Role of Endogenous Regulators of Hem- And Lymphangiogenesis in Corneal Transplantation
Abstract
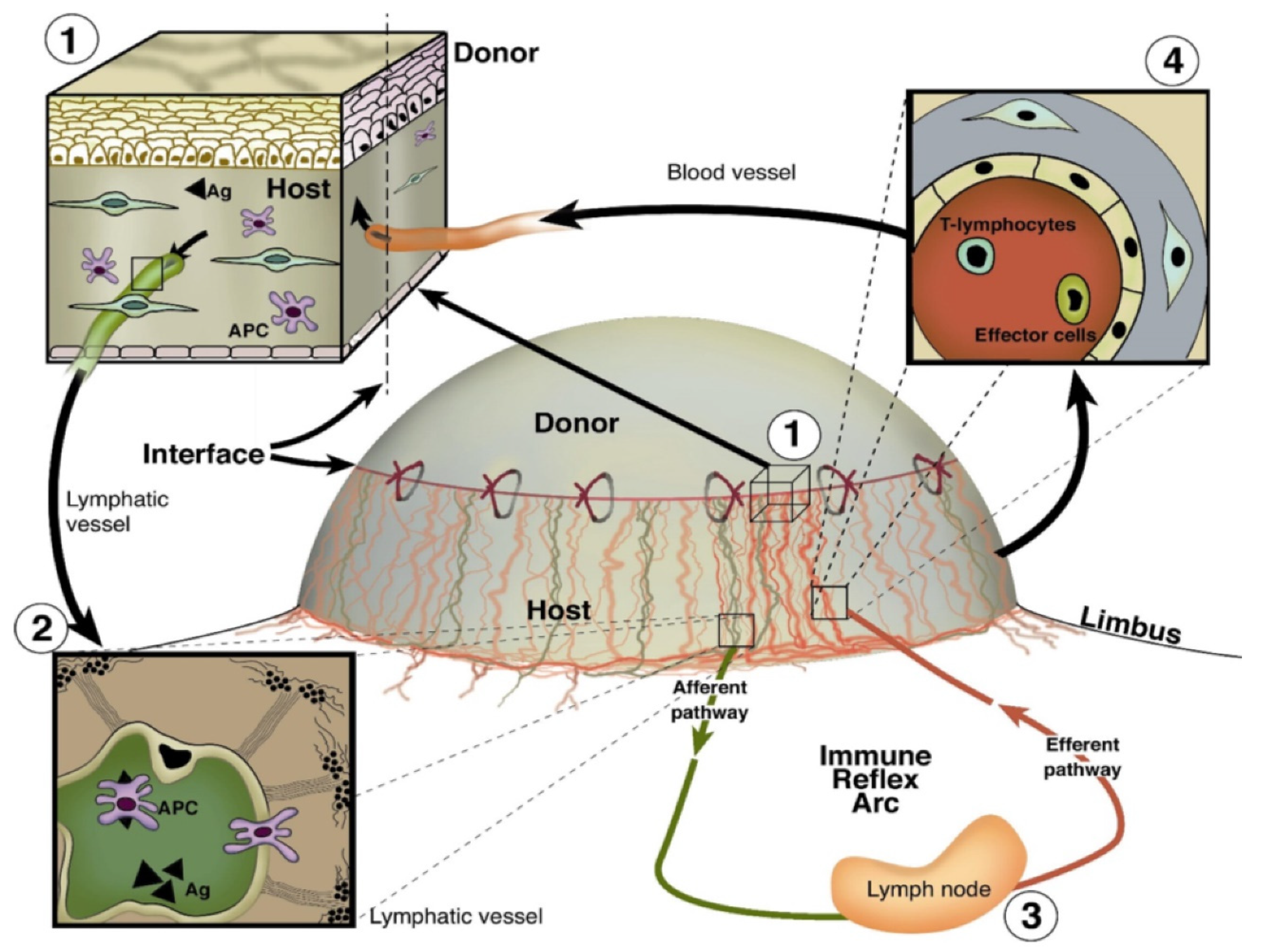
1. Introduction
2. Endogenous Regulators of (lymph)angiogenesis
3. Endogenous Regulators of Lymphangiogenesis in Corneal Transplantation
4. Identification of Novel Endogenous Regulators of Lymphangiogenesis
4.1. Proteins and Peptides in Lymphangiogenesis
4.2. Non-Coding RNAs in Lymphangiogenesis
5. Murine Cornea is a Suitable Model for Identification of Novel Endogenous Modulators of Lymphangiogenesis
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cursiefen, C.; Schlotzer-Schrehardt, U.; Kuchle, M.; Sorokin, L.; Breiteneder-Geleff, S.; Alitalo, K.; Jackson, D. Lymphatic vessels in vascularized human corneas: Immunohistochemical investigation using LYVE-1 and podoplanin. Invest. Ophthalmol Vis. Sci 2002, 43, 2127–2135. [Google Scholar] [PubMed]
- Chen, L. Ocular lymphatics: State-of-the-art review. Lymphology 2009, 42, 66–76. [Google Scholar] [PubMed]
- Dietrich, T.; Bock, F.; Yuen, D.; Hos, D.; Bachmann, B.O.; Zahn, G.; Wiegand, S.; Chen, L.; Cursiefen, C. Cutting edge: Lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J. Immunol 2010, 184, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Chen, L.; Dana, M.R.; Streilein, J.W. Corneal lymphangiogenesis: Evidence, mechanisms, and implications for corneal transplant immunology. Cornea 2003, 22, 273–281. [Google Scholar] [CrossRef]
- Streilein, J.W. Ocular immune privilege: Therapeutic opportunities from an experiment of nature. Nat. Rev. Immunol 2003, 3, 879–889. [Google Scholar] [CrossRef]
- Oh, S.J.; Jeltsch, M.M.; Birkenhager, R.; McCarthy, J.E.; Weich, H.A.; Christ, B.; Alitalo, K.; Wilting, J. VEGF and VEGF-C: Specific induction of angiogenesis and lymphangiogenesis in the differentiated avian chorioallantoic membrane. Dev. Biol 1997, 188, 96–109. [Google Scholar] [CrossRef]
- Otrock, Z.K.; Makarem, J.A.; Shamseddine, A.I. Vascular endothelial growth factor family of ligands and receptors: Review. Blood Cells Mol. Dis 2007, 38, 258–268. [Google Scholar] [CrossRef]
- Vlahakis, N.E.; Young, B.A.; Atakilit, A.; Sheppard, D. The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin alpha9beta1. J. Biol Chem 2005, 280, 4544–4552. [Google Scholar] [CrossRef]
- Heldin, C.H.; Westermark, B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999, 79, 1283–1316. [Google Scholar] [CrossRef]
- Bussolino, F.; Albini, A.; Camussi, G.; Presta, M.; Viglietto, G.; Ziche, M.; Persico, G. Role of soluble mediators in angiogenesis. Eur J. Cancer 1996, 32A, 2401–2412. [Google Scholar] [CrossRef]
- Berdugo, M.; Andrieu-Soler, C.; Doat, M.; Courtois, Y.; BenEzra, D.; Behar-Cohen, F. Downregulation of IRS-1 expression causes inhibition of corneal angiogenesis. Invest. Ophthalmol Vis. Sci 2005, 46, 4072–4078. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Achen, M.G.; Jeltsch, M.; Kukk, E.; Makinen, T.; Vitali, A.; Wilks, A.F.; Alitalo, K.; Stacker, S.A. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc. Natl. Acad. Sci. USA 1998, 95, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Bock, F.; Onderka, J.; Dietrich, T.; Bachmann, B.; Kruse, F.E.; Paschke, M.; Zahn, G.; Cursiefen, C. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest. Ophthalmol Vis. Sci 2007, 48, 2545–2552. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Chen, L.; Borges, L.P.; Jackson, D.; Cao, J.; Radziejewski, C.; D’Amore, P.A.; Dana, M.R.; Wiegand, S.J.; Streilein, J.W. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Invest. 2004, 113, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Li, M.; Cursiefen, C.; Jackson, D.G.; Keino, H.; Tomita, M.; Van Rooijen, N.; Takenaka, H.; D’Amore, P.A.; Stein-Streilein, J.; et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Invest. 2005, 115, 2363–2372. [Google Scholar] [CrossRef] [PubMed]
- Pepper, M.S.; Wasi, S.; Ferrara, N.; Orci, L.; Montesano, R. In vitro angiogenic and proteolytic properties of bovine lymphatic endothelial cells. Exp Cell Res 1994, 210, 298–305. [Google Scholar] [CrossRef]
- Jitariu, A.A.; Cimpean, A.M.; Kundnani, N.R.; Raica, M. Platelet-derived growth factors induced lymphangiogenesis: Evidence, unanswered questions and upcoming challenges. Arch. Med. Sci 2015, 11, 57–66. [Google Scholar] [CrossRef]
