Effects of Circulating HMGB-1 and Histones on Cardiomyocytes–Hemadsorption of These DAMPs as Therapeutic Strategy after Multiple Trauma
Abstract
:1. Introduction
2. Material and Methods
2.1. In Vitro Incubation of Human Cardiomyocytes (CMs) with HMGB-1 and Histones
2.2. In Vitro Incubation of HL-1 Cells with HMGB-1
2.3. Calcium Measurements
2.4. Mitochondrial Respiration
2.5. Plasma Samples of Polytrauma Patients
2.6. Hemadsorption in Plasma of Multiple Injured Patients
2.7. Statistical Procedures
3. Results
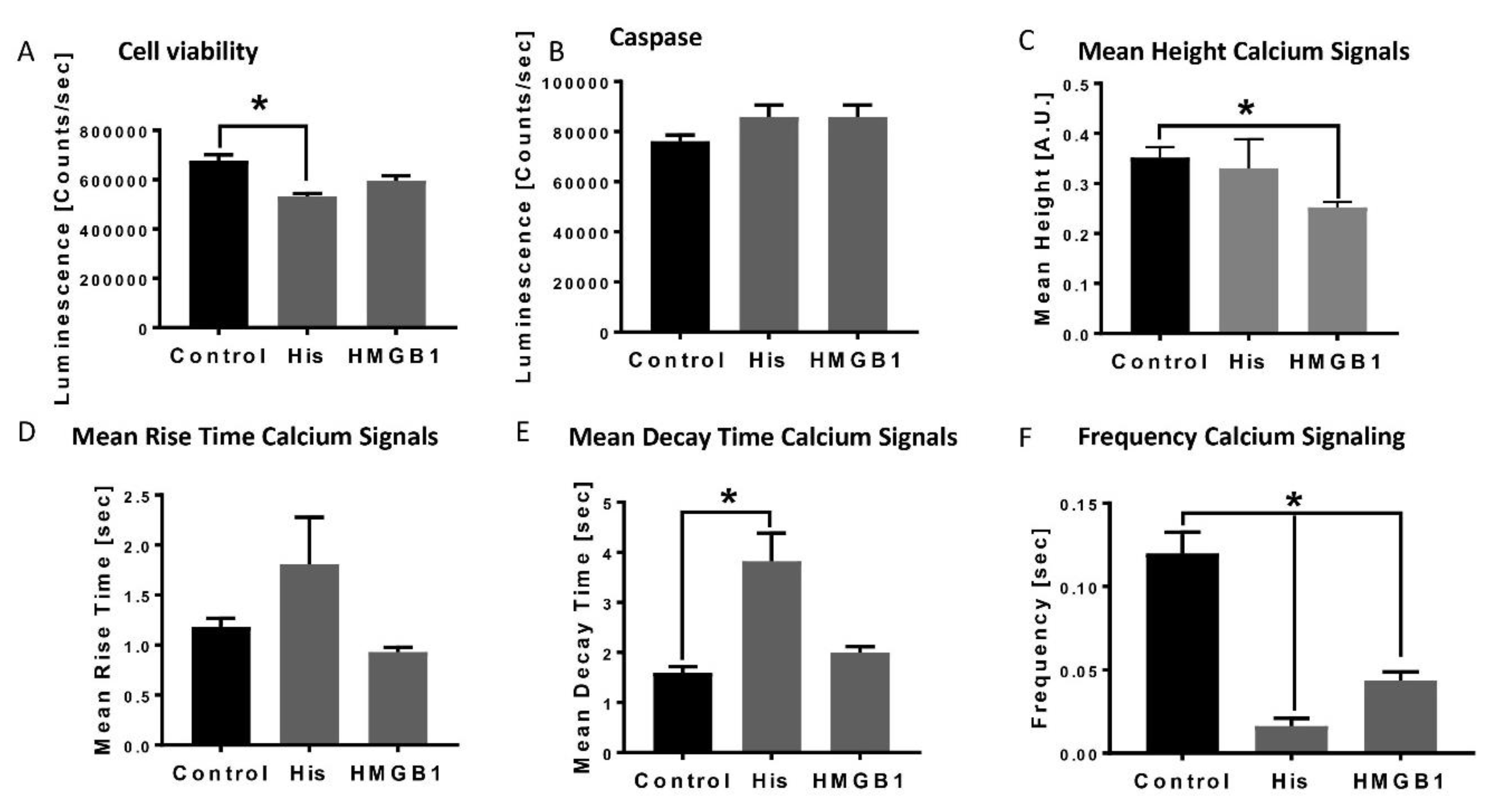
3.1. Decreased Apoptosis in Human Cardiomyocytes and Alterations of Calcium Handling in Presence of HMGB-1 and Histones
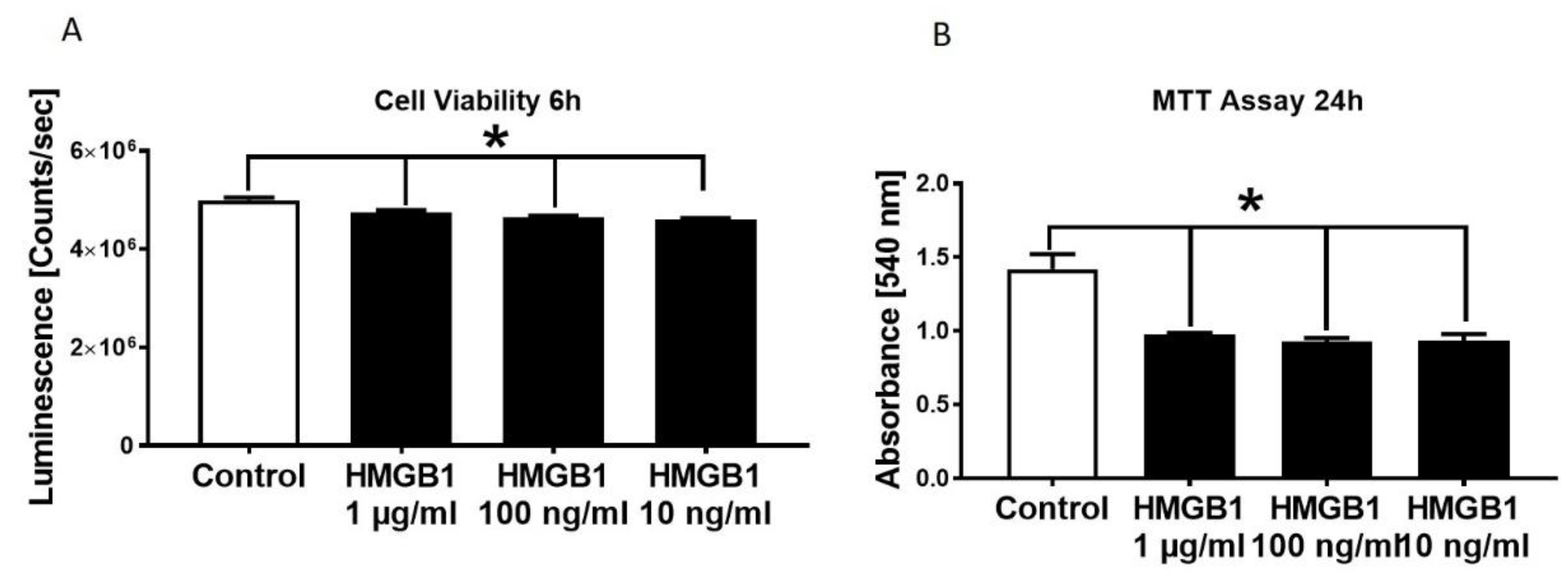
3.2. Decrease in HL-1 Cell Viability and Metabolic Activity in Presence of Different HMGB-1 Concentrations
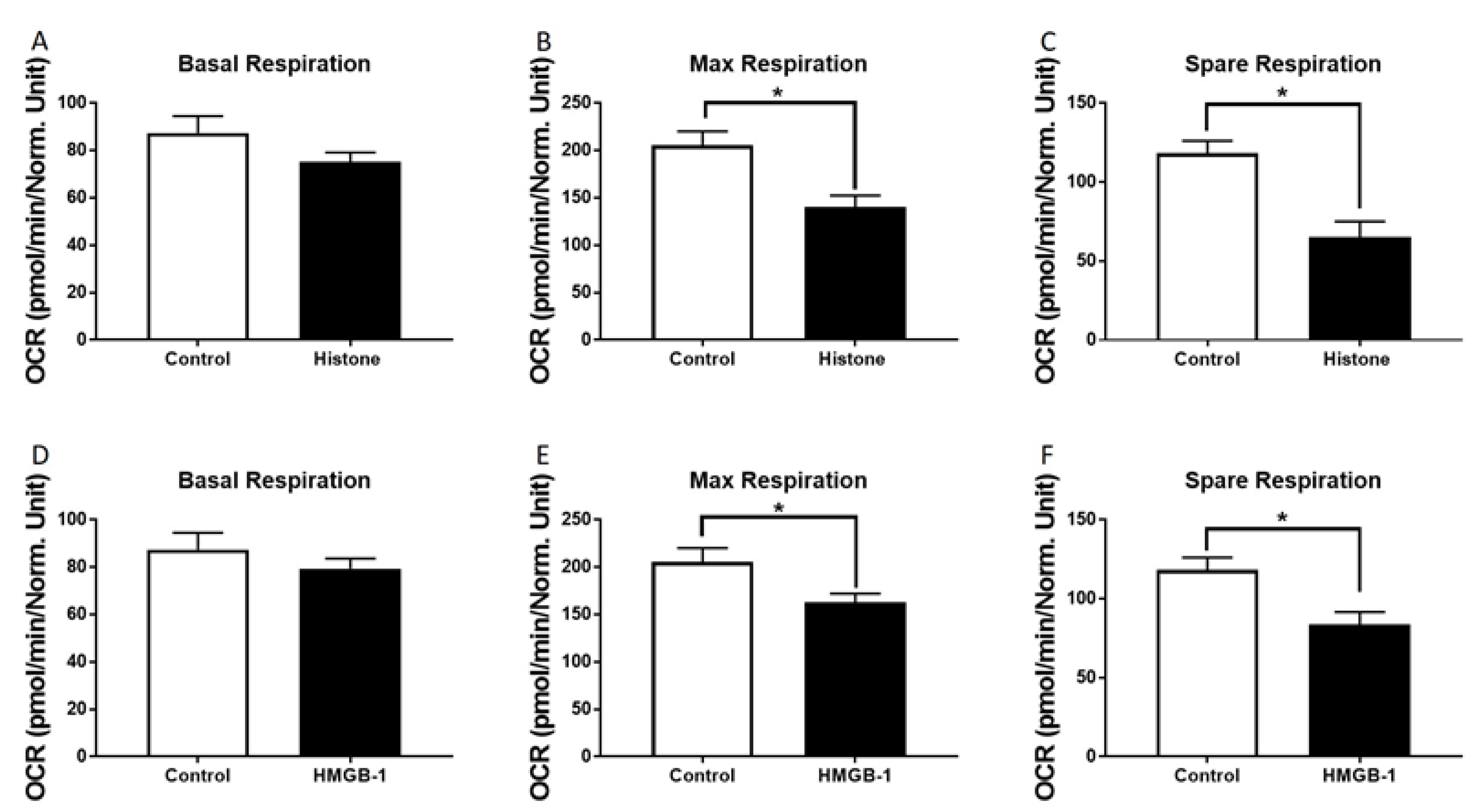
3.3. Metabolic Alterations of Human Cardiomyocytes in Presence of Histones
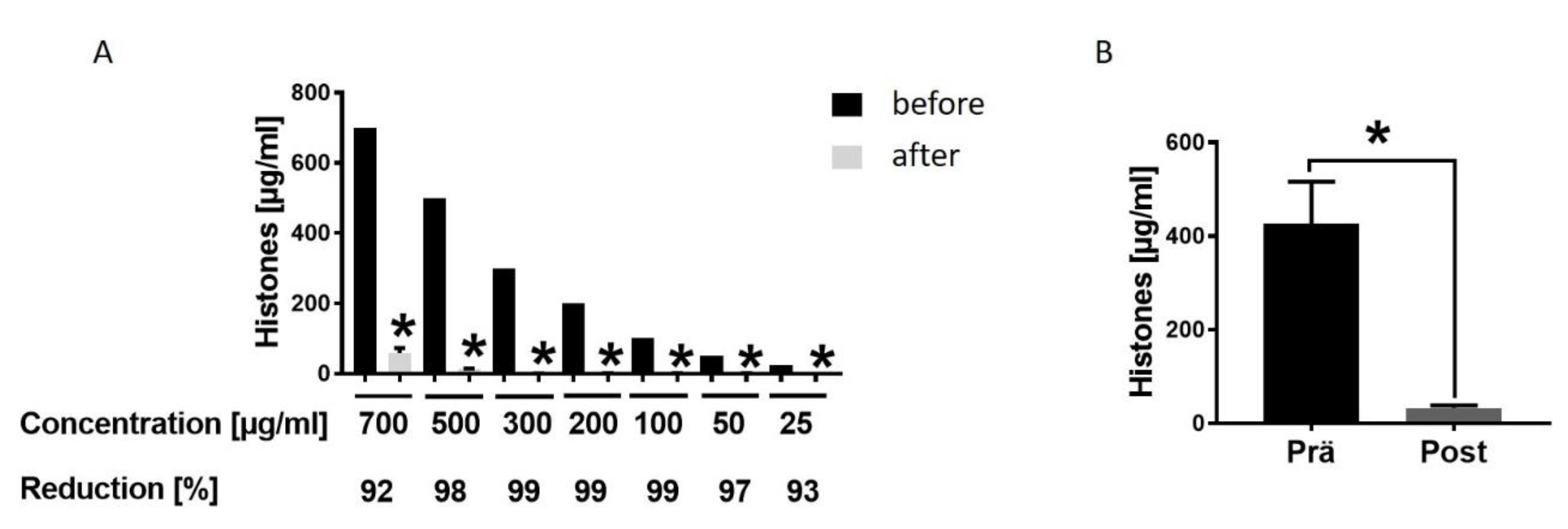
3.4. Hemadsorption—A Therapeutic Option to Eliminate Systemic Extracellular Histones
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kalbitz, M.; Pressmar, J.; Stecher, J.; Weber, B.; Weiss, M.; Schwarz, S.; Miltner, E.; Gebhard, F.; Huber-Lang, M. The Role of Troponin in Blunt Cardiac Injury After Multiple Trauma in Humans. World J. Surg. 2017, 41, 162–169. [Google Scholar] [CrossRef]
- Martin, M.; Mullenix, P.; Rhee, P.; Belzberg, H.; Demetriades, D.; Salim, A. Troponin increases in the critically injured patient: Mechanical trauma or physiologic stress? J. Trauma 2005, 59, 1086–1091. [Google Scholar] [CrossRef]
- Kalbitz, M.; Schwarz, S.; Weber, B.; Bosch, B.; Pressmar, J.; Hoenes, F.M.; Braun, C.K.; Horst, K.; Simon, T.P.; Pfeifer, R.; et al. Cardiac Depression in Pigs after Multiple Trauma—Characterization of Posttraumatic Structural and Functional Alterations. Sci. Rep. 2017, 7, 17861. [Google Scholar] [CrossRef]
