Engagement in Lifestyle Activities is Associated with Increased Alzheimer’s Disease-Associated Cortical Thickness and Cognitive Performance in Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Lifestyle Activities Questionnaire
2.3. MRI Acquisition
2.4. Cortical Thickness Measurement
2.5. Cognitive Performance
2.6. Statistical Analysis
3. Results
3.1. Participants Characteristics
3.2. Association of AD Signature Cortical Thickness and Cognitive Function with Lifestyle Activities
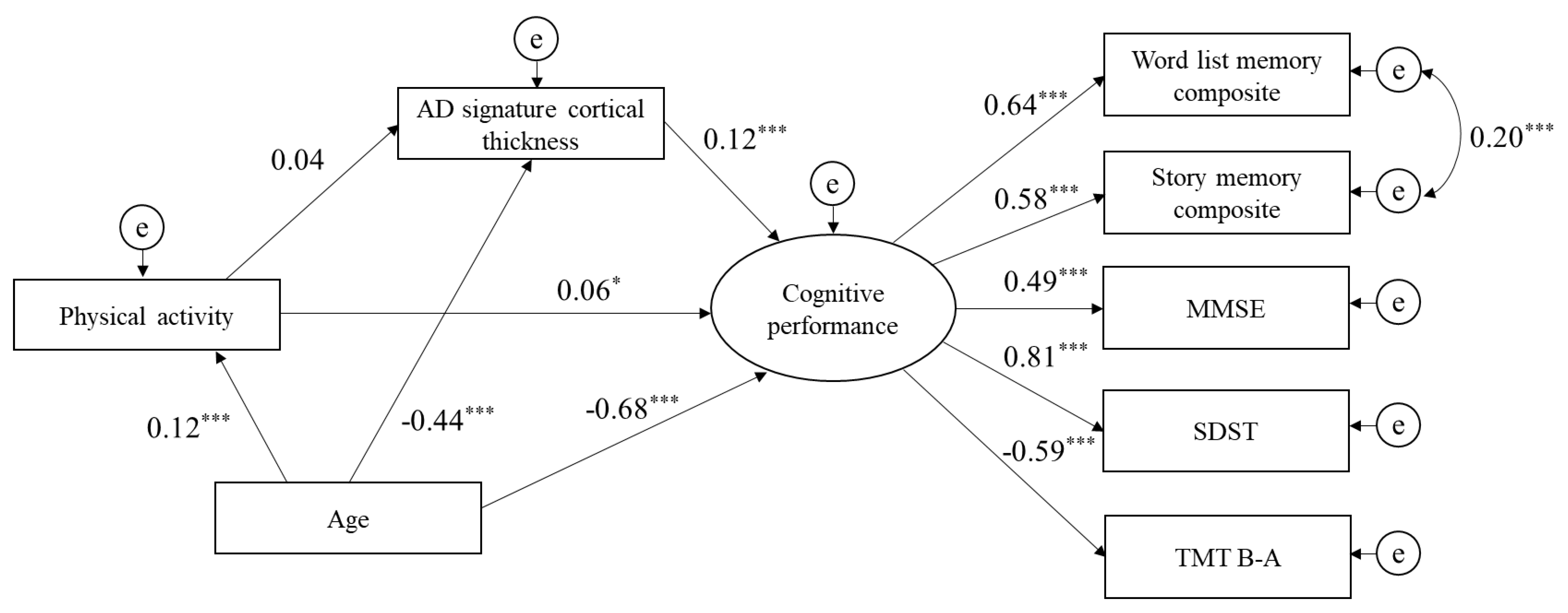
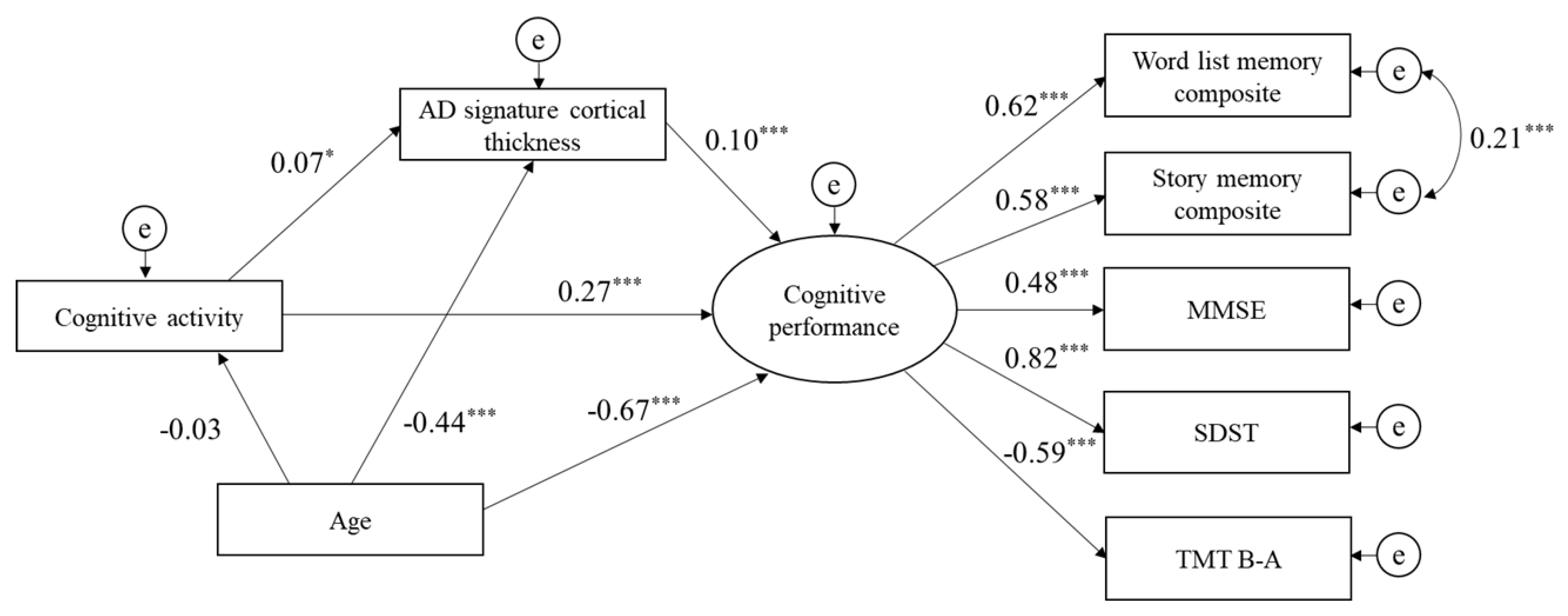
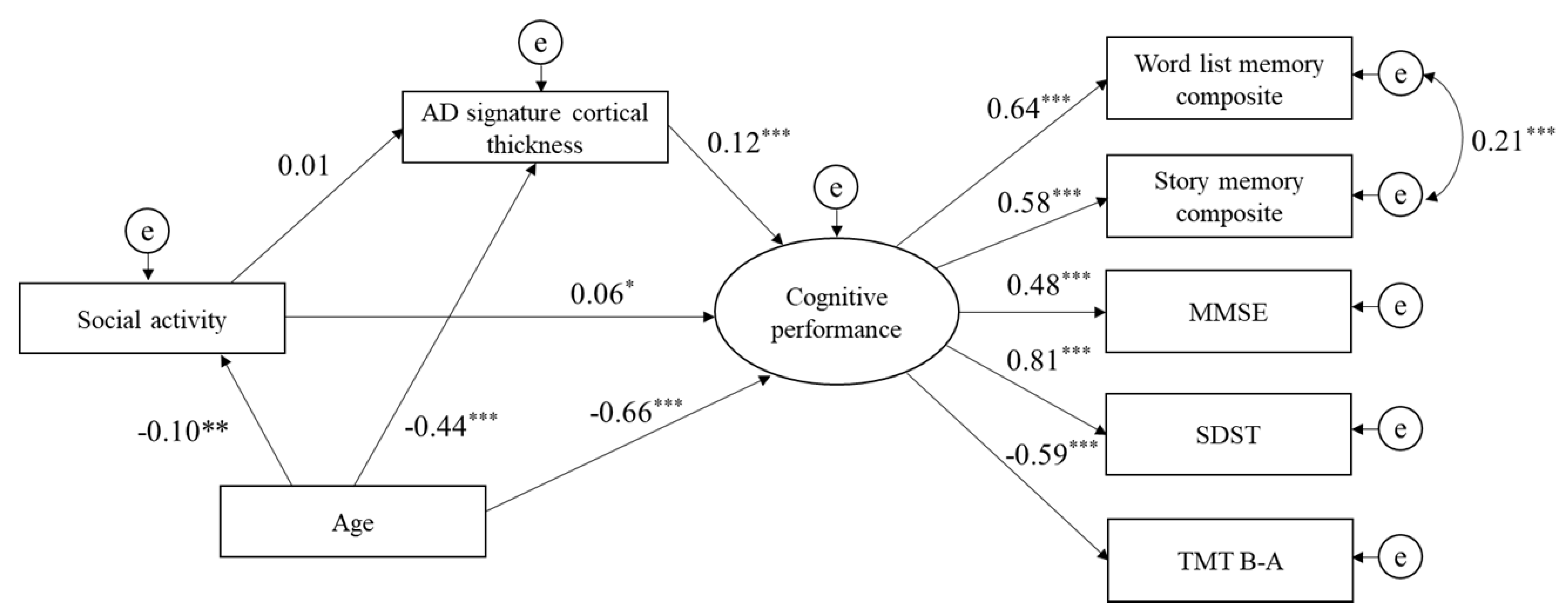
3.3. Structural Equation Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Association. Alzheimer’s Disease Facts and Figures; 1552–5279 (Electronic) 1552–5260 (Linking); Elsevier Science: Amsterdam, The Netherlands, 2015; pp. 332–384. [Google Scholar]
- Carlson, M.C.; Parisi, J.M.; Xia, J.; Xue, Q.L.; Rebok, G.W.; Bandeen-Roche, K.; Fried, L.P. Lifestyle activities and memory: Variety may be the spice of life. The women’s health and aging study II. J. Int. Neuropsychol. Soc. 2012, 18, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Carlson, M.C.; McGill, S.; Seeman, T.; Xue, Q.L.; Frick, K.; Tan, E.; Tanner, E.K.; Barron, J.; Frangakis, C.; et al. Experience Corps: A dual trial to promote the health of older adults and children’s academic success. Contemp. Clin. Trials 2013, 36, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gow, A.J.; Pattie, A.; Deary, I.J. Lifecourse Activity Participation From Early, Mid, and Later Adulthood as Determinants of Cognitive Aging: The Lothian Birth Cohort 1921. J. Gerontol. B Psychol. Sci. Soc. Sci. 2017, 72, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Carlson, M.C.; Kuo, J.H.; Chuang, Y.F.; Varma, V.R.; Harris, G.; Albert, M.S.; Erickson, K.I.; Kramer, A.F.; Parisi, J.M.; Xue, Q.L.; et al. Impact of the Baltimore Experience Corps Trial on cortical and hippocampal volumes. Alzheimers Dement. 2015, 11, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Suo, C.; Leon, I.; Brodaty, H.; Trollor, J.; Wen, W.; Sachdev, P.; Valenzuela, M.J. Supervisory experience at work is linked to low rate of hippocampal atrophy in late life. Neuroimage 2012, 63, 1542–1551. [Google Scholar] [CrossRef]
- Valenzuela, M.J.; Sachdev, P.; Wen, W.; Chen, X.; Brodaty, H. Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS ONE 2008, 3, e2598. [Google Scholar] [CrossRef]
- Barnes, L.L.; Mendes de Leon, C.F.; Wilson, R.S.; Bienias, J.L.; Evans, D.A. Social resources and cognitive decline in a population of older African Americans and whites. Neurology 2004, 63, 2322–2326. [Google Scholar] [CrossRef]