- Tammela, T.; Saaristo, A.; Lohela, M.; Morisada, T.; Tornberg, J.; Norrmen, C.; Oike, Y.; Pajusola, K.; Thurston, G.; Suda, T.; et al. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood 2005, 105, 4642–4648. [Google Scholar] [CrossRef]
- Yuen, D.; Grimaldo, S.; Sessa, R.; Ecoiffier, T.; Truong, T.; Huang, E.; Bernas, M.; Daley, S.; Witte, M.; Chen, L. Role of angiopoietin-2 in corneal lymphangiogenesis. Invest. Ophthalmol Vis. Sci. 2014, 55, 3320–3327. [Google Scholar] [CrossRef]
- Kajiya, K.; Hirakawa, S.; Ma, B.; Drinnenberg, I.; Detmar, M. Hepatocyte growth factor promotes lymphatic vessel formation and function. EMBO J. 2005, 24, 2885–2895. [Google Scholar] [CrossRef]
- Alitalo, K.; Tammela, T.; Petrova, T.V. Lymphangiogenesis in development and human disease. Nature 2005, 438, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Nicolls, M.R.; Tian, W.; Rockson, S.G. Lymphatic Dysfunction, Leukotrienes, and Lymphedema. Annu Rev. Physiol 2018, 80, 49–70. [Google Scholar] [CrossRef] [PubMed]
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol 2009, 78, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Tecchio, C.; Micheletti, A.; Cassatella, M.A. Neutrophil-derived cytokines: Facts beyond expression. Front. Immunol 2014, 5, 508. [Google Scholar] [CrossRef]
- Peppicelli, S.; Bianchini, F.; Calorini, L. Inflammatory cytokines induce vascular endothelial growth factor-C expression in melanoma-associated macrophages and stimulate melanoma lymph node metastasis. Oncol Lett 2014, 8, 1133–1138. [Google Scholar] [CrossRef]
- Savetsky, I.L.; Ghanta, S.; Gardenier, J.C.; Torrisi, J.S.; Garcia Nores, G.D.; Hespe, G.E.; Nitti, M.D.; Kataru, R.P.; Mehrara, B.J. Th2 cytokines inhibit lymphangiogenesis. PLoS ONE 2015, 10, e0126908. [Google Scholar] [CrossRef]
- Shin, K.; Kataru, R.P.; Park, H.J.; Kwon, B.I.; Kim, T.W.; Hong, Y.K.; Lee, S.H. TH2 cells and their cytokines regulate formation and function of lymphatic vessels. Nat. Commun 2015, 6, 6196. [Google Scholar] [CrossRef]
- Ambati, B.K.; Nozaki, M.; Singh, N.; Takeda, A.; Jani, P.D.; Suthar, T.; Albuquerque, R.J.; Richter, E.; Sakurai, E.; Newcomb, M.T.; et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature 2006, 443, 993–997. [Google Scholar] [CrossRef]
- Azar, D.T. Corneal angiogenic privilege: Angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis). Trans. Am. Ophthalmol Soc. 2006, 104, 264–302. [Google Scholar]
- Cursiefen, C. Immune privilege and angiogenic privilege of the cornea. Chem Immunol Allergy 2007, 92, 50–57. [Google Scholar] [CrossRef]
- Lawler, J. The functions of thrombospondin-1 and-2. Curr Opin Cell Biol 2000, 12, 634–640. [Google Scholar] [CrossRef]
- O’Reilly, M.S.; Boehm, T.; Shing, Y.; Fukai, N.; Vasios, G.; Lane, W.S.; Flynn, E.; Birkhead, J.R.; Olsen, B.R.; Folkman, J. Endostatin: An endogenous inhibitor of angiogenesis and tumor growth. Cell 1997, 88, 277–285. [Google Scholar] [CrossRef]
- Chen, S.; Mienaltowski, M.J.; Birk, D.E. Regulation of corneal stroma extracellular matrix assembly. Exp. Eye Res. 2015, 133, 69–80. [Google Scholar] [CrossRef]
- Ishizaki, M.; Shimoda, M.; Wakamatsu, K.; Ogro, T.; Yamanaka, N.; Kao, C.W.; Kao, W.W. Stromal fibroblasts are associated with collagen IV in scar tissues of alkali-burned and lacerated corneas. Curr Eye Res. 1997, 16, 339–348. [Google Scholar] [CrossRef]
- Frikeche, J.; Maiti, G.; Chakravarti, S. Small leucine-rich repeat proteoglycans in corneal inflammation and wound healing. Exp. Eye Res. 2016, 151, 142–149. [Google Scholar] [CrossRef]
- Amjadi, S.; Mai, K.; McCluskey, P.; Wakefield, D. The role of lumican in ocular disease. ISRN Ophthalmol 2013, 2013, 632302. [Google Scholar] [CrossRef]
- Faye, C.; Moreau, C.; Chautard, E.; Jetne, R.; Fukai, N.; Ruggiero, F.; Humphries, M.J.; Olsen, B.R.; Ricard-Blum, S. Molecular interplay between endostatin, integrins, and heparan sulfate. J. Biol Chem 2009, 284, 22029–22040. [Google Scholar] [CrossRef]
- Su, J.; Stenbjorn, R.S.; Gorse, K.; Su, K.; Hauser, K.F.; Ricard-Blum, S.; Pihlajaniemi, T.; Fox, M.A. Target-derived matricryptins organize cerebellar synapse formation through alpha3beta1 integrins. Cell Rep. 2012, 2, 223–230. [Google Scholar] [CrossRef]
