Abstract
Corneal grafts interact with their hosts via complex immunobiological processes that sometimes lead to graft failure. Prediction of graft failure is often a tedious task due to the genetic and nongenetic heterogeneity of patients. As in other areas of medicine, a reliable prediction method would impact therapeutic decision-making in corneal transplantation. Valuable insights into the clinically observed heterogeneity of host responses to corneal grafts have emerged from multidisciplinary approaches, including genomics analyses, mechanical studies, immunobiology, and theoretical modeling. Here, we review the emerging concepts, tools, and new biomarkers that may allow for the prediction of graft survival.
1. Introduction
The cornea is the most frequently transplanted human tissue [1]; however, 10% of recipients with uninflamed graft beds will experience graft failure. Despite maximal immune suppression, the failure rate dramatically increases to 50% in recipients with inflamed graft beds. The likelihood of acute rejection and/or graft failure in patients in this “high risk” category is comparable to or larger than that of the commonly transplanted solid organs [2]. Despite intensive study, the heterogeneity of responses to corneal grafts is a poorly understood subject. Interdisciplinary approaches have been increasingly applied to resolve this heterogeneity and to find new biomarkers for disease prediction. Novel genetic, immunological, and physical biomarkers have recently been introduced. Here, we review the recent developments in the field with a special focus on new innovations and emerging frontiers.
2. Emerging Immunological Markers
With the high frequency of corneal transplants, numerous studies have attempted to decode the overarching etiologies of corneal allograft rejection. As with other solid organ transplants, the immune system has been shown to be one of the most important contributors to corneal allograft rejection, along with immune-enabling factors such as blood and lymph flow, in the relatively immune-tolerant cornea. Major and minor histocompatibility complexes, immune cell milieu, native corneal cell dynamics, and proteins such as cytokines and cell surface proteins have been investigated for their contributions to allograft rejection. Although efforts to understand the pathophysiology of corneal allograft rejection continue, here we discuss contributory factors already identified by this field of study (Table 1).

Table 1.
Candidate biomarkers for detection and prediction of corneal allograft rejection.
2.1. Importance of Immune Processes in Cornea Transplantation
Although corneal graft rejection involves a complex pathophysiology, the host immune response seems to be the most important modulator. Specifically, the transfer of donor immune cells and the strong cluster of differentiation (CD)4+ T helper (Th)1 cell-mediated delayed-type hypersensitivity reactions brought on by allogeneic components of the donor cornea are thought to be the main culprits [3,42]. The anterior chamber of the eye is one of the few organs that carries “immune privilege” This privilege is partially due to the lack of draining lymphatic vessels and to a unique interplay between immunomodulatory molecules that suppress angiogenesis [17,18,19,20]. However, the neovascularization and lymphangiogenesis initiated by ocular inflammation post-operation allows for the transfer of donor antigens to draining cervical lymph nodes (Figure 1). The subsequent recognition of donor antigens as foreign molecules leads to the activation and proliferation of naïve T cells to effector T cells (Teff) that trigger the destruction of graft cells [42].
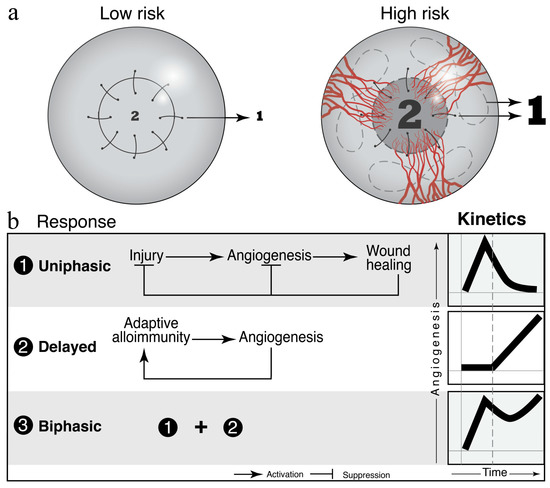
Figure 1.
Vascular dynamics in the grafted cornea and in the host bed as predictive and prognostic biomarkers for graft survival in murine models. (a) The density of pre-existing vessels correlates with the risk of allograft rejection. “Low risk” and “high risk” murine models are commonly used to study the host response to grafted corneas. (b) Wound healing and adaptive immune processes contribute to the angiogenic response to cornea grafts. Figure taken from Azimzade, Y. et al. with permission [43].