- McGue, M.; Christensen, K. Social activity and healthy aging: A study of aging Danish twins. Twin Res. Hum. Genet. 2007, 10, 255–265. [Google Scholar] [CrossRef]
- Newson, R.S.; Kemps, E.B. General lifestyle activities as a predictor of current cognition and cognitive change in older adults: A cross-sectional and longitudinal examination. J. Gerontol. B Psychol. Sci. Soc. Sci. 2005, 60, P113–P120. [Google Scholar] [CrossRef]
- Opdebeeck, C.; Martyr, A.; Clare, L. Cognitive reserve and cognitive function in healthy older people: A meta-analysis. NeuroPsychol. Dev Cogn. B Aging NeuroPsychol. Cogn. 2016, 23, 40–60. [Google Scholar] [CrossRef]
- Vemuri, P.; Lesnick, T.G.; Przybelski, S.A.; Knopman, D.S.; Roberts, R.O.; Lowe, V.J.; Kantarci, K.; Senjem, M.L.; Gunter, J.L.; Boeve, B.F.; et al. Effect of lifestyle activities on Alzheimer disease biomarkers and cognition. Ann. Neurol. 2012, 72, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Bugg, J.M.; Head, D. Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiol. Aging 2011, 32, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Makizako, H.; Shimada, H.; Tsutsumimoto, K.; Hotta, R.; Nakakubo, S.; Park, H.; Suzuki, T. Objectively measured physical activity, brain atrophy, and white matter lesions in older adults with mild cognitive impairment. Exp. Gerontol. 2015, 62, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rovio, S.; Spulber, G.; Nieminen, L.J.; Niskanen, E.; Winblad, B.; Tuomilehto, J.; Nissinen, A.; Soininen, H.; Kivipelto, M. The effect of midlife physical activity on structural brain changes in the elderly. Neurobiol. Aging 2010, 31, 1927–1936. [Google Scholar] [CrossRef]
- Colcombe, S.J.; Erickson, K.I.; Scalf, P.E.; Kim, J.S.; Prakash, R.; McAuley, E.; Elavsky, S.; Marquez, D.X.; Hu, L.; Kramer, A.F. Aerobic exercise training increases brain volume in aging humans. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1166–1170. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]
- Schultz, S.A.; Larson, J.; Oh, J.; Koscik, R.; Dowling, M.N.; Gallagher, C.L.; Carlsson, C.M.; Rowley, H.A.; Bendlin, B.B.; Asthana, S.; et al. Participation in cognitively-stimulating activities is associated with brain structure and cognitive function in preclinical Alzheimer’s disease. Brain Imaging Behav. 2015, 9, 729–736. [Google Scholar] [CrossRef][Green Version]
- Stine-Morrow, E.A.; Parisi, J.M.; Morrow, D.G.; Park, D.C. The effects of an engaged lifestyle on cognitive vitality: A field experiment. Psychol. Aging 2008, 23, 778–786. [Google Scholar] [CrossRef]