- Walia, A.; Yang, J.F.; Huang, Y.H.; Rosenblatt, M.I.; Chang, J.H.; Azar, D.T. Endostatin’s emerging roles in angiogenesis, lymphangiogenesis, disease, and clinical applications. Biochim Biophys Acta 2015, 1850, 2422–2438. [Google Scholar] [CrossRef]
- Bix, G.; Iozzo, R.V. Matrix revolutions: “tails” of basement-membrane components with angiostatic functions. Trends Cell Biol 2005, 15, 52–60. [Google Scholar] [CrossRef]
- Mundel, T.M.; Kalluri, R. Type IV collagen-derived angiogenesis inhibitors. Microvasc Res. 2007, 74, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Tarui, T.; Miles, L.A.; Takada, Y. Specific interaction of angiostatin with integrin alpha(v)beta(3) in endothelial cells. J. Biol Chem 2001, 276, 39562–39568. [Google Scholar] [CrossRef] [PubMed]
- Troyanovsky, B.; Levchenko, T.; Mansson, G.; Matvijenko, O.; Holmgren, L. Angiomotin: An angiostatin binding protein that regulates endothelial cell migration and tube formation. J. Cell Biol 2001, 152, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Masli, S.; Ng, T.F.; Dana, M.R.; Bornstein, P.; Lawler, J.; Streilein, J.W. Roles of thrombospondin-1 and -2 in regulating corneal and iris angiogenesis. Invest. Ophthalmol Vis. Sci 2004, 45, 1117–1124. [Google Scholar] [CrossRef]
- Lawler, J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J. Cell Mol. Med. 2002, 6, 1–12. [Google Scholar] [CrossRef]
- Kendall, R.L.; Thomas, K.A. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc. Natl. Acad. Sci. USA 1993, 90, 10705–10709. [Google Scholar] [CrossRef]
- Albuquerque, R.J.; Hayashi, T.; Cho, W.G.; Kleinman, M.E.; Dridi, S.; Takeda, A.; Baffi, J.Z.; Yamada, K.; Kaneko, H.; Green, M.G.; et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat. Med. 2009, 15, 1023–1030. [Google Scholar] [CrossRef]
- Singh, N.; Tiem, M.; Watkins, R.; Cho, Y.K.; Wang, Y.; Olsen, T.; Uehara, H.; Mamalis, C.; Luo, L.; Oakey, Z.; et al. Soluble vascular endothelial growth factor receptor 3 is essential for corneal alymphaticity. Blood 2013, 121, 4242–4249. [Google Scholar] [CrossRef]
- Makinen, T.; Jussila, L.; Veikkola, T.; Karpanen, T.; Kettunen, M.I.; Pulkkanen, K.J.; Kauppinen, R.; Jackson, D.G.; Kubo, H.; Nishikawa, S.; et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat. Med. 2001, 7, 199–205. [Google Scholar] [CrossRef]
- Cursiefen, C.; Chen, L.; Saint-Geniez, M.; Hamrah, P.; Jin, Y.; Rashid, S.; Pytowski, B.; Persaud, K.; Wu, Y.; Streilein, J.W.; et al. Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision. Proc. Natl. Acad. Sci. USA 2006, 103, 11405–11410. [Google Scholar] [CrossRef]
- Emami-Naeini, P.; Dohlman, T.H.; Omoto, M.; Hattori, T.; Chen, Y.; Lee, H.S.; Chauhan, S.K.; Dana, R. Soluble vascular endothelial growth factor receptor-3 suppresses allosensitization and promotes corneal allograft survival. Graefes Arch. Clin. Exp. Ophthalmol 2014, 252, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Cao, J.; Chen, L.; Liu, Y.; Maruyama, K.; Jackson, D.; Kruse, F.E.; Wiegand, S.J.; Dana, M.R.; Streilein, J.W. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest. Ophthalmol Vis. Sci 2004, 45, 2666–2673. [Google Scholar] [CrossRef] [PubMed]
- Hos, D.; Regenfuss, B.; Bock, F.; Onderka, J.; Cursiefen, C. Blockade of insulin receptor substrate-1 inhibits corneal lymphangiogenesis. Invest. Ophthalmol Vis. Sci 2011, 52, 5778–5785. [Google Scholar] [CrossRef]
- Dohlman, T.H.; Omoto, M.; Hua, J.; Stevenson, W.; Lee, S.M.; Chauhan, S.K.; Dana, R. VEGF-trap aflibercept significantly improves long-term graft survival in high-risk corneal transplantation. Transplantation 2015, 99, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Dastjerdi, M.H.; Saban, D.R.; Okanobo, A.; Nallasamy, N.; Sadrai, Z.; Chauhan, S.K.; Hajrasouliha, A.R.; Dana, R. Effects of topical and subconjunctival bevacizumab in high-risk corneal transplant survival. Invest. Ophthalmol Vis. Sci 2010, 51, 2411–2417. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gupta, A.A.; Mammo, D.A.; Page, M.A. Intrastromal bevacizumab in the management of corneal neovascularization: A retrospective review. Graefes Arch. Clin. Exp. Ophthalmol 2019. [Google Scholar] [CrossRef]