Although immunosuppressive therapies, including steroids, cyclosporin, and tacrolimus, have demonstrated benefits in preventing acute graft rejection, the risk of corneal graft rejection remains as high as 40–90% in high-risk host beds. Infections at the surgical site, complications due to existing autoimmune diseases, and angio/lymphangiogenesis from previous grafts are detrimental to graft outcomes and increase the risk of long-term side effects of immunosuppressive medications, including drug toxicity, glaucoma, and cataracts [44,45,46,47]. Additionally, the severity of side effects associated with immunosuppressants has led to disagreements regarding the timing of when therapy should be discontinued. Therefore, the identification of graft rejection biomarkers is imperative for the development of preventative and precision pharmacotherapies to avoid rejection and to help determine effective immunosuppressant dosing in cases where corneal graft rejection does occur (Table 1). In the following sections, we explore the current understanding of anatomical changes in the cornea post-transplant and the cellular and molecular interactions between the aforementioned immune cells, antigen-presenting cells (APCs), cytokines, and major histocompatibility complexes (MHC).
2.2. In Vivo Confocal Microscopy Evaluation of Immune Cells in the Corneal Graft
Slit lamp biomicroscopy has been the gold standard for examining clinical signs of corneal allograft rejection. However, research has indicated that the early detection of immune responses is crucial for the prompt diagnosis of graft rejection, and this limits the use of slit lamp biomicroscopy. Specifically, conventional light microscopy produces light that is reflected from structures surrounding the focal area. The resultant fringing effect leads to images that are low-contrast and ultimately of lower resolution [48]. The resolution power of slit lamp biomicroscopy is often constrained at 20 µm. Therefore, the visualization of immune-mediated signs of graft rejection, such as delayed-type hypersensitivity reactions against allogeneic cells and leukocytic infiltrations at the graft site, are limited [9]. These limitations may lead to a failure to recognize early signs of graft rejection, a delay in the diagnosis of graft rejection, and irreversible chronic damage.
In vivo confocal microscopy (IVCM) is now utilized to overcome these limitations. IVCM also overcomes some additional limitations of conventional light and electron microscopy, including eliminating the requirements for an in vitro study environment and for the fixation processes that chemically and physically disrupt the cells taken in vivo. By isolating a single focal plane and obscuring the planes that are not in focus, IVCM improves both the lateral and axial resolution to 1–2 µm and 5–10 µm, respectively, and allows for the visualization of leukocytic infiltrates that can be as small as 7 µm [48]. This level of resolution—in the context of corneal layer examinations—allows for the visualization of additional minute microstructural changes to keratocytes and cuboidal cells in the basal epithelium, corneal stroma, and endothelium.
Previously, Chirapapaisan C et al. reported that there was a significant increase in immune cell density following corneal graft rejection, along with a correlation between sub-basal layer immune cell density and clinical signs of graft rejection [10]. Of note, the density of immune cells was significantly increased in the sub-basal and endothelial layers in rejected grafts compared with that in nonrejected grafts. A measurement of seven nonrejected grafts at one year post-operation showed that there was a significant decrease in immune cells in all layers. This finding suggests that there are long-term differences in immune cell dynamics between rejected and nonrejected grafts. A history of dry eye disease in the donor has also been shown to contribute to the rejection of corneal allografts in a mouse model. It is possible that increased leukocyte maturation and T-cell dysregulation triggered by dry eye disease may participate in the pathogenesis of allograft rejection [16]. Recently, studies have reported that atypical hyper-reflective cells and activated keratocytes in the corneal stroma are crucial biomarkers of corneal allograft rejection [10,11,16].
As quiescent keratocytes remain dormant in their native state, activated keratocytes appear to reflect the level of intrastromal inflammation in response to cytokines and other inflammatory mediators, including interleukin (IL)-1 and tumor necrosis factor (TNF)-α [13,14,23,25]. Moreover, Beauregard et al. [17] reported that an inflammation-independent alloimmune response caused by delayed-type hypersensitivity reactions leads to apoptosis of keratocytes in the stroma and contributes to graft failure [12]. The increased level of activated keratocytes can be observed as early as two months before clinical diagnosis of corneal allograft rejection, and reaches its peak at the time of diagnosis. The subsequent administration of topical steroids and/or cyclosporin at diagnosis has been shown to successfully decrease activated keratocyte levels within one month of therapy [9]. Therefore, the current understanding of corneal allograft rejection pathogenesis dictates that corneal immune cell infiltration may be a decisive early biomarker. Thus, new or increased immune suppressant therapy should be considered following IVCM examinations that demonstrate increases in this immune cell population.