- Gow, A.J.; Bastin, M.E.; Munoz Maniega, S.; Valdes Hernandez, M.C.; Morris, Z.; Murray, C.; Royle, N.A.; Starr, J.M.; Deary, I.J.; Wardlaw, J.M. Neuroprotective lifestyles and the aging brain: Activity, atrophy, and white matter integrity. Neurology 2012, 79, 1802–1808. [Google Scholar] [CrossRef]
- James, B.D.; Glass, T.A.; Caffo, B.; Bobb, J.F.; Davatzikos, C.; Yousem, D.; Schwartz, B.S. Association of social engagement with brain volumes assessed by structural MRI. J. Aging Res. 2012, 2012, 512714. [Google Scholar] [CrossRef]
- Parent, A.; Carpenter, M.B. Human Neuroanatomy; Williams & Wilkins: Baltimore, MD, USA, 1995. [Google Scholar]
- Fischer, B.; Dale, A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. USA 2000, 97, 11050–11055. [Google Scholar] [CrossRef]
- Lerch, J.P.; Evans, A.C. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage 2005, 24, 163–173. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Wiste, H.J.; Weigand, S.D.; Knopman, D.S.; Mielke, M.M.; Vemuri, P.; Lowe, V.; Senjem, M.L.; Gunter, J.L.; Reyes, D.; et al. Different definitions of neurodegeneration produce similar amyloid/neurodegeneration biomarker group findings. Brain 2015, 138, 3747–3759. [Google Scholar] [CrossRef] [PubMed]
- Lezak, M.D.; Lezak, M.D. Neuropsychological Assessment, 4th ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2004; p. xiv. 1016p. [Google Scholar]
- Dickerson, B.C.; Fenstermacher, E.; Salat, D.H.; Wolk, D.A.; Maguire, R.P.; Desikan, R.; Pacheco, J.; Quinn, B.T.; Van der Kouwe, A.; Greve, D.N.; et al. Detection of cortical thickness correlates of cognitive performance: Reliability across MRI scan sessions, scanners, and field strengths. Neuroimage 2008, 39, 10–18. [Google Scholar] [CrossRef]
- MacPherson, S.E.; Cox, S.R.; Dickie, D.A.; Karama, S.; Starr, J.M.; Evans, A.C.; Bastin, M.E.; Wardlaw, J.M.; Deary, I.J. Processing speed and the relationship between Trail Making Test-B performance, cortical thinning and white matter microstructure in older adults. Cortex 2017, 95, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Kranz, M.B.; Voss, M.W.; Cooke, G.E.; Banducci, S.E.; Burzynska, A.Z.; Kramer, A.F. The cortical structure of functional networks associated with age-related cognitive abilities in older adults. PLoS ONE 2018, 13, e0204280. [Google Scholar] [CrossRef] [PubMed]
- Pettigrew, C.; Soldan, A.; Zhu, Y.; Wang, M.C.; Moghekar, A.; Brown, T.; Miller, M.; Albert, M.; Team, B.R. Cortical thickness in relation to clinical symptom onset in preclinical AD. Neuroimage Clin. 2016, 12, 116–122. [Google Scholar] [CrossRef]