- Kalbitz, M.; Amann, E.M.; Bosch, B.; Palmer, A.; Schultze, A.; Pressmar, J.; Weber, B.; Wepler, M.; Gebhard, F.; Schrezenmeier, H.; et al. Experimental blunt chest trauma-induced myocardial inflammation and alteration of gap-junction protein connexin 43. PLoS ONE 2017, 12, e0187270. [Google Scholar] [CrossRef] [Green Version]
- Braun, C.K.; Kalbitz, M.; Halbgebauer, R.; Eisele, P.; Messerer, D.A.C.; Weckbach, S.; Schultze, A.; Braumuller, S.; Gebhard, F.; Huber-Lang, M.S. Early structural changes of the heart after experimental polytrauma and hemorrhagic shock. PLoS ONE 2017, 12, e0187327. [Google Scholar] [CrossRef] [Green Version]
- Cohen, M.J.; Brohi, K.; Calfee, C.S.; Rahn, P.; Chesebro, B.B.; Christiaans, S.C.; Carles, M.; Howard, M.; Pittet, J.-F. Early release of high mobility group box nuclear protein 1 after severe trauma in humans: Role of injury severity and tissue hypoperfusion. Crit. Care 2009, 13, 174. [Google Scholar] [CrossRef] [Green Version]
- Ward, P.A.; Grailer, J.J. Acute lung injury and the role of histones. Transl. Respir. Med. 2014, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Abrams, S.T.; Zhang, N.; Manson, J.; Liu, T.; Dart, C.; Baluwa, F.; Wang, S.S.; Brohi, K.; Kipar, A.; Yu, W.; et al. Circulating histones are mediators of trauma-associated lung injury. Am. J. Respir. Crit. Care Med. 2013, 187, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Kalbitz, M.; Grailer, J.J.; Fattahi, F.; Jajou, L.; Herron, T.J.; Campbell, K.F.; Zetoune, F.S.; Bosmann, M.; Sarma, J.V.; Huber-Lang, M.; et al. Role of extracellular histones in the cardiomyopathy of sepsis. FASEB J. 2015, 29, 2185–2193. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Tohme, S.; Al-Khafaji, A.B.; Tai, S.; Loughran, P.; Chen, L.; Wang, S.; Kim, J.; Billiar, T.; Wang, Y.; et al. Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology 2015, 62, 600–614. [Google Scholar] [CrossRef] [Green Version]
- Park, J.S.; Svetkauskaite, D.; He, Q.; Kim, J.-Y.; Strassheim, D.; Ishizaka, A.; Abraham, E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 2004, 279, 7370–7377. [Google Scholar] [CrossRef] [Green Version]