2.3. Cytokines and Regulatory T cells
Other components of the immune milieu, including various cytokines and mediator cells, contribute to the decompensation of the corneal endothelium when their balance shifts [42,49]. Corneal edema and bullous keratopathy often signal graft failure, and these conditions are often characterized by dystrophic changes in the Descemet’s membrane and stroma that are partially, if not largely, caused by immune responses [50]. Keratocytes within the epithelium and stroma, endothelial cells, and immune cells brought to the cornea through homing mechanisms all contribute to the corneal immune response through cytokine and chemokine secretion, assisted by the expression of various adhesion proteins in the corneal cells [21,24,26,28,29,30,34,35,36,37]. Very similar immune components have been identified in the anterior chamber after penetrating keratoplasty, suggesting that graft rejections are likely initiated within the corneal microcosm and that identifying minor dysregulations holds the key to timely detection of allograft rejection.
Yoon et al. proposed that CD8+/interferon (IFN)-γ+ cell and C3a levels within the aqueous humor (AqH) at weeks 2–4 post-keratoplasty may be predictors of graft rejection. This proposition was supported by the results of a receiver operator characteristic (ROC) curve analysis with an area under the curve (AUC) of 0.715 and 0.847, respectively [32]. Although this analysis was from a retrospective study that used a xenotransplantation model, the high reported specificity of 0.94 and 1.0, respectively, at the optimal cutoff warrants further investigation. Yamauchi et al. identified numerous candidate biomarkers that were correlated with the development of bullous keratopathy and low endothelial cell density, including IL-1a, IL-8, IL-17A, TNF-a, granulocyte-macrophage colony-stimulating factor (GM-CSF), macrophage inflammatory protein (MIP)-1a, IFN-c, and E-selectin levels in the AqH [31]. Intriguingly, Maier et al. investigated the risk of rejection before corneal transplantation and reported that the levels of protective factors (IL-2 and IL-5) and hazardous factors (IL-4 and IFN-γ) within the AqH before operation aided in prognosticating postoperative immune responses [27].
Regulatory T cells (Treg) were first identified in 1995 and are a major subgroup of T cells of paramount importance in the development of self-tolerance and downregulation of immune responses [51]. With respect to corneal allografts, IL-10 and TGF-β secreted by Foxp3high-expressing Tregs found in the draining lymph nodes of allograft recipients have been associated with reduced graft rejection [52]. Similarly, Foxp3 expression in T cells is reduced in recipients who experience corneal allograft rejection. These findings highlight the role of Tregs in the prevention of corneal graft rejection. Unfortunately, the differentiation and proliferation pathways of Tregs are very susceptible to changes in the environment, with fluctuations altering the immunosuppressive capabilities of these cells. Previous studies have demonstrated that high-risk host beds, often characterized by inflammation, neovascularization, and lymphangiogenesis, are correlated with a stark decrease in the functionality and expression of Foxp3 in Tregs in draining lymph nodes after corneal allograft placement [16,40]. Similarly, studies evaluated the use of Foxp3 expression level as a biomarker for kidney allograft prognosis and reported that there is a positive correlation between increased Foxp3 expression at 6 months post-transplantation and increased kidney function at 2 years post-transplantation [41]. It is possible that localization and expression of Foxp3+ Tregs—in concert with the various aforementioned cytokines and chemokines—could be potent predictive biomarkers for corneal graft rejection.
2.4. Major Histocompatibility Complex and Antigen-Presenting Cells
A healthy cornea exhibits a unique distribution of dendritic cells. MHC class I-positive dendritic cells are present throughout its entirety, while MHC class II-positive cells only reside in the limbus and peripheral cornea [53,54]. Despite the distinct separation in occupying regions, both classes of MHC are thought to contribute to the development of graft rejection [42]. This hypothesis is further underscored by the increased expression of Class II MHCs in the resident corneal dendritic cells and epithelial Langerhans cells within the central cornea during states of inflammation, including those caused by corneal transplantation [7,38,39,55].
Yet, unlike in kidney and heart transplants, human leukocyte antigen (HLA) matching is not routinely performed in corneal transplantation because the largest randomized studies that compared the outcomes of HLA matching (i.e., the Collaborative Corneal Transplantation Studies (CCTS)) reported that there were no significant differences in graft rejection rates [3]. Although the absence of meaningful extenders of corneal graft survival time and the predetermined notion that the cornea is immune-privileged have pushed clinical practice away from the utilization of HLA matching, continued conflicting reports have flooded the field and fueled further research [5,6]. New observations of increased Class II MHCs and co-stimulatory molecules, such as CD40, CD80, and CD86, in grafts from high-risk beds highlight the importance of MHC discrepancies in recipients who are at a high risk for graft rejection [4,8].