- Dubois, B.; Hampel, H.; Feldman, H.H.; Scheltens, P.; Aisen, P.; Andrieu, S.; Bakardjian, H.; Benali, H.; Bertram, L.; Blennow, K.; et al. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016, 12, 292–323. [Google Scholar] [CrossRef]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R., Jr.; Kaye, J.; Montine, T.J.; et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef]
- Fagan, A.M.; Head, D.; Shah, A.R.; Marcus, D.; Mintun, M.; Morris, J.C.; Holtzman, D.M. Decreased cerebrospinal fluid Abeta(42) correlates with brain atrophy in cognitively normal elderly. Ann. Neurol. 2009, 65, 176–183. [Google Scholar] [CrossRef]
- Storandt, M.; Mintun, M.A.; Head, D.; Morris, J.C. Cognitive decline and brain volume loss as signatuRes. of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: Cognitive decline associated with Abeta deposition. Arch Neurol. 2009, 66, 1476–1481. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Petersen, R.C.; O’Brien, P.C.; Tangalos, E.G. MR-based hippocampal volumetry in the diagnosis of Alzheimer’s disease. Neurology 1992, 42, 183–188. [Google Scholar] [CrossRef] [PubMed]
- van Maurik, I.S.; Zwan, M.D.; Tijms, B.M.; Bouwman, F.H.; Teunissen, C.E.; Scheltens, P.; Wattjes, M.P.; Barkhof, F.; Berkhof, J.; van der Flier, W.M.; et al. Interpreting Biomarker Results in Individual Patients With Mild Cognitive Impairment in the Alzheimer’s Biomarkers in Daily Practice (ABIDE) Project. JAMA Neurol. 2017, 74, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, B.C.; Wolk, D.A.; Alzheimer’s Disease Neuroimaging Initiative. MRI cortical thickness biomarker predicts AD-like CSF and cognitive decline in normal adults. Neurology 2012, 78, 84–90. [Google Scholar] [CrossRef]
- Schwarz, C.G.; Gunter, J.L.; Wiste, H.J.; Przybelski, S.A.; Weigand, S.D.; Ward, C.P.; Senjem, M.L.; Vemuri, P.; Murray, M.E.; Dickson, D.W.; et al. A large-scale comparison of cortical thickness and volume methods for measuring Alzheimer’s disease severity. Neuroimage Clin. 2016, 11, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Bakkour, A.; Morris, J.C.; Dickerson, B.C. The cortical signature of prodromal AD: Regional thinning predicts mild AD dementia. Neurology 2009, 72, 1048–1055. [Google Scholar] [CrossRef]
- Busovaca, E.; Zimmerman, M.E.; Meier, I.B.; Griffith, E.Y.; Grieve, S.M.; Korgaonkar, M.S.; Williams, L.M.; Brickman, A.M. Is the Alzheimer’s disease cortical thickness signature a biological marker for memory? Brain Imaging Behav. 2016, 10, 517–523. [Google Scholar] [CrossRef]