- Tsung, A.; Sahai, R.; Tanaka, H.; Nakao, A.; Fink, M.P.; Lotze, M.T.; Yang, H.; Li, J.; Tracey, K.J.; Geller, D.A.; et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J. Exp. Med. 2005, 201, 1135–1143. [Google Scholar] [CrossRef]
- Kawai, C.; Kotani, H.; Miyao, M.; Ishida, T.; Jemail, L.; Abiru, H.; Tamaki, K. Circulating Extracellular Histones Are Clinically Relevant Mediators of Multiple Organ Injury. Am. J. Pathol. 2016, 186, 829–843. [Google Scholar] [CrossRef] [Green Version]
- Asavarut, P.; Zhao, H.; Gu, J.; Ma, D. The role of HMGB1 in inflammation-mediated organ injury. Acta Anaesthesiol. Taiwan 2013, 51, 28–33. [Google Scholar] [CrossRef]
- Van Beijnum, J.R.; Buurman, W.A.; Griffioen, A.W. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1). Angiogenesis 2008, 11, 91–99. [Google Scholar] [CrossRef]
- Meng, X.-Y.; Wu, L.; Zhou, Y.-H.; Liu, T.; Han, Q.-F.; Zhang, D.-Y.; Wang, L.-H.; Yao, H.-C. High mobility group box 1 might be a novel therapeutic target in ischemia heart disease. Int. J. Cardiol. 2016, 206, 42–43. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, M.; Jiang, H.; Yu, Y.; Yu, P.; Tong, R.; Wu, J.; Zhang, S.; Yao, K.; Zou, Y.; et al. Extracellular high-mobility group box 1 mediates pressure overload-induced cardiac hypertrophy and heart failure. J. Cell. Mol. Med. 2016, 20, 459–470. [Google Scholar] [CrossRef]
- Tian, Y.; Charles, E.J.; Yan, Z.; Wu, D.; French, B.A.; Kron, I.L.; Yang, Z. The myocardial infarct-exacerbating effect of cell-free DNA is mediated by the high-mobility group box 1-receptor for advanced glycation end products-Toll-like receptor 9 pathway. J. Thorac. Cardiovasc. Surg. 2018, 157, 2256–2269. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.-Y.; Carter, M.J.; Kellum, J.A. Effects of hemoadsorption on cytokine removal and short-term survival in septic rats. Crit. Care Med. 2008, 36, 1573–1577. [Google Scholar] [CrossRef]
- Kellum, J.A.; Song, M.; Venkataraman, R. Hemoadsorption removes tumor necrosis factor, interleukin-6, and interleukin-10, reduces nuclear factor-kappaB DNA binding, and improves short-term survival in lethal endotoxemia. Crit. Care Med. 2004, 32, 801–805. [Google Scholar] [CrossRef]
