; Kunz, E.; Ramshekar, A.; Kennedy, C.; Cardenas, I.; Hartnett, M.E. Erythropoietin Receptor Signaling Supports Retinal Function after Vascular Injury. Am. J. Pathol. 2020, 190, 630–641. [Google Scholar] [CrossRef]
- Petropoulos, I.N.; Ponirakis, G.; Khan, A.; Almuhannadi, H.; Gad, H.; Malik, R.A. Diagnosing Diabetic Neuropathy: Something Old, Something New. Diabetes Metab. J. 2018, 42, 255–269. [Google Scholar] [CrossRef]
- Labbé, A.; Liang, Q.; Wang, Z.; Zhang, Y.; Xu, L.; Baudouin, C.; Sun, X. Corneal nerve structure and function in patients with non-sjogren dry eye: clinical correlations. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5144–5150. [Google Scholar] [CrossRef]
- Patel, N.S.; Kerr-Peterson, H.L.; Brines, M.; Collino, M.; Rogazzo, M.; Fantozzi, R.; Wood, E.G.; Johnson, F.L.; Yaqoob, M.M.; Cerami, A.; et al. Delayed administration of pyroglutamate helix B surface peptide (pHBSP), a novel nonerythropoietic analog of erythropoietin, attenuates acute kidney injury. Mol. Med. 2012, 18, 719–727. [Google Scholar] [CrossRef]
- Lan, T.; Morgan, D.A.; Rahmouni, K.; Sonoda, J.; Fu, X.; Burgess, S.C.; Holland, W.L.; Kliewer, S.A.; Mangelsdorf, D.J. FGF19, FGF21, and an FGFR1/beta-Klotho-Activating Antibody Act on the Nervous System to Regulate Body Weight and Glycemia. Cell Metab. 2017, 26, 709–718, e703. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Gallego-Escuredo, J.M.; Catalán, V.; Rodríguez, A.; Domingo, P.; Moncada, R.; Valentí, V.; Salvador, J.; Giralt, M.; Villarroya, F.; et al. FGF19 and FGF21 serum concentrations in human obesity and type 2 diabetes behave differently after diet- or surgically-induced weight loss. Clin. Nutr. 2017, 36, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Niesters, M.; Swartjes, M.; Heij, L.; Brines, M.; Cerami, A.; Dunne, A.; Hoitsma, E.; Dahan, A. The erythropoietin-analogue ARA 290 for treatment of sarcoidosis-induced chronic neuropathic pain. Exp. Opin. Orphan Drugs 2013, 1, 77–87. [Google Scholar] [CrossRef][Green Version]

| Characteristics | Baseline n = 9(100%) | Baseline * n = 8(88.9%) | |
|---|---|---|---|
| Gender | Male | 5 (55.6%) | 5 (62.5%) |
| Female | 4 (44.4%) | 3 (37.5%) | |
| Study Eye | Left | 2 (22.2%) | 2 (25.0%) |
| Right | 7 (77.8%) | 6 (75.0%) | |
| Fellow Eye with DME present | ≥400 microns | 2 (22.2%) | 1 (12.5%) |
| <400 microns | 6 (66.7%) | 6 (75.0%) | |
| Type of diabetes | Type 1 | 1 (11.1%) | 1 (12.5%) |
| Type 2 | 8 (88.9%) | 7 (87.5%) | |
| Duration of diabetes (years, mean ± SD and median [IQR]) | 14.4 ± 8.5 16.1 [9.8, 18.0] | 15.0 ± 8.9 16.7 [8.8, 20.5] | |
| Age (years, mean ± SD) | 57.6 ± 13.9 | 57.6 ± 14.9 | |
| Weight (kg, mean ± SD) | 95.5 ± 20.5 | 90.6 ± 15.3 | |