- Dickerson, B.C.; Bakkour, A.; Salat, D.H.; Feczko, E.; Pacheco, J.; Greve, D.N.; Grodstein, F.; Wright, C.I.; Blacker, D.; Rosas, H.D.; et al. The cortical signature of Alzheimer’s disease: Regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb. Cortex 2009, 19, 497–510. [Google Scholar] [CrossRef]
- Dickerson, B.C.; Stoub, T.R.; Shah, R.C.; Sperling, R.A.; Killiany, R.J.; Albert, M.S.; Hyman, B.T.; Blacker, D.; Detoledo-Morrell, L. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology 2011, 76, 1395–1402. [Google Scholar] [CrossRef]
- Dickerson, B.C.; Wolk, D.A.; Alzheimer’s Disease Neuroimaging Initiative. Dysexecutive versus amnesic phenotypes of very mild Alzheimer’s disease are associated with distinct clinical, genetic and cortical thinning characteristics. J. Neurol. Neurosurg. Psychiatry 2011, 82, 45–51. [Google Scholar] [CrossRef]
- Putcha, D.; Brickhouse, M.; O’Keefe, K.; Sullivan, C.; Rentz, D.; Marshall, G.; Dickerson, B.; Sperling, R. Hippocampal hyperactivation associated with cortical thinning in Alzheimer’s disease signature regions in non-demented elderly adults. J. NeuroSci. 2011, 31, 17680–17688. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, B.C.; Wolk, D.A.; Alzheimer’s Disease Neuroimaging Initiative. Biomarker-based prediction of progression in MCI: Comparison of AD signature and hippocampal volume with spinal fluid amyloid-beta and tau. Front. Aging NeuroSci. 2013, 5, 55. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Makizako, H.; Doi, T.; Tsutsumimoto, K.; Lee, S.; Suzuki, T. Cognitive Impairment and Disability in Older Japanese Adults. PLoS ONE 2016, 11, e0158720. [Google Scholar] [CrossRef] [PubMed]
- Foundation Sasakawa Sports. The 2014 SSF National Sports-Life Survey: Sports Life Data; Sasakawa Sports Foundation: Tokyo, Japan, 2014. [Google Scholar]
- Scarmeas, N.; Levy, G.; Tang, M.X.; Manly, J.; Stern, Y. Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology 2001, 57, 2236–2242. [Google Scholar] [CrossRef] [PubMed]
- Schinka, J.A.; McBride, A.; Vanderploeg, R.D.; Tennyson, K.; Borenstein, A.R.; Mortimer, J.A. Florida Cognitive Activities Scale: Initial development and validation. J. Int. NeuroPsychol. Soc. 2005, 11, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Verghese, J.; Lipton, R.B.; Katz, M.J.; Hall, C.B.; Derby, C.A.; Kuslansky, G.; Ambrose, A.F.; Sliwinski, M.; Buschke, H. Leisure activities and the risk of dementia in the elderly. N. Engl. J. Med. 2003, 348, 2508–2516. [Google Scholar] [CrossRef]