- Hinz, B.; Jauch, O.; Noky, T.; Friesecke, S.; Abel, P.; Kaiser, R. CytoSorb, a novel therapeutic approach for patients with septic shock: A case report. Int. J. Artif. Organs 2015, 38, 461–464. [Google Scholar] [CrossRef]
- Artimovich, E.; Jackson, R.K.; Kilander, M.B.C.; Lin, Y.-C.; Nestor, M.W. PeakCaller: An automated graphical interface for the quantification of intracellular calcium obtained by high-content screening. BMC Neurosci. 2017, 18, 72. [Google Scholar] [CrossRef]
- Horst, K.; Hildebrand, F.; Pfeifer, R.; Hubenthal, S.; Almahmoud, K.; Sassen, M.; Steinfeldt, T.; Wulf, H.; Ruchholtz, S.; Pape, H.C.; et al. Impact of haemorrhagic shock intensity on the dynamic of alarmins release in porcine poly-trauma animal model. Eur. J. Trauma Emerg. Surg. 2016, 42, 67–75. [Google Scholar] [CrossRef]
- Wang, H.; Bloom, O.; Zhang, M.; Vishnubhakat, J.M.; Ombrellino, M.; Che, J.; Frazier, A.; Yang, H.; Ivanova, S.; Borovikova, L.; et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999, 285, 248–251. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Mu, T. HMGB1 Neutralizing Antibody Attenuates Cardiac Injury and Apoptosis Induced by Hemorrhagic Shock/Resuscitation in Rats. Biol. Pharm. Bull. 2015, 38, 1150–1160. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Thota, V.; Dee, L.; Olson, J.; Uretz, E.; Parrillo, J.E. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J. Exp. Med. 1996, 183, 949–958. [Google Scholar] [CrossRef]
- Yang, S.; Zheng, R.; Hu, S.; Ma, Y.; Choudhry, M.A.; Messina, J.L.; Rue, L.W., III; Bland, K.I.; Chaudry, I.H. Mechanism of cardiac depression after trauma-hemorrhage: Increased cardiomyocyte IL-6 and effect of sex steroids on IL-6 regulation and cardiac function. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, 2183–2191. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Hu, S.; Hsieh, Y.-C.; Choudhry, M.A.; Rue, L.W., III; Bland, K.I.; Chaudry, I.H. Mechanism of IL-6-mediated cardiac dysfunction following trauma-hemorrhage. J. Mol. Cell. Cardiol. 2006, 40, 570–579. [Google Scholar] [CrossRef]