MHC class I-related chain A (MICA) has also been identified as a potential biomarker of graft rejection [33]. MICA is a protein that is expressed in the cytoplasms of corneal epithelial and endothelial cells; however, there is not much known regarding its regulatory mechanisms or impact on allograft rejection. Nevertheless, IFN-γ, a key cytokine released by CD4+ Th1 cells, has been shown to cause an upregulation of MICA in the epithelium, and this upregulation subsequently stimulates CD8+ T cells and natural killer cells [33]. This link between increased IFN-γ levels observed in rejected grafts and the enhanced immune response against the corneal epithelium suggests that changes in MICA levels may be used to preemptively recognize epithelial damage caused by graft rejection.
Dendritic cells (DCs) are bone marrow-derived APCs that are capable of expressing both MHC class I and II molecules; thus, they are thought to play an important role in the pathogenesis of graft rejection. Recent studies have alluded to the role of DCs as biomarkers for corneal epithelial inflammation in patients with type 2 diabetes mellitus, suggesting they may also be used as biomarkers for graft-related inflammation [56]. Nuanced variations in the presence, phenotype, and maturity of APC and DC populations have been shown to have varying associations on corneal allograft transplantation outcomes, implying they may reveal underlying immune dynamics [16,57,58]. Hamrah et al. reported that the number and distribution of mature CD11c+ DC populations is increased in the anterior stroma and that there is an upregulation of MHC class II molecules within 24 h after inflammation is induced. Additionally, CD11c-CD11b+ monocytes/macrophages, which are normally confined to the posterior stroma, have been found throughout the stroma during inflammation [38]. APCs, including corneal DCs, monocytes, and macrophages, are the cornerstone of immune responses and reflect the immune state; therefore, future studies should investigate the role of these cells in corneal graft rejection.
3. Endothelial Cell Density and Morphological Indicators as Graft Response Predictors
Human corneal endothelial cells (HCECs) cover the posterior surface of the cornea on Descemet’s membrane in a single layer with a well-arranged mosaic pattern. They play an important role in keeping the cornea clear by pumping out water into the anterior chamber using Na+-and K+-dependent ATPase in the basolateral membrane [15]. Unlike the vascular endothelium that originates from the mesoderm, HCECs originate from the cranial neural crest (neuroectoderm) [59,60]. HCECs have a limited proliferative capacity in vivo, and damage to HCECs by trauma; surgery; or primary corneal endotheliopathies, such as Fuchs’ corneal endothelial dystrophy, can lead to bullous keratopathy or corneal endothelial blindness. Corneal endothelial dysfunction is the main cause for corneal transplantation, responsible for 40% to 50% of transplants [61,62]. Endothelial cell density (ECD, cell counts/mm2) and endothelial cell morphology (hexagonality and coefficient of variation of mean cell area) are markers for corneal endothelial dysfunction [15,63]. The first direct visualization of the endothelium by Vogt was performed using the specular reflection method with the slit lamp in 1918, and the specular microscope was developed by David Maurice in the 1960s to evaluate ECD and morphology for ophthalmic research [64]. The specular microscope is now widely used clinically to evaluate corneal endothelial function in diverse corneal disease conditions and in the preoperative evaluation for intraocular surgery. For corneal transplantation, minimum donor ECD has been generally established to be 2000 cells/mm2 for penetrating keratoplasty. In a longitudinal cohort study of 500 consecutive penetrating keratoplasties, a low endothelial cell density before surgery (p = 0.007) and 2 months postoperatively (p = 0.002) were identified as significant risk factors for developing late endothelial failure [65]. However, a recent study conducted by the Corneal Donor Study Investigator Group revealed that graft failure from endothelial decompensation was not related to donor ECD; nevertheless, they reported that graft failure was strongly correlated with ECD at 6 months after penetrating keratoplasty [66,67]. Among endothelial cell morphology indices, only lower hexagonality at 6 months after penetrating keratoplasty showed a suggestive trend of higher graft failure (p = 0.02) [67]. Recently, newer surgical techniques for endothelial dysfunction, including Descemet’s stripping automated endothelial keratoplasty (DSAEK), Descemet’s membrane endothelial keratoplasty (DMEK), and Pre-Descemet’s endothelial keratoplasty (PDEK) have been used to replace the standard technique of penetrating keratoplasty [68]. Studies have reported that cell loss is greater in the first six months after endothelial keratoplasty than in the first six months after penetrating keratoplasty; therefore, the minimum donor ECD requirement is 2300–2500 cells/mm2) [69]. In a recent study evaluating the factors associated with graft survival and ECD after DSAEK, lower graft ECD was identified as a significant predisposing factor for lower postoperative ECD, but was not a predisposing factor for graft failure [70]. For DMEK, lower graft ECD was also found as a significant risk factor for higher postoperative ECD loss by multinominal regression analysis comparing groups of eyes with low and high endothelial cell loss [71]. In a genome-wide association study of specular microscopic findings in 6125 Icelanders, an intergenic variant (rs78658973(A), frequency = 28.3%) close to ANAPC1 (anaphase-promoting complex subunit 1) was strongly associated with decreased ECD [72]. ANAPC1 encodes a cell cycle-regulated E3 ubiquitin ligase that controls the progression through mitosis and the G1 phase of the cell cycle. Sequence variation at ANAPC1 accounts for 24% of the variability in corneal ECD [72].