| Height (m, mean ± SD) | 1.6 ± 0.12 | 1.7 ± 0.1 | |
| Body Mass Index (kg/m2, mean ± SD) | 37.18 ± 12.1 | 33.6 ± 5.9 | |
| HbA1c (mmol/L, mean ± SD) | 74.0 ± 19.7 | 75 ± 20.9 | |
| Baseline | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | A | B | C | D | E | F | G | H | I |
| Gender | Female | Male | Male | Male | Male | Male | Female | Female | Female |
| Study Eye | Right | Left | Right | Left | Right | Right | Right | Right | Right |
| Type of diabetes | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Duration of diabetes (years) | 2.7 | 18 | 23 | 14.9 | 17.3 | 16.1 | 9.8 | 26.5 | 1.1 |
| Age (years) | 74.5 | 31.8 | 43.7 | 50.8 | 58.5 | 59.7 | 57.7 | 72 | 69.5 |
| Weight (kg) | 74.2 | 81.5 | 118 | 92.2 | 102 | 94.4 | 134.6 | 91.2 | 71 |
| Height (m) | 1.57 | 1.75 | 1.65 | 1.66 | 1.72 | 1.76 | 1.43 | 1.49 | 1.56 |
| BMI (kg/m2) | 30.1 | 26.6 | 43.3 | 33.5 | 34.5 | 30.5 | 65.8 | 41.1 | 29.2 |
| HbA1c (mmol/L) | 63 | 83 | 56 | 117 | 88 | 74 | 66 | 65 | 54 |
| Diabetes Medications | Metformin | Novorapid | Sitagliptin | Metformin | Dapagliflozin | Novorapid insulin | Metformin | Metformin | Gliclazide |
| Lantus Solostar | Gliclazide | Novo Rapid | Novomix 30 | Levemir | Novorapid Flexpen | Novomix 30 | Metformin | ||
| Levemir | Metformin | Liraglutide | |||||||
| Novomix 30 (PM) | Lantus | ||||||||
| Baseline | Baseline * | Week 12 * | Change * | |
|---|---|---|---|---|
| n = 9 (100%) | n = 8 (88.9%) | n = 8 (88.9%) | n = 8 (88.9%) | |
| Best corrected distance visual acuity (mean ± SD and 95% CI) | 68.1± 7.9 (62.0, 74.2) | 69.1 ± 7.8 (62.6, 75.6) | 66.3 ± 9.5 (58.3, 74.2) | −2.9 ± 5.0 (−7.1, 1.3) |
| Central subfield thickness (microns, mean ± SD and 95% CI) | 490.3 ± 61.9 (442.8, 537.9) | 488.5 ± 65.9 (433.4, 543.6) | 498.5 ± 127.1 (392.2, 604.8) | 10.0 ± 94.6 (−69.1, 89.1) |
| Central retinal sensitivity (dB, mean ± SD and 95% CI) | 23.3 ± 2.2 (21.5, 25.0) | 23.7 ± 1.9 (22.1, 25.3) | 23.2 ± 2.3 (21.3, 25.1) | −0.53 ± 1.9 (−2.1, 1.1) |
| Tear production (mm, mean ± SD and 95% CI) | 13.4 ± 8.8 (6.7, 20.2) | 13.9 ± 9.3 (6.1, 21.7) | 13.8 ± 2.4 (11.8, 15.7) | −0.13 ± 7.7 (−6.6, 6.3) |
| VFQ-25 Composite Score (mean ± SD and 95% CI) | 79.0 ± 20.1 (63.6, 94.5) | 84.8 ± 11.3 (75.4, 94.2) | 87.5 ± 6.9 (81.8, 93.2) | 2.7 ± 3.1 (−4.5, 10.0) |
| EQ-5D-5L Index (mean ± SD and 95% CI) EQ-5D 5L Visual Analogue Score (mean ± SD and 95% CI) | 0.7 ± 0.4 (0.5, 1.0) 68.9 ± 21.3 (52.5, 85.3) | 0.8 ± 0.2 (0.7, 1.0) 68.8 ± 22.8 (49.7, 87.8) | 0.8 ± 0.3 (0.6, 1.0) 75.0 ± 12.8 (64.3, 85.7) | −0.03 ± 0.2 (−0.2, 0.1) 6.3 ± 21.5 (−11.7, 24.2) |