- Duncan, D.J.; Yang, Z.; Hopkins, P.M.; Steele, D.S.; Harrison, S.M. TNF-alpha and IL-1beta increase Ca2+ leak from the sarcoplasmic reticulum and susceptibility to arrhythmia in rat ventricular myocytes. Cell Calcium 2010, 47, 378–386. [Google Scholar] [CrossRef] [Green Version]
- Monnerat, G.; Alarcon, M.L.; Vasconcellos, L.R.; Hochman-Mendez, C.; Brasil, G.; Bassani, R.A.; Casis, O.; Malan, D.; Travassos, L.H.; Sepulveda, M.; et al. Macrophage-dependent IL-1beta production induces cardiac arrhythmias in diabetic mice. Nat. Commun. 2016, 7, 13344. [Google Scholar] [CrossRef] [Green Version]
- Bangert, A.; Andrassy, M.; Muller, A.-M.; Bockstahler, M.; Fischer, A.; Volz, C.H.; Leib, C.; Goser, S.; Korkmaz-Icoz, S.; Zittrich, S.; et al. Critical role of RAGE and HMGB1 in inflammatory heart disease. Proc. Natl. Acad. Sci. USA 2016, 113, 155–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, T.; Kawahara, K.; Nakamura, T.; Yamada, S.; Abeyama, K.; Hashiguchi, T.; Maruyama, I. High-mobility group box 1 protein promotes development of microvascular thrombosis in rats. J. Thromb. Haemost. 2007, 5, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Lackner, I.; Weber, B.; Baur, M.; Fois, G.; Gebhard, F.; Pfeifer, R.; Cinelli, P.; Halvachizadeh, S.; Lipiski, M.; Cesarovic, N.; et al. Complement Activation and Organ Damage After Trauma—Differential Immune Response Based on Surgical Treatment Strategy. Front. Immunol. 2020, 11, 64. [Google Scholar] [CrossRef] [Green Version]
- Kalbitz, M.; Fattahi, F.; Herron, T.J.; Grailer, J.J.; Jajou, L.; Lu, H.; Huber-Lang, M.; Zetoune, F.S.; Sarma, J.V.; Day, S.M.; et al. Complement Destabilizes Cardiomyocyte Function In Vivo after Polymicrobial Sepsis and In Vitro. J. Immunol. 2016, 197, 2353–2361. [Google Scholar] [CrossRef] [Green Version]