- Office, C. Annual Report on the Aging Society; Cabinet Office: Tokyo, Japan, 2013. [Google Scholar]
- Hashimoto, S.; Aoki, R.; Tamakoshi, A.; Shibazaki, S.; Nagai, M.; Kawakami, N.; Ikari, A.; Ojima, T.; Ohno, Y. Development of index of social activities for the elderly. Nihon Koshu Eisei Zasshi 1997, 44, 760–768. [Google Scholar]
- Fischl, B. FreeSurfer. Neuroimage 2012, 62, 774–781. [Google Scholar] [CrossRef]
- Desikan, R.S.; Segonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006, 31, 968–980. [Google Scholar] [CrossRef]
- Lin, F.; Ren, P.; Wang, X.; Anthony, M.; Tadin, D.; Heffner, K.L. Cortical thickness is associated with altered autonomic function in cognitively impaired and non-impaired older adults. J. Physiol. 2017, 595, 6969–6978. [Google Scholar] [CrossRef]
- Makizako, H.; Shimada, H.; Park, H.; Doi, T.; Yoshida, D.; Uemura, K.; Tsutsumimoto, K.; Suzuki, T. Evaluation of multidimensional neurocognitive function using a tablet personal computer: Test-retest reliability and validity in community-dwelling older adults. Geriatr. Gerontol. Int. 2013, 13, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Vazzana, R.; Bandinelli, S.; Lauretani, F.; Volpato, S.; Lauretani, F.; Di Iorio, A.; Abate, M.; Corsi, A.M.; Milaneschi, Y.; Guralnik, J.M.; et al. Trail Making Test predicts physical impairment and mortality in older persons. J. Am. Geriatr. Soc. 2010, 58, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. Mini-mental state. J. Psychiat. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Beran, T.N.; Violato, C. Structural equation modeling in medical research: A primer. BMC Res. Notes 2010, 3, 267. [Google Scholar] [CrossRef]
- Barrett, P. Structural equation modelling: Adjudging model fit. Personal. Individ. Differ. 2007, 42, 815–824. [Google Scholar] [CrossRef]
- Steiger, J.H. Understanding the limitations of global fit assessment in structural equation modeling. Personal. Individ. Differ. 2007, 42, 893–898. [Google Scholar] [CrossRef]
- Newson, R.S.; Kemps, E.B. The influence of physical and cognitive activities on simple and complex cognitive tasks in older adults. Exp. Aging Res. 2006, 32, 341–362. [Google Scholar] [CrossRef]
- Craik, F.I.; Winocur, G.; Palmer, H.; Binns, M.A.; Edwards, M.; Bridges, K.; Glazer, P.; Chavannes, R.; Stuss, D.T. Cognitive rehabilitation in the elderly: Effects on memory. J. Int. NeuroPsychol. Soc. 2007, 13, 132–142. [Google Scholar] [CrossRef]
- Ghisletta, P.; Bickel, J.F.; Lovden, M. Does activity engagement protect against cognitive decline in old age? Methodological and analytical considerations. J. Gerontol. B Psychol. Sci. Soc. Sci. 2006, 61, P253–P261. [Google Scholar] [CrossRef]