- Sarah, B.; Ibtissam, H.; Mohammed, B.; Hasna, S.; Abdeljalil, M. Intrastromal Injection of Bevacizumab in the Management of Corneal Neovascularization: About 25 Eyes. J. Ophthalmol 2016, 2016, 6084270. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.N.; Lichtinger, A.; Kim, P.; Amiran, M.D.; Slomovic, A.R. Combined use of subconjunctival and intracorneal bevacizumab injection for corneal neovascularization. Cornea 2011, 30, 1110–1114. [Google Scholar] [CrossRef]
- Bhatti, N.; Qidwai, U.; Hussain, M.; Kazi, A. Efficacy of topical bevacizumab in high-risk corneal transplant survival. Pak. J. Med. Sci 2013, 29, 519–522. [Google Scholar] [CrossRef]
- Dekaris, I.; Gabric, N.; Draca, N.; Pauk-Gulic, M.; Milicic, N. Three-year corneal graft survival rate in high-risk cases treated with subconjunctival and topical bevacizumab. Graefes Arch. Clin. Exp. Ophthalmol 2015, 253, 287–294. [Google Scholar] [CrossRef]
- Koenig, Y.; Bock, F.; Horn, F.; Kruse, F.; Straub, K.; Cursiefen, C. Short- and long-term safety profile and efficacy of topical bevacizumab (Avastin) eye drops against corneal neovascularization. Graefes Arch. Clin. Exp. Ophthalmol 2009, 247, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, N.; Qidwai, U.; Hussain, M.; Kazi, A. Efficacy of sub-conjunctival and topical bevacizumab in high-risk corneal transplant survival. J. Pak. Med. Assoc. 2013, 63, 1256–1259. [Google Scholar] [PubMed]
- Hos, D.; Le, V.N.H.; Hellmich, M.; Siebelmann, S.; Roters, S.; Bachmann, B.O.; Cursiefen, C. Risk of Corneal Graft Rejection After High-risk Keratoplasty Following Fine-needle Vessel Coagulation of Corneal Neovascularization Combined With Bevacizumab: A Pilot Study. Transplant. Direct 2019, 5, e452. [Google Scholar] [CrossRef] [PubMed]
- White, M.F. The IRS-signaling system: A network of docking proteins that mediate insulin and cytokine action. Recent Prog Horm Res. 1998, 53, 119–138. [Google Scholar]
- Cursiefen, C.; Viaud, E.; Bock, F.; Geudelin, B.; Ferry, A.; Kadlecova, P.; Levy, M.; Al Mahmood, S.; Colin, S.; Thorin, E.; et al. Aganirsen antisense oligonucleotide eye drops inhibit keratitis-induced corneal neovascularization and reduce need for transplantation: The I-CAN study. Ophthalmology 2014, 121, 1683–1692. [Google Scholar] [CrossRef]
- Niederkorn, J.Y. Immune mechanisms of corneal allograft rejection. Curr Eye Res. 2007, 32, 1005–1016. [Google Scholar] [CrossRef]
- Hajrasouliha, A.R.; Funaki, T.; Sadrai, Z.; Hattori, T.; Chauhan, S.K.; Dana, R. Vascular endothelial growth factor-C promotes alloimmunity by amplifying antigen-presenting cell maturation and lymphangiogenesis. Invest. Ophthalmol Vis. Sci 2012, 53, 1244–1250. [Google Scholar] [CrossRef]
- Acton, S.E.; Astarita, J.L.; Malhotra, D.; Lukacs-Kornek, V.; Franz, B.; Hess, P.R.; Jakus, Z.; Kuligowski, M.; Fletcher, A.L.; Elpek, K.G.; et al. Podoplanin-rich stromal networks induce dendritic cell motility via activation of the C-type lectin receptor CLEC-2. Immunity 2012, 37, 276–289. [Google Scholar] [CrossRef]
- Colonna, M.; Samaridis, J.; Angman, L. Molecular characterization of two novel C-type lectin-like receptors, one of which is selectively expressed in human dendritic cells. Eur J. Immunol 2000, 30, 697–704. [Google Scholar] [CrossRef]
- Kerjaschki, D.; Regele, H.M.; Moosberger, I.; Nagy-Bojarski, K.; Watschinger, B.; Soleiman, A.; Birner, P.; Krieger, S.; Hovorka, A.; Silberhumer, G.; et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J. Am. Soc. Nephrol 2004, 15, 603–612. [Google Scholar] [CrossRef]
- Turley, S.J.; Fletcher, A.L.; Elpek, K.G. The stromal and haematopoietic antigen-presenting cells that reside in secondary lymphoid organs. Nat. Rev. Immunol 2010, 10, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, Y.; Maruyama, K.; Kato, Y.; Kajiya, K.; Moritoh, S.; Yamamoto, K.; Matsumoto, Y.; Sawane, M.; Kerjaschki, D.; Nakazawa, T.; et al. The effect of podoplanin inhibition on lymphangiogenesis under pathological conditions. Invest. Ophthalmol Vis. Sci 2014, 55, 4813–4822. [Google Scholar] [CrossRef] [PubMed]
- Garmy-Susini, B.; Varner, J.A. Roles of integrins in tumor angiogenesis and lymphangiogenesis. Lymphat Res. Biol 2008, 6, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Mishima, K.; Watabe, T.; Saito, A.; Yoshimatsu, Y.; Imaizumi, N.; Masui, S.; Hirashima, M.; Morisada, T.; Oike, Y.; Araie, M.; et al. Prox1 induces lymphatic endothelial differentiation via integrin alpha9 and other signaling cascades. Mol. Biol Cell 2007, 18, 1421–1429. [Google Scholar] [CrossRef]