Transplantation of cultured HCECs or possible precursor cells has been performed to overcome the shortage of donor tissue [59,73,74,75,76]. Diverse research groups have identified markers for HCECs, including CD166, glypican 4 (GPC4), CD200, CD56, Integrin Subunit Alpha 3 (ITGA3), and CD49c [77,78,79,80,81]. To discriminate HCECs from other cell types, molecular markers have been evaluated by integrating the published ribonucleic acid (RNA)-seq data of corneal endothelial cells (CECs) with the FANTOM5 atlas, which contains a diverse range of cell types. CLRN1, MRGPRX3, HTR1D, GRIP1, and ZP4 were identified as markers of CECs [82]. Recently, Kinoshita et al. reported promising clinical results by injecting cultured HCECs supplemented with a rho-associated protein kinase inhibitor into the anterior chamber [76]. To assess the quality of in vitro cultured HCECs, surface markers were analyzed using flow cytometry, and CD166+/CD24–/CD105–/CD44– cells were defined as effector cells in this group [61]. However, to measure the quality of cultured HCECs noninvasively, they developed the “spring constant K” as a physical biomarker, which represents the collective order of HCECs and is calculated by the second derivative of the function summated for the number of neighbor cells according to the distance from each reference cell [61]. The quantitative analysis of spring constant K from the effective interaction potential can be used preoperatively in vitro using phase contrast microscopy images and postoperatively in vivo using specular microscopy images. While preoperative spring constant K showed a clear positive correlation with effector cell fraction (r2 = 0.86) in vitro, its best classification accuracy (AUC = 0.96) was found with postoperative ECD in vivo at 6 months in comparison with other parameters, including effector cell fraction, preoperative ECD, and preoperative hexagonality [61]. This biomarker may enable preemptive interventions from passive monitoring in patients with severe corneal disorders [61]. The biomarkers for graft response covered in this section are summarized in Table 2.

Table 2.
Biomarkers for graft response related with corneal endothelial cells.
4. Vascular Dynamics and Graft Survival
4.1. Prior to Transplantation
The normal cornea is devoid of blood and lymphatic vessels and actively maintains a state of “angiogenic privilege” [83]. Corneal grafting onto vascularized and inflamed host beds (i.e., high-risk beds) leads to increased angiogenesis that further increases the chance of graft rejection [57]. Thereafter, the usually high success rate of corneal transplantation is completely overshadowed by rejection of larger grafts. Host bed vascularity is the principal risk factor for allograft rejection because corneal neo-vessels are critical in delivering the immune effector cells to the graft site and in driving the immune rejection [84].
The likelihood of the birth of a new vessel branch at any location is suggested to be proportional to the concentration of angiogenic factors and inversely proportional to the distance to the source. When angiogenesis develops with limited reversibility within a short time scale, prompt assessments of corneal neovascularization biomarkers in the early stage are critical for adequate and successful management. Corneal angiogenesis can occur from excessive levels of pro-angiogenic factors, including vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), matrix metalloproteinases (MMPs), and others [22,85,86,87,88,89]. The main markers implicated in angiogenesis and lymphangiogenesis are listed in Table 3. Members of the VEGF family (VEGF A, C, and D) are directly involved in both corneal angiogenesis and lymphangiogenesis [22,85,89,90,91]. Corneal angiogenesis can also occur secondary to a relative paucity of anti-angiogenic factors [92,93], such as soluble VEGF receptors (sVEGFR)-1, -2, -3; pigment epithelium-derived factor; angiostatin (created by the proteolytic cleavage of plasminogen); and endostatin (a type XVIII collagen proteolytic product) [94,95,96,97]. In response to hypoxia, the expression of VEGF increases 30-fold within minutes. Additionally, studies have reported that VEGF-A is directly involved in the pro-angiogenic circle of “bad” neovascularization, which allows a direct immune response to the graft site. This neovascularization ultimately induces allograft reactions and rejections [83]. Conversely, studies have revealed that IFN-γ has high antiangiogenic activity, though it is a powerful promoter of T cell-mediated immune rejection [83].