- Wilson, R.; Barnes, L.; Bennett, D. Assessment of lifetime participation in cognitively stimulating activities. J. Clin. Exp. NeuroPsychol. 2003, 25, 634–642. [Google Scholar] [CrossRef]
- Engvig, A.; Fjell, A.M.; Westlye, L.T.; Moberget, T.; Sundseth, O.; Larsen, V.A.; Walhovd, K.B. Effects of memory training on cortical thickness in the elderly. Neuroimage 2010, 52, 1667–1676. [Google Scholar] [CrossRef]
- Jiang, L.; Cao, X.; Li, T.; Tang, Y.; Li, W.; Wang, J.; Chan, R.C.; Li, C. Cortical Thickness Changes Correlate with Cognition Changes after Cognitive Training: Evidence from a Chinese Community Study. Front. Aging NeuroSci. 2016, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Maviel, T.; Durkin, T.P.; Menzaghi, F.; Bontempi, B. Sites of neocortical reorganization critical for remote spatial memory. Science 2004, 305, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Trachtenberg, J.T.; Chen, B.E.; Knott, G.W.; Feng, G.; Sanes, J.R.; Welker, E.; Svoboda, K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature 2002, 420, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y. What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 2002, 8, 448–460. [Google Scholar] [CrossRef]
- Lee, J.S.; Shin, H.Y.; Kim, H.J.; Jang, Y.K.; Jung, N.Y.; Lee, J.; Kim, Y.J.; Chun, P.; Yang, J.J.; Lee, J.M.; et al. Combined effects of physical exercise and education on age-related cortical thinning in cognitively normal individuals. Sci. Rep. 2016, 6, 24284. [Google Scholar] [CrossRef]
- Walhovd, K.B.; Storsve, A.B.; Westlye, L.T.; Drevon, C.A.; Fjell, A.M. Blood markers of fatty acids and vitamin D, cardiovascular measures, body mass index, and physical activity relate to longitudinal cortical thinning in normal aging. Neurobiol. Aging 2014, 35, 1055–1064. [Google Scholar] [CrossRef]
- Seider, T.R.; Fieo, R.A.; O’Shea, A.; Porges, E.C.; Woods, A.J.; Cohen, R.A. Cognitively Engaging Activity Is Associated with Greater Cortical and Subcortical Volumes. Front. Aging NeuroSci. 2016, 8, 94. [Google Scholar] [CrossRef]
| Characteristic | Mean or % (SD) | Range |
|---|---|---|
| Age, years | 70.6 (6.3) | 60 to 95 |
| Sex, male | 51.1 | |
| Physical activity, score | 8.5 (6.2) | 0 to 34 |
| Cognitive activity, score | 15.4 (7.7) | 0 to 46 |
| Social activity, score | 11.6 (6.4) | 0 to 41 |
| AD signature cortical thickness, mm | 2.95 (0.13) | 2.29 to 3.33 |
| Entorhinal, mm | 3.47 (0.28) | 2.06 to 4.30 |
| Inferior temporal, mm | 2.81 (0.12) | 2.22 to 3.25 |
| Middle temporal, mm | 2.78 (0.12) | 2.34 to 3.21 |