- Fattahi, F.; Frydrych, L.M.; Bian, G.; Kalbitz, M.; Herron, T.J.; Malan, E.A.; Delano, M.J.; Ward, P.A. Role of complement C5a and histones in septic cardiomyopathy. Mol. Immunol. 2018, 102, 32–41. [Google Scholar] [CrossRef]
- Limana, F.; Germani, A.; Zacheo, A.; Kajstura, J.; Di Carlo, A.; Borsellino, G.; Leoni, O.; Palumbo, R.; Battistini, L.; Rastaldo, R.; et al. Exogenous high-mobility group box 1 protein induces myocardial regeneration after infarction via enhanced cardiac C-kit+ cell proliferation and differentiation. Circ. Res. 2005, 97, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Abarbanell, A.M.; Hartley, J.A.; Herrmann, J.L.; Weil, B.R.; Wang, Y.; Manukyan, M.C.; Poynter, J.A.; Meldrum, D.R. Exogenous high-mobility group box 1 improves myocardial recovery after acute global ischemia/reperfusion injury. Surgery 2011, 149, 329–335. [Google Scholar] [CrossRef]
- Kitahara, T.; Takeishi, Y.; Harada, M.; Niizeki, T.; Suzuki, S.; Sasaki, T.; Ishino, M.; Bilim, O.; Nakajima, O.; Kubota, I. High-mobility group box 1 restores cardiac function after myocardial infarction in transgenic mice. Cardiovasc. Res. 2008, 80, 40–46. [Google Scholar] [CrossRef]
- Calogero, S.; Grassi, F.; Aguzzi, A.; Voigtlander, T.; Ferrier, P.; Ferrari, S.; Bianchi, M.E. The lack of chromosomal protein Hmg1 does not disrupt cell growth but causes lethal hypoglycaemia in newborn mice. Nat. Genet. 1999, 22, 276–280. [Google Scholar] [CrossRef]
- Yao, L.; Lv, X.; Wang, X. MicroRNA 26a inhibits HMGB1 expression and attenuates cardiac ischemia-reperfusion injury. J. Pharmacol. Sci. 2016, 131, 6–12. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ouyang, M.; Wang, Q.; Jian, Z. MicroRNA-142-3p inhibits hypoxia/reoxygenationinduced apoptosis and fibrosis of cardiomyocytes by targeting high mobility group box 1. Int. J. Mol. Med. 2016, 38, 1377–1386. [Google Scholar] [CrossRef] [Green Version]
- ESICM LIVES 2018: Paris, France. 20-24 October 2018. Intensive Care Med. Exp. 2018, 6, 40. [CrossRef] [Green Version]