Several reports underline the critical proangiogenic actions of IL-17 and IL-1a, local biomarkers of corneal angiogenic response to insults such as grafting. Importantly, the survival of corneal transplants can be significantly affected by the degree of the hemangiogenic, as well as lymphangiogenic, response [83]. This finding is related to lymphatic and blood vessels acting as mediators of the afferent and efferent arms of the immune system, respectively. Lymphatics mediate the trafficking of alloantigen and immune cells from the graft to lymphoid tissues, and blood vessels facilitate the homing of immune cells to the graft [83]. Both angiogenesis and lymphangiogenesis are mainly driven by the VEGF family of receptors and ligands. The binding of VEGF-A and VEGF-B to VEGFR-1 and VEGFR-2 drives angiogenesis, whereas the binding of VEGF-C and VEGF-D to VEGFR-2 and VEGFR-3 drives lymphangiogenesis [91,98,99,100]. The complex interactions between VEGFs and VEGFRs exhibit sophisticated control over angiogenesis and lymphangiogenesis [101].
In addition, macrophages (MPs), a subtype of innate immune cells, contribute to proliferation of lymphatic vessels in inflammation-associated lymphangiogenesis [102,103]. Corneal lymphangiogenesis has been shown to be associated with CD11b+ MPs [103], and under inflammatory conditions, CD11b+ MPs secrete VEGF-A and VEGF-C in the peripheral cornea (limbus) [104]. The presence of lymphatic vessels in the cornea is usually detected by positive localization of podoplanin, lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1), VEGF-C, or VEGFR-3 and is associated with blood vessels, inflammatory cells, and disorganized stromal architecture in the immediate vicinity. Hence, VEGFR-3 is a specific biomarker for lymphangiogenesis. Additionally, the central corneal presence of CD11b+ macrophages may also play a direct role in corneal lymphangiogenesis.
Finally, studies have also suggested that very late antigen 1 (VLA-1) correlates with allogeneic corneal transplant survival. VLA-1 is an integrin that mediates intercellular and matrix interactions, and VLA-1 blockade markedly promotes survival of corneal allografts [102]. Chen L, et al. has been implicated in inflammatory reactions involving hemangiogenesis, macrophages, and T cells. Recently, VLA-1 has been identified in the corneal stroma.

Table 3.
Markers of angiogenesis and lymphangiogenesis.
Table 3.
Markers of angiogenesis and lymphangiogenesis.
| Antiangiogenic | Proangiogenic |
|---|---|
| IFN-γ [83] | VEGF-A, C, D [22,85,89,90,91,98,99,100] |
| sVEGFR-1,2,3 [92,93,94,95,96,97,98,99,100] | bFGF [22,85,86,87,88,89] |
| PEDF [94,95,96,97] | VLA-1 [102] |
| Endostatin [94,95,96,97] | PDGF [105,106] |
| ANG2 [107,108] |
Abbreviation listed as followed. IFN-γ: interferon gamma; sVEGFR: soluble VEGF receptors; PEDF: pigment epithelium-derived factor; VEGF: vascular endothelial growth factor; bFGF: basic fibroblast growth factor; VLA-1: very late antigen 1; PDGF: platelet-derived growth factor; ANG2: angiopoietin 2.
4.2. After Transplantation
Previous studies that focused on post-transplantation angiogenic responses revealed that low-risk (LR) and high-risk (HR) hosts have comparable lymphatic ingrowth early on; however, the amount of blood vessel ingrowth is significantly different [83]. These data demonstrate that angiogenic homeostatic mechanisms are restored much earlier in LR as opposed to HR graft recipients. Specifically, lymphatic responses regress early in noninflamed LR hosts, and this regression critically reduces the risk of allograft rejection. Corneal neovascularization is established in vitro by CD31 immunoreactive profiles. Simultaneous immunofluorescence using CD31 and podoplanin distinguishes lymphatic vessels from blood vessels. Moreover, confirming the presence of lymphatic mRNA by identifying at least two of the following mRNAs: podoplanin, VEGFR-3, and LYVE-1, may improve the accuracy of detection post-transplantation [109].