| Fusiform, mm | 2.72 (0.13) | 2.30 to 3.10 |
| Word List Memory test composite, score | 11.5 (3.1) | 1.3 to 20.0 |
| Story Memory test composite, score | 14.0 (3.6) | 0 to 20 |
| MMSE, score | 27.7 (2.2) | 16 to 30 |
| SDST, score | 46.9 (9.7) | 8 to 78 |
| TMT B-A, second | 19.4 (17.3) | −9 to 186 |
| Partial Correlation Coefficient | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| 1. Physical activity, score | ||||||||
| 2. Cognitive activity, score | 0.187 *** | |||||||
| 3. Social activity, score | 0.218 *** | 0.304 *** | ||||||
| 4. AD signature cortical thickness, mm | 0.048 | 0.079 ** | 0.009 | |||||
| 5. Word List Memory test composite, score | 0.058 | 0.156 *** | 0.051 | 0.15 *** | ||||
| 6. Story Memory test composite, score | 0.035 | 0.209 *** | 0.021 | 0.07 * | 0.382 *** | |||
| 7. MMSE, score | 0.087 ** | 0.146 *** | −0.009 | 0.051 | 0.289 *** | 0.28 *** | ||
| 8. SDST, score | 0.037 | 0.28 *** | 0.069 * | 0.11 *** | 0.345 *** | 0.239 *** | 0.251 *** | |
| 9. TMT B-A, second | −0.044 | −0.182 *** | −0.044 | −0.025 | −0.297 *** | −0.226 *** | −0.265 *** | −0.313 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, S.; Lee, S.; Harada, K.; Makino, K.; Chiba, I.; Katayama, O.; Shinkai, Y.; Park, H.; Shimada, H. Engagement in Lifestyle Activities is Associated with Increased Alzheimer’s Disease-Associated Cortical Thickness and Cognitive Performance in Older Adults. J. Clin. Med. 2020, 9, 1424. https://doi.org/10.3390/jcm9051424
Bae S, Lee S, Harada K, Makino K, Chiba I, Katayama O, Shinkai Y, Park H, Shimada H. Engagement in Lifestyle Activities is Associated with Increased Alzheimer’s Disease-Associated Cortical Thickness and Cognitive Performance in Older Adults. Journal of Clinical Medicine. 2020; 9(5):1424. https://doi.org/10.3390/jcm9051424
Chicago/Turabian StyleBae, Seongryu, Sangyoon Lee, Kenji Harada, Keitaro Makino, Ippei Chiba, Osamu Katayama, Yohei Shinkai, Hyuntae Park, and Hiroyuki Shimada. 2020. "Engagement in Lifestyle Activities is Associated with Increased Alzheimer’s Disease-Associated Cortical Thickness and Cognitive Performance in Older Adults" Journal of Clinical Medicine 9, no. 5: 1424. https://doi.org/10.3390/jcm9051424
APA StyleBae, S., Lee, S., Harada, K., Makino, K., Chiba, I., Katayama, O., Shinkai, Y., Park, H., & Shimada, H. (2020). Engagement in Lifestyle Activities is Associated with Increased Alzheimer’s Disease-Associated Cortical Thickness and Cognitive Performance in Older Adults. Journal of Clinical Medicine, 9(5), 1424. https://doi.org/10.3390/jcm9051424