- Kang, G.J.; Truong, T.; Huang, E.; Su, V.; Ge, S.; Chen, L. Integrin Alpha 9 Blockade Suppresses Lymphatic Valve Formation and Promotes Transplant Survival. Invest. Ophthalmol Vis. Sci 2016, 57, 5935–5939. [Google Scholar] [CrossRef][Green Version]
- Garmy-Susini, B.; Makale, M.; Fuster, M.; Varner, J.A. Methods to study lymphatic vessel integrins. Methods Enzymol 2007, 426, 415–438. [Google Scholar] [CrossRef]
- Nasarre, P.; Gemmill, R.M.; Drabkin, H.A. The emerging role of class-3 semaphorins and their neuropilin receptors in oncology. Onco Targets Ther 2014, 7, 1663–1687. [Google Scholar] [CrossRef]
- Suto, F.; Ito, K.; Uemura, M.; Shimizu, M.; Shinkawa, Y.; Sanbo, M.; Shinoda, T.; Tsuboi, M.; Takashima, S.; Yagi, T.; et al. Plexin-a4 mediates axon-repulsive activities of both secreted and transmembrane semaphorins and plays roles in nerve fiber guidance. J. Neurosci 2005, 25, 3628–3637. [Google Scholar] [CrossRef]
- Takahashi, T.; Fournier, A.; Nakamura, F.; Wang, L.H.; Murakami, Y.; Kalb, R.G.; Fujisawa, H.; Strittmatter, S.M. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell 1999, 99, 59–69. [Google Scholar] [CrossRef]
- Buehler, A.; Sitaras, N.; Favret, S.; Bucher, F.; Berger, S.; Pielen, A.; Joyal, J.S.; Juan, A.M.; Martin, G.; Schlunck, G.; et al. Semaphorin 3F forms an anti-angiogenic barrier in outer retina. FEBS Lett 2013, 587, 1650–1655. [Google Scholar] [CrossRef]
- Sun, Y.; Liegl, R.; Gong, Y.; Buhler, A.; Cakir, B.; Meng, S.S.; Burnim, S.B.; Liu, C.H.; Reuer, T.; Zhang, P.; et al. Sema3f Protects Against Subretinal Neovascularization In Vivo. EBioMedicine 2017, 18, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Reuer, T.; Schneider, A.C.; Cakir, B.; Buhler, A.D.; Walz, J.M.; Lapp, T.; Lange, C.; Agostini, H.; Schlunck, G.; Cursiefen, C.; et al. Semaphorin 3F Modulates Corneal Lymphangiogenesis and Promotes Corneal Graft Survival. Invest. Ophthalmol Vis. Sci 2018, 59, 5277–5284. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Maruyama, K.; Bock, F.; Saban, D.; Sadrai, Z.; Lawler, J.; Dana, R.; Masli, S. Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. J. Exp. Med. 2011, 208, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Heishi, T.; Hosaka, T.; Suzuki, Y.; Miyashita, H.; Oike, Y.; Takahashi, T.; Nakamura, T.; Arioka, S.; Mitsuda, Y.; Takakura, T.; et al. Endogenous angiogenesis inhibitor vasohibin1 exhibits broad-spectrum antilymphangiogenic activity and suppresses lymph node metastasis. Am. J. Pathol 2010, 176, 1950–1958. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, L.; Mak, J.; Pardanaud, L.; Caunt, M.; Kasman, I.; Larrivee, B.; Del Toro, R.; Suchting, S.; Medvinsky, A.; et al. Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3. J. Cell Biol 2010, 188, 115–130. [Google Scholar] [CrossRef]
- Watanabe, K.; Hasegawa, Y.; Yamashita, H.; Shimizu, K.; Ding, Y.; Abe, M.; Ohta, H.; Imagawa, K.; Hojo, K.; Maki, H.; et al. Vasohibin as an endothelium-derived negative feedback regulator of angiogenesis. J. Clin. Invest. 2004, 114, 898–907. [Google Scholar] [CrossRef]
- Tang, X.L.; Sun, J.F.; Wang, X.Y.; Du, L.L.; Liu, P. Blocking neuropilin-2 enhances corneal allograft survival by selectively inhibiting lymphangiogenesis on vascularized beds. Mol. Vis. 2010, 16, 2354–2361. [Google Scholar]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef]
- McCawley, L.J.; Matrisian, L.M. Matrix metalloproteinases: Multifunctional contributors to tumor progression. Mol. Med. Today 2000, 6, 149–156. [Google Scholar] [CrossRef]
- Barbolina, M.V.; Stack, M.S. Membrane type 1-matrix metalloproteinase: Substrate diversity in pericellular proteolysis. Semin Cell Dev. Biol 2008, 19, 24–33. [Google Scholar] [CrossRef]