- Hawchar, F.; Laszlo, I.; Oveges, N.; Trasy, D.; Ondrik, Z.; Molnar, Z. Extracorporeal cytokine adsorption in septic shock: A proof of concept randomized, controlled pilot study. J. Crit. Care 2019, 49, 172–178. [Google Scholar] [CrossRef] [Green Version]
- Gruda, M.C.; Ruggeberg, K.-G.; O’Sullivan, P.; Guliashvili, T.; Scheirer, A.R.; Golobish, T.D.; Capponi, V.J.; Chan, P.P. Broad adsorption of sepsis-related PAMP and DAMP molecules, mycotoxins, and cytokines from whole blood using CytoSorb(R) sorbent porous polymer beads. PLoS ONE 2018, 13, e0191676. [Google Scholar] [CrossRef]
- Ekaney, M.L.; Otto, G.P.; Sossdorf, M.; Sponholz, C.; Boehringer, M.; Loesche, W.; Rittirsch, D.; Wilharm, A.; Kurzai, O.; Bauer, M.; et al. Impact of plasma histones in human sepsis and their contribution to cellular injury and inflammation. Crit. Care 2014, 18, 543. [Google Scholar] [CrossRef] [Green Version]
- Silk, E.; Zhao, H.; Weng, H.; Ma, D. The role of extracellular histone in organ injury. Cell Death Dis. 2017, 8, e2812. [Google Scholar] [CrossRef] [Green Version]
- Zima, A.V.; Blatter, L.A. Redox regulation of cardiac calcium channels and transporters. Cardiovasc. Res. 2006, 71, 310–321. [Google Scholar] [CrossRef]
- Xu, K.Y.; Zweier, J.L.; Becker, L.C. Hydroxyl radical inhibits sarcoplasmic reticulum Ca(2+)-ATPase function by direct attack on the ATP binding site. Circ. Res. 1997, 80, 76–81. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, X.; Pelayo, R.; Monestier, M.; Ammollo, C.T.; Semeraro, F.; Taylor, F.B.; Esmon, N.L.; Lupu, F.; Esmon, C.T. Extracellular histones are major mediators of death in sepsis. Nat. Med. 2009, 15, 1318–1321. [Google Scholar] [CrossRef] [Green Version]
- Kleine, T.J.; Lewis, P.N.; Lewis, S.A. Histone-induced damage of a mammalian epithelium: The role of protein and membrane structure. Am. J. Physiol. 1997, 273, 1925–1936. [Google Scholar] [CrossRef]
- Kleine, T.J.; Gladfelter, A.; Lewis, P.N.; Lewis, S.A. Histone-induced damage of a mammalian epithelium: The conductive effect. Am. J. Physiol. 1995, 268, 1114–1125. [Google Scholar] [CrossRef]