| Best Corrected Distance Visual Acuity | Central Subfield Thickness (Microns) | Central Retinal Sensitivity (dB) | Tear Production (mm) | EQ5D-5L Index Score | VFQ Composite Score | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Eye | Baseline | Week 12 | Change | Baseline | Week 12 | Change | Baseline | Week 12 | Change | Baseline | Week 12 | Change | Baseline | Week 12 | Change | Baseline | Week 12 | Change |
| A | Study Eye | 73 | 69 | −4 | 602 | 646 | 44 | 22.7 | 24.4 | 1.7 | 10 | 16 | 6 | 0.61 | 0.29 | −0.32 | 87.4 | 89.0 | 1.7 |
| Non Study Eye | 79 | 77 | −2 | 375 | 424 | 49 | 24.1 | 25.5 | 1.4 | - | - | - | |||||||
| B | Study Eye | 68 | 70 | 2 | 538 | 653 | 115 | 25.6 | 22.7 | −2.9 | 35 | 17 | −18 | 0.94 | 1.00 | 0.06 | 87.7 | 80.6 | −7.1 |
| Non Study Eye | 79 | 80 | 1 | 363 | 380 | 17 | 26.5 | 27.6 | 1.1 | - | - | - | |||||||
| C | Study Eye | 59 | 56 | −3 | 428 | 588 | 160 | 23.9 | 20.6 | −3.3 | 11 | 14 | 3 | 0.95 | 0.95 | 0.00 | 86.6 | 95.9 | 9.3 |
| Non Study Eye | 85 | 88 | 3 | 305 | 315 | 10 | 25.4 | 21.3 | −4.1 | - | - | - | |||||||
| D | Study Eye | 84 | 81 | −3 | 429 | 425 | −4 | 23.7 | 24.2 | 0.5 | 6 | 11 | 5 | 1.00 | 1.00 | 0.00 | 85.1 | 81.6 | −3.5 |
| Non Study Eye | 69 | 68 | −1 | 485 | 473 | −12 | 23.1 | 22.2 | −0.9 | - | - | - | |||||||
| E | Study Eye | 72 | 68 | −4 | 477 | 470 | −7 | 23.6 | 22.2 | −1.4 | 17 | 15 | −2 | 0.89 | 0.84 | −0.05 | 98.2 | 91.4 | −6.8 |
| Non Study Eye | 70 | 78 | 8 | 356 | 364 | 8 | 21.1 | 22.3 | 1.2 | - | - | - | |||||||
| F | Study Eye | 61 | 59 | −2 | 490 | 424 | −66 | 27 | 26.9 | −0.1 | 6 | 10 | 4 | 0.65 | 0.89 | 0.24 | 73.3 | 82.9 | 9.7 |
| Non Study Eye | 78 | 84 | 6 | 311 | 304 | −725 | 28 | 26.9 | −1.1 | - | - | - | |||||||
| H | Study Eye | 70 | 74 | 4 | 412 | 280 | −132 | 22.5 | 24.4 | 1.9 | 14 | 14 | 0 | 0.63 | 0.48 | −0.15 | 64.0 | 81.2 | 17.2 |
| 41 | 41 | 58 | 17 | 239 | 230 | −9 | 21.9 | 22.8 | 0.9 | - | - | - | |||||||
| I | Study Eye | 66 | 53 | −13 | 532 | 502 | −30 | 20.6 | 20 | −0.6 | 12 | 13 | 1 | 1.00 | 1.00 | 0.00 | 95.9 | 97.4 | 1.5 |
| Non Study Eye | 72 | 74 | 2 | 297 | 293 | −4 | 22.8 | 24.8 | 2 | - | - | - | |||||||
| BMI (kg/m2) | Systolic Blood pressure (mmHg) | Diastolic Blood Pressure | HbA1c (mmol/L) | HDL (mmol/L) | LDL (mmol/L) | Triglycerides (mmol/L) | Albumin Creatinine Ratio | Carbamoylated Albumin | Glycated Albumin | FGF-19 | FGF-21 | Albumin (g/L) | Estimated GFR (mL/min) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Baseline | Week 12 | Baseline | Week 12 | Baseline | Week 12 | Baseline | Week 12 | Baseline | Week 12 | Baseline | Week 12 | Baseline | Week 12 | Baseline | Week 12 | Baseline | Week 12 | Baseline | Week 12 | Baseline | Week 12 | Baseline | Week 12 | Baseline | Week 12 | Baseline | Week 12 |