- Han, K.Y.; Dugas-Ford, J.; Lee, H.; Chang, J.H.; Azar, D.T. MMP14 Cleavage of VEGFR1 in the Cornea Leads to a VEGF-Trap Antiangiogenic Effect. Invest. Ophthalmol Vis. Sci 2015, 56, 5450–5456. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.L.; Cao, R.; Jin, G.; Chan, K.M.; Cao, Y.; Zhou, Z. When MT1-MMP meets ADAMs. Cell Cycle 2012, 11, 2793–2798. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Apte, S.S.; Soininen, R.; Cao, R.; Baaklini, G.Y.; Rauser, R.W.; Wang, J.; Cao, Y.; Tryggvason, K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc. Natl. Acad. Sci. USA 2000, 97, 4052–4057. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.L.; Jin, G.; Cao, R.; Zhang, S.; Cao, Y.; Zhou, Z. MT1-MMP sheds LYVE-1 on lymphatic endothelial cells and suppresses VEGF-C production to inhibit lymphangiogenesis. Nat. Commun 2016, 7, 10824. [Google Scholar] [CrossRef]
- Du, H.T.; Du, L.L.; Tang, X.L.; Ge, H.Y.; Liu, P. Blockade of MMP-2 and MMP-9 inhibits corneal lymphangiogenesis. Graefes Arch. Clin. Exp. Ophthalmol 2017, 255, 1573–1579. [Google Scholar] [CrossRef]
- Bock, F.; Onderka, J.; Braun, G.; Schneider, A.C.; Hos, D.; Bi, Y.; Bachmann, B.O.; Cursiefen, C. Identification of Novel Endogenous Anti(lymph)angiogenic Factors in the Aqueous Humor. Invest. Ophthalmol Vis. Sci 2016, 57, 6554–6560. [Google Scholar] [CrossRef]
- Nacher, J.C. Community structure of non-coding RNA interaction network. J. Integr Bioinform 2013, 10, 217. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Filipowicz, W.; Bhattacharyya, S.N.; Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat. Rev. Genet. 2008, 9, 102–114. [Google Scholar] [CrossRef]
- Khan, A.; Nasr, P.; El-Charabaty, E.; El-Sayegh, S. An Insight Into the Immunologic Events and Risk Assessment in Renal Transplantation. J. Clin. Med. Res. 2016, 8, 367–372. [Google Scholar] [CrossRef]
- Oghumu, S.; Bracewell, A.; Nori, U.; Maclean, K.H.; Balada-Lasat, J.M.; Brodsky, S.; Pelletier, R.; Henry, M.; Satoskar, A.R.; Nadasdy, T.; et al. Acute pyelonephritis in renal allografts: A new role for microRNAs? Transplantation 2014, 97, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wu, J.; Ma, M.; Wu, X.; Wen, J.; Yu, J. An integrated deep sequencing analysis of microRNAs in transplanted corneas. Mol. Immunol 2018, 101, 429–439. [Google Scholar] [CrossRef]
- Wang, T.; Li, F.; Geng, W.; Ruan, Q.; Shi, W. MicroRNA-122 ameliorates corneal allograft rejection through the downregulation of its target CPEB1. Cell Death Discov 2017, 3, 17021. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grimaldo, S.; Yuen, D.; Theis, J.; Ng, M.; Ecoiffier, T.; Chen, L. MicroRNA-184 Regulates Corneal Lymphangiogenesis. Invest. Ophthalmol Vis. Sci 2015, 56, 7209–7213. [Google Scholar] [CrossRef]
- Hong, Y.K.; Detmar, M. Prox1, master regulator of the lymphatic vasculature phenotype. Cell Tissue Res. 2003, 314, 85–92. [Google Scholar] [CrossRef]
- Hong, Y.K.; Harvey, N.; Noh, Y.H.; Schacht, V.; Hirakawa, S.; Detmar, M.; Oliver, G. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev. Dyn 2002, 225, 351–357. [Google Scholar] [CrossRef]
- Wigle, J.T.; Harvey, N.; Detmar, M.; Lagutina, I.; Grosveld, G.; Gunn, M.D.; Jackson, D.G.; Oliver, G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002, 21, 1505–1513. [Google Scholar] [CrossRef]
- Kazenwadel, J.; Michael, M.Z.; Harvey, N.L. Prox1 expression is negatively regulated by miR-181 in endothelial cells. Blood 2010, 116, 2395–2401. [Google Scholar] [CrossRef]
- Pedrioli, D.M.; Karpanen, T.; Dabouras, V.; Jurisic, G.; van de Hoek, G.; Shin, J.W.; Marino, D.; Kalin, R.E.; Leidel, S.; Cinelli, P.; et al. miR-31 functions as a negative regulator of lymphatic vascular lineage-specific differentiation in vitro and vascular development in vivo. Mol. Cell Biol 2010, 30, 3620–3634. [Google Scholar] [CrossRef]
- Seo, M.; Choi, J.S.; Rho, C.R.; Joo, C.K.; Lee, S.K. MicroRNA miR-466 inhibits Lymphangiogenesis by targeting prospero-related homeobox 1 in the alkali burn corneal injury model. J. Biomed. Sci 2015, 22, 3. [Google Scholar] [CrossRef]
- Dumont, D.J.; Jussila, L.; Taipale, J.; Lymboussaki, A.; Mustonen, T.; Pajusola, K.; Breitman, M.; Alitalo, K. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science 1998, 282, 946–949. [Google Scholar] [CrossRef] [PubMed]
- Tammela, T.; Alitalo, K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell 2010, 140, 460–476. [Google Scholar] [CrossRef] [PubMed]