Different markers, including podoplanin, LYVE-1, and VEGFR-3, are commonly used to identify lymphatic channels. CD31 is a traditional marker of blood vessels that is weakly expressed on lymphatics [110]. However, podoplanin is a more specific marker of lymphatic endothelium since it is absent from blood vessel endothelium [111]. Conversely, LYVE-1 has been found to be expressed in some macrophages and blood vessels, including liver sinusoids [110]. Similarly, VEGFR-3 is found in some blood vessels, including in cases of inflammation-induced angiogenesis [110]. However, a recent “Consensus Statement on the Immunohistochemical Detection of Ocular Lymphatic Vessels” suggests that the use of a single lymphatic marker is sufficient for areas in which the presence of lymphatics has been well established, including the inflamed cornea [112].
In human beings and mice, angiopoietin 2 (ANG2) is mainly expressed in the epithelium and mildly expressed in the endothelium of the avascular cornea. It is, however, expressed in the epithelium, endothelium, and stroma of vascularized corneas. Disruption of the Bowman membrane is associated with a significant increase of ANG2 stromal expression and proangiogenic macrophage infiltration in the corneal stroma [107]. Selective epithelial staining patterns for ANG2 are similar to those for sVEGFR-1, which is a potent soluble inhibitor of VEGF and angiogenesis [108]. Proangiogenic ANG2 is sequestered just above the basal epithelial membrane, and membrane disruption may be associated with ANG2 diffusion into the stroma. Interestingly, ANG2 stromal expression is associated with the massive infiltration of proangiogenic macrophages, whose numbers nearly tripled after Bowman removal [107].
Finally, platelet-derived growth factor (PDGF) is another late-phase vascular biomarker. PDGF has an angiogenic effect on sprouting endothelial cells, promotes capillary maturation by recruiting pericytes to growing vessels, and is necessary for pericyte viability [105,106].
5. Physical Properties of the Cornea as Biomarkers of Graft Survival
Central corneal thickness (CCT) is a widely utilized physical measurement of the cornea used for the accurate measurement of intraocular pressure (IOP) in glaucoma patients and for calculating the appropriate laser ablation that is needed for refractive surgeries to prevent resultant side effects, including corneal ectasia. These applications, however, are mainly concerned with the one-dimensional effects of thickness, such as variations in CCT causing artifacts in Goldmann applanation tonometer IOP measurements or the iatrogenic decrease in CCT seen in laser-assisted in situ keratomileusis (LASIK) procedures [113,114,115]. To explore the implications of CCT on corneal allograft placements, there have been numerous attempts to understand the physiology affecting CCT and the changes in CCT that can reveal underlying pathologies (Table 4).

Table 4.
Clinical factors and gene loci associated with CCT.
Studies have revealed associations between CCT in patients with diabetes mellitus and hyperglycemia [118], speculating that the hyperglycemia-induced collagen disulfide crosslinks and osmotic fluid in the intracorneal spaces contribute to the shift in endothelial dynamics [119]. Further exploiting the corneal stromal permeability to fluids, CCT can also signify the level of hydration and activity of endothelial pumps that push fluids out of the stroma to prevent edematous buildup [120,121,122]. These pumps are altered in decompensated states, including Fuchs’ endothelial corneal dystrophy. Together with additional corneal physical properties, including hysteresis, resistance factor, and air pressure curves, the screening of subclinical presentations, such as forme fruste keratoconus, have become more reliable [127]. These findings suggest that corneal thickness reflects a wide range of underlying physiological mechanisms and this property can be applied to a multitude of other disease states, including corneal allograft rejection.