| A | 30.1 | - | 167 | 144 | 72 | 70 | 63 | 53 | 1.4 | 1.4 | 1.4 | 1 | 1.33 | 1.82 | 5.5 | 3.41 | 2.4 | 2.6 | 18.1 | 17.6 | 7.2 | 9.7 | 5.5 | 4.6 | 48 | 47 | >60 | >60 |
| B | 26.6 | - | 129 | 124 | 76 | 80 | 83 | 75 | 1.3 | 1.2 | 3.5 | 2.5 | 0.68 | 0.53 | 46.5 | 40.64 | 2.8 | 3.1 | 22.1 | 20.3 | 6.6 | 6.4 | 2.8 | 5.6 | 43 | 45 | >60 | >60 |
| C | 43.3 | - | 131 | 160 | 71 | 73 | 56 | 50 | 1 | 0.9 | 1.6 | 1.5 | 2.71 | 2.9 | 220.65 | 195.79 | 4.9 | 4.8 | 16.9 | 14.7 | 7.8 | - | 4.1 | - | 36 | 40 | 41 | 38 |
| D | 33.5 | - | 126 | 128 | 81 | 81 | 117 | 123 | 1.1 | 1 | 1.7 | 1.3 | 1.43 | 2.32 | 1.6 | 2.14 | 2.5 | 2.3 | 23.5 | 26.0 | 7.8 | 9.0 | 9.3 | 6.8 | 46 | 46 | >60 | >60 |
| E | 34.5 | - | 142 | 125 | 79 | 66 | 88 | 85 | 0.8 | 0.9 | 2.7 | 2.5 | 2.52 | 2.62 | 4.52 | 3.59 | 1.7 | 1.7 | 17.4 | 18.5 | - | 7.7 | - | 6.1 | 43 | 47 | >60 | >60 |
| F | 30.5 | - | 152 | 126 | 56 | 62 | 74 | 68 | 0.9 | 1 | 1.3 | 1.7 | 1.49 | 1.41 | 1.38 | 0.73 | 1.8 | 2.0 | 23.9 | 24.8 | 6.1 | 10.6 | 5.7 | 2.6 | 40 | 40 | >60 | >60 |
| H | 41.1 | - | 150 | 127 | 71 | 64 | 65 | 64 | 1.3 | 1.4 | 1.5 | 1.5 | 2.49 | 2.6 | 3.1 | 2.71 | 2.5 | 3.1 | 18.9 | 21.2 | 6.8 | 7.6 | 6.9 | 6.0 | 43 | 43 | >60 | >60 |
| I | 29.2 | - | 136 | 122 | 74 | 71 | 54 | 56 | 1.6 | 1.5 | 3.3 | 3 | 1.91 | 1.91 | 3.04 | 3.1 | 2.6 | 2.7 | 14.5 | 14.3 | 6.8 | 7.2 | 2.2 | 5.9 | 47 | 45 | >60 | >60 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lois, N.; Gardner, E.; McFarland, M.; Armstrong, D.; McNally, C.; Lavery, N.J.; Campbell, C.; Kirk, R.I.; Bajorunas, D.; Dunne, A.; et al. A Phase 2 Clinical Trial on the Use of Cibinetide for the Treatment of Diabetic Macular Edema. J. Clin. Med. 2020, 9, 2225. https://doi.org/10.3390/jcm9072225
Lois N, Gardner E, McFarland M, Armstrong D, McNally C, Lavery NJ, Campbell C, Kirk RI, Bajorunas D, Dunne A, et al. A Phase 2 Clinical Trial on the Use of Cibinetide for the Treatment of Diabetic Macular Edema. Journal of Clinical Medicine. 2020; 9(7):2225. https://doi.org/10.3390/jcm9072225
Chicago/Turabian StyleLois, Noemi, Evie Gardner, Margaret McFarland, David Armstrong, Christine McNally, Nuala Jane Lavery, Christina Campbell, Rita I Kirk, Daiva Bajorunas, Ann Dunne, and et al. 2020. "A Phase 2 Clinical Trial on the Use of Cibinetide for the Treatment of Diabetic Macular Edema" Journal of Clinical Medicine 9, no. 7: 2225. https://doi.org/10.3390/jcm9072225
APA StyleLois, N., Gardner, E., McFarland, M., Armstrong, D., McNally, C., Lavery, N. J., Campbell, C., Kirk, R. I., Bajorunas, D., Dunne, A., Cerami, A., & Brines, M. (2020). A Phase 2 Clinical Trial on the Use of Cibinetide for the Treatment of Diabetic Macular Edema. Journal of Clinical Medicine, 9(7), 2225. https://doi.org/10.3390/jcm9072225