- Flister, M.J.; Wilber, A.; Hall, K.L.; Iwata, C.; Miyazono, K.; Nisato, R.E.; Pepper, M.S.; Zawieja, D.C.; Ran, S. Inflammation induces lymphangiogenesis through up-regulation of VEGFR-3 mediated by NF-kappaB and Prox1. Blood 2010, 115, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Li, Y.; He, Y.; Xu, Z.; Chen, H.; Min, W. Mirtron microRNA-1236 inhibits VEGFR-3 signaling during inflammatory lymphangiogenesis. Arterioscler Thromb Vasc Biol 2012, 32, 633–642. [Google Scholar] [CrossRef]
- Chakraborty, S.; Zawieja, D.C.; Davis, M.J.; Muthuchamy, M. MicroRNA signature of inflamed lymphatic endothelium and role of miR-9 in lymphangiogenesis and inflammation. Am. J. Physiol Cell Physiol 2015, 309, C680–692. [Google Scholar] [CrossRef]
- Kontarakis, Z.; Rossi, A.; Ramas, S.; Dellinger, M.T.; Stainier, D.Y.R. Mir-126 is a conserved modulator of lymphatic development. Dev. Biol 2018, 437, 120–130. [Google Scholar] [CrossRef]
- Borza, C.M.; Pozzi, A. Discoidin domain receptors in disease. Matrix Biol 2014, 34, 185–192. [Google Scholar] [CrossRef]
- Oh, S.; Seo, M.; Choi, J.S.; Joo, C.K.; Lee, S.K. MiR-199a/b-5p Inhibits Lymphangiogenesis by Targeting Discoidin Domain Receptor 1 in Corneal Injury. Mol. Cells 2018, 41, 93–102. [Google Scholar] [CrossRef]
- Yamanaka, R.; Arao, T.; Yajima, N.; Tsuchiya, N.; Homma, J.; Tanaka, R.; Sano, M.; Oide, A.; Sekijima, M.; Nishio, K. Identification of expressed genes characterizing long-term survival in malignant glioma patients. Oncogene 2006, 25, 5994–6002. [Google Scholar] [CrossRef]
- Shrivastava, A.; Radziejewski, C.; Campbell, E.; Kovac, L.; McGlynn, M.; Ryan, T.E.; Davis, S.; Goldfarb, M.P.; Glass, D.J.; Lemke, G.; et al. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol. Cell 1997, 1, 25–34. [Google Scholar] [CrossRef]
- Vogel, W.; Gish, G.D.; Alves, F.; Pawson, T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol. Cell 1997, 1, 13–23. [Google Scholar] [CrossRef]
- Xiao, Q.; Jiang, Y.; Liu, Q.; Yue, J.; Liu, C.; Zhao, X.; Qiao, Y.; Ji, H.; Chen, J.; Ge, G. Minor Type IV Collagen alpha5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1. PLoS Genet. 2015, 11, e1005249. [Google Scholar] [CrossRef] [PubMed]
- Amaral, P.P.; Mattick, J.S. Noncoding RNA in development. Mamm Genome 2008, 19, 454–492. [Google Scholar] [CrossRef] [PubMed]
- Taft, R.J.; Pang, K.C.; Mercer, T.R.; Dinger, M.; Mattick, J.S. Non-coding RNAs: Regulators of disease. J. Pathol 2010, 220, 126–139. [Google Scholar] [CrossRef]
- Wilusz, J.E.; Sunwoo, H.; Spector, D.L. Long noncoding RNAs: Functional surprises from the RNA world. Genes Dev. 2009, 23, 1494–1504. [Google Scholar] [CrossRef]
- Evans, J.R.; Feng, F.Y.; Chinnaiyan, A.M. The bright side of dark matter: lncRNAs in cancer. J. Clin. Invest. 2016, 126, 2775–2782. [Google Scholar] [CrossRef]
- Chen, W.; Yang, S.; Zhou, Z.; Zhao, X.; Zhong, J.; Reinach, P.S.; Yan, D. The Long Noncoding RNA Landscape of the Mouse Eye. Invest. Ophthalmol Vis. Sci 2017, 58, 6308–6317. [Google Scholar] [CrossRef]
- Sun, B.; Ding, Y.; Jin, X.; Xu, S.; Zhang, H. Long non-coding RNA H19 promotes corneal neovascularization by targeting microRNA-29c. Biosci Rep. 2019, 39. [Google Scholar] [CrossRef]
- Sun, Z.; Ou, C.; Ren, W.; Xie, X.; Li, X.; Li, G. Downregulation of long non-coding RNA ANRIL suppresses lymphangiogenesis and lymphatic metastasis in colorectal cancer. Oncotarget 2016, 7, 47536–47555. [Google Scholar] [CrossRef]
- He, W.; Zhong, G.; Jiang, N.; Wang, B.; Fan, X.; Chen, C.; Chen, X.; Huang, J.; Lin, T. Long noncoding RNA BLACAT2 promotes bladder cancer-associated lymphangiogenesis and lymphatic metastasis. J. Clin. Invest. 2018, 128, 861–875. [Google Scholar] [CrossRef]
- Rohan, R.M.; Fernandez, A.; Udagawa, T.; Yuan, J.; D’Amato, R.J. Genetic heterogeneity of angiogenesis in mice. FASEB J. 2000, 14, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.S.; Rohan, R.M.; Birsner, A.E.; D’Amato, R.J. Genetic loci that control vascular endothelial growth factor-induced angiogenesis. FASEB J. 2003, 17, 2112–2114. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.S.; Rohan, R.M.; Birsner, A.E.; D’Amato, R.J. Genetic loci that control the angiogenic response to basic fibroblast growth factor. FASEB J. 2004, 18, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Regenfuss, B.; Onderka, J.; Bock, F.; Hos, D.; Maruyama, K.; Cursiefen, C. Genetic heterogeneity of lymphangiogenesis in different mouse strains. Am. J. Pathol 2010, 177, 501–510. [Google Scholar] [CrossRef]