- Alhamdi, Y.; Abrams, S.T.; Cheng, Z.; Jing, S.; Su, D.; Liu, Z.; Lane, S.; Welters, I.; Wang, G.; Toh, C.-H. Circulating Histones Are Major Mediators of Cardiac Injury in Patients with Sepsis. Crit. Care Med. 2015, 43, 2094–2103. [Google Scholar] [CrossRef]
- Bosmann, M.; Ward, P.A. Protein-based therapies for acute lung injury: Targeting neutrophil extracellular traps. Expert Opin. Ther. Targets 2014, 18, 703–714. [Google Scholar] [CrossRef] [Green Version]
- Lackner, I.; Weber, B.; Baur, M.; Haffner-Luntzer, M.; Eiseler, T.; Fois, G.; Gebhard, F.; Relja, B.; Marzi, I.; Pfeifer, R.; et al. Midkine Is Elevated After Multiple Trauma and Acts Directly on Human Cardiomyocytes by Altering Their Functionality and Metabolism. Front. Immunol. 2019, 10, 1920. [Google Scholar] [CrossRef]
- Lackner, I.; Weber, B.; Knecht, D.; Horst, K.; Relja, B.; Gebhard, F.; Pape, H.-C.; Huber-Lang, M.; Hildebrand, F.; Kalbitz, M. Cardiac Glucose and Fatty Acid Transport After Experimental Mono- and Polytrauma. Shock 2019. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weber, B.; Lackner, I.; Baur, M.; Fois, G.; Gebhard, F.; Marzi, I.; Schrezenmeier, H.; Relja, B.; Kalbitz, M. Effects of Circulating HMGB-1 and Histones on Cardiomyocytes–Hemadsorption of These DAMPs as Therapeutic Strategy after Multiple Trauma. J. Clin. Med. 2020, 9, 1421. https://doi.org/10.3390/jcm9051421
Weber B, Lackner I, Baur M, Fois G, Gebhard F, Marzi I, Schrezenmeier H, Relja B, Kalbitz M. Effects of Circulating HMGB-1 and Histones on Cardiomyocytes–Hemadsorption of These DAMPs as Therapeutic Strategy after Multiple Trauma. Journal of Clinical Medicine. 2020; 9(5):1421. https://doi.org/10.3390/jcm9051421
Chicago/Turabian StyleWeber, Birte, Ina Lackner, Meike Baur, Giorgio Fois, Florian Gebhard, Ingo Marzi, Hubert Schrezenmeier, Borna Relja, and Miriam Kalbitz. 2020. "Effects of Circulating HMGB-1 and Histones on Cardiomyocytes–Hemadsorption of These DAMPs as Therapeutic Strategy after Multiple Trauma" Journal of Clinical Medicine 9, no. 5: 1421. https://doi.org/10.3390/jcm9051421