- Regenfuss, B.; Dreisow, M.L.; Hos, D.; Masli, S.; Bock, F.; Cursiefen, C. The Naive Murine Cornea as a Model System to Identify Novel Endogenous Regulators of Lymphangiogenesis: TRAIL and rtPA. Lymphat Res. Biol 2015, 13, 76–84. [Google Scholar] [CrossRef]
- Bock, F.; Onderka, J.; Hos, D.; Horn, F.; Martus, P.; Cursiefen, C. Improved semiautomatic method for morphometry of angiogenesis and lymphangiogenesis in corneal flatmounts. Exp. Eye Res. 2008, 87, 462–470. [Google Scholar] [CrossRef]
- Buttner, C.; Clahsen, T.; Regenfuss, B.; Dreisow, M.L.; Steiber, Z.; Bock, F.; Reis, A.; Cursiefen, C. Tyrosinase Is a Novel Endogenous Regulator of Developmental and Inflammatory Lymphangiogenesis. Am. J. Pathol 2019, 189, 440–448. [Google Scholar] [CrossRef]



| Proteins in Lymphangiogenesis | |||
|---|---|---|---|
| Protein | Function | ||
| Endostatin | Endostatin | inhibition of angiogenesis | [32,37,38,39] |
| Tumstatin | inhibition of angiogenesis | [40,41] | |
| Arrestin | inhibition of angiogenesis | [41] | |
| Plasminogen | Angiostatin | inhibition angiogenesis | [42,43] |
| Thrombospondin | TSP-1 | inhibition of angiogenesis and lymphangiogenesis | [44,83] |
| TSP-2 | inhibition of angiogenesis | [31] | |
| soluble VEGFR | sVEGFR-1 | decoy receptor for VEGF-A; inhibition of angiogenesis | [28,46] |
| sVEGFR-2 | decoy receptor for VEGF-C and -D; inhibition of lymphangiogenesis | [47] | |
| sVEGFR-3 | decoy receptor for VEGF-C and -D; inhibition of lymphangiogenesis | [48] | |
| adapter protein | IRS-1 | treatment with antisense oligonucleotide inhibits hem- and lymphangiogenesis | [65] |
| Glycoprotein | Podoplanin | implication in lymphocyte trafficking, blocking antibody inhibits lymphangiogenesis | [71,72] |
| Integrine | Integrin α5β1 | treatment with antagonist JSM6227 inhibits lymphangiogenesis | [3] |
| Integrin α9β1 | blocking antibody improves graft survival | [75] | |
| Semaphorine | Semaphorin-3F | contributing to anti-(lymph) angiogenic barrier | [82] |
| Vasohibin | VASH-1 | negative feedback; regulator inhibition of angiogenesis and lymphangiogenesis | [84,86] |
| transmembrane Receptor | Neuropilin-2 | associated with VEGFR-3, artificial miRNA improves graft | [87] |
| Metalloproteases | MT-MMP1 | cleavage of VEGFR-1 and LYVE-1 | [91,94] |
| MMP-2 & MMP9 | blockade with SB-3CT inhibits lymphangiogenesis | [95] | |
| Peptide hormone | VIP | inhibition of lymphangiogenesis | [96] |
| α-MSH | inhibition of lymphangiogenesis | [96] | |
| TNF/TNFR-Superfamily | Trail | inhibition of lymphangiogenesis | [135] |
| Proteases | tPA | inhibition of lymphangiogenesis | [135] |
| Membrane protein | Tyrosinase | inhibition of lymphangiogenesis | [137] |
| ncRNAs in Lymphangiogenesis | |||
| Targets | Function | ||
| miRNA-184 | LECs | suppresses migration and adhesion | [104] |
| miRNA-181a | Prox-1 | degradation of Prox-1 | [108] |
| miRNA-31 | Prox-1 | degradation of Prox-1 | [109] |
| miRNA-466 | Prox-1 | degradation of Prox-1 | [110] |
| miRNA-1236 | VEGFR-3 | inhibition of VEGFR-3 | [114] |
| miRNA-9 | VEGFR-3 | increased VEGFR-3 expression | [115] |
| miRNA-126 | VEGFR-2/VEGFR-3 | modulates VEGFR-2 and VEGFR-3 signal transduction | [116] |
| miRNA-199a/b5p | DDR1 | degradation of DDR1 | [118] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clahsen, T.; Büttner, C.; Hatami, N.; Reis, A.; Cursiefen, C. Role of Endogenous Regulators of Hem- And Lymphangiogenesis in Corneal Transplantation. J. Clin. Med. 2020, 9, 479. https://doi.org/10.3390/jcm9020479
Clahsen T, Büttner C, Hatami N, Reis A, Cursiefen C. Role of Endogenous Regulators of Hem- And Lymphangiogenesis in Corneal Transplantation. Journal of Clinical Medicine. 2020; 9(2):479. https://doi.org/10.3390/jcm9020479
Chicago/Turabian StyleClahsen, Thomas, Christian Büttner, Niloofar Hatami, André Reis, and Claus Cursiefen. 2020. "Role of Endogenous Regulators of Hem- And Lymphangiogenesis in Corneal Transplantation" Journal of Clinical Medicine 9, no. 2: 479. https://doi.org/10.3390/jcm9020479
APA StyleClahsen, T., Büttner, C., Hatami, N., Reis, A., & Cursiefen, C. (2020). Role of Endogenous Regulators of Hem- And Lymphangiogenesis in Corneal Transplantation. Journal of Clinical Medicine, 9(2), 479. https://doi.org/10.3390/jcm9020479