A report from the Corneal Donor Study, which searched for causes and associations of corneal allograft failure, posited that CCT has the potential to predict diagnoses of corneal edema, increases in IOP > 25 mmHg during the first postoperative month, and graft failure [116,117]. Interestingly, endothelial cell density (ECD), another measurement used for graft failure assessment, has shown little correlation with CCT. ECD only accounted for <10% of the variance in CCT, yet both parameters were independently predictive of graft failure. Although the authors caution against the use of CCT as a proxy for ECD, these results suggest that CCT may represent a novel and distinct niche in predicting graft failure and may justify further investigation on the mechanisms of its predictive strength. Currently, concomitant examination of corneal thickness kinetics and the use of specular endothelial micrography could expand the range of endothelial damage observed by clinicians, from the structural changes in the endothelium to the more extensive effects of inflammation and immune responses. Hence, the importance of deciphering the implications of a given CCT is increasing and is fueled by the rising concrete evidence that supports the highly genetic and inheritable component of CCT. The heritability of CCT seems to lie between 60% and 90%, suggesting that CCT is one of most highly heritable traits [128]. The connection between CCT and glaucoma has led to multiple genome-wide studies (GWS) conducted to determine loci that affect CCT. Lu et al. reported that there was a significant association between CCT variance and single-nucleotide polymorphisms (SNPs) near the ZNF469 gene (16q24), which is implicated in the development of Brittle Cornea Syndrome (BCS) [123]. BCS is a heritable disease characterized by extreme thinning of the cornea and skin and joint changes due to collagen dysfunction, thus providing a potential connection between the heritability of CCT and factors that affect corneal collagen formation. This finding aligns with the corneal thinning observed in certain connective tissue disorders that cause primary or secondary collagen malformation, including Ehlers–Danlos syndrome and osteogenesis imperfecta, further underscoring the importance of collagen in determining corneal architecture [129,130,131]. Not surprisingly, studies have reported that the COL5A1 gene, which also causes a variant of Ehlers–Danlos syndrome, and the COL8A2 gene are loci that both contribute to CCT variance [124,125]. COL8A2 mutations have been previously reported in patients with posterior polymorphous corneal dystrophy and Fuchs’ endothelial corneal dystrophy, both of which induce dystrophic changes to the cornea. Although these mutation hotspots are not directly linked to graft rejection, the connection between these loci and CCT may become valuable as the links between increased CCT and graft rejection are revealed.
Genome-wide association studies (GWAS) have also identified loci that are directly connected to corneal rejection, including certain minor histocompatibility antigens (mHag) loci, suggesting benefit to mHag matching in corneal allograft placements. Nicholls et al. conducted a GWAS on a swine model that underwent mismatched corneal transplantation and revealed four mHag loci that were associated with allograft rejection [126]. While three were novel findings, one block of SNPs spanned a region on chromosome 1 that contained the Zfp106 gene that encodes H-3a epitopes, and has previously been shown to mediate corneal graft rejection. Notably, a β-2M homologue was also located within the block of SNPs that has been shown to contribute to skin graft rejections [132]. Together with findings from the CCTS regarding the role of minor histocompatibility complex antigens in corneal graft rejection [133], genomic SNP analyses of corneal rejection-associated hotspots hold promise in prognosticating corneal allograft rejection. Furthermore, full genomic analysis of donors and recipients that examines individual corneal functional capabilities and immunologic characteristics could open up the possibility of precision gene therapy, such as individualized anti-angiogenic and anti-apoptotic gene therapy, to target rejection-promotive genes that vary in every patient [134].
6. Markers of Wound Healing
Neovessel formation is often a physiological process that is involved in tissue repair. Tissue damage is typically followed by an inflammatory response that protects the tissue from infection and promotes wound healing [135]. The innate arm of the immune system, particularly macrophages [136,137], facilitates wound healing by generating proangiogenic factors. Several studies have shown that corneal injury leads to the recruitment of VEGF-A-secreting macrophages and the induction of hemangiogenesis [138]. Blood vessels sprout at the edge of the injury and enable the formation of granulation tissue, which consists of fibroblasts and collagen [139]. Growth and maturation of blood vessels at the site of injury is usually a tightly controlled process and, once the tissue is repaired, the blood vessels regress [140]. Numerous physiological antiangiogenic factors, such as angiostatin, endostatin, tumstatin, vasohibin, fragments of prolactin, and growth hormones, facilitate this regression [141]. The regression of the vessels is then associated with the resolution of the inflammation [142]. Postgrafting wound healing involves the integrated actions of multiple growth factors, cytokines, and proteases that are produced by epithelial cells, stromal keratocytes, inflammatory cells, and lacrimal gland cells [143]. Table 5 lists the main markers of wound healing.
Multiple cytokines are released from the injured epithelium and epithelial basement membrane, including IL-1, TNF-alpha [144], bone morphogenic proteins (BMP) 2 and 4, epidermal growth factor (EGF), and PDGF [145]. These factors, along with others derived from the tears, trigger a variety of responses in underlying stromal keratocytes, including IL-1-mediated synthesis of Fas ligand. Keratocyte Fas ligand binds to the Fas receptor on nearby keratocytes and induces apoptosis [144] with minimal collateral damage from local cell lysis and liposomal enzyme release. In addition, it can induce autocrine suicide in keratocytes that already express Fas. A compromised epithelial barrier potentiates the effects of epithelial and lacrimal cytokines by providing direct access to the stroma.
Soon after wounding, extracellular matrix (ECM) proteins, such as fibronectin, fibrinogen/fibrin, laminin, and tenascin, are produced by both the basal cells and stromal keratocytes and appear on the denuded surface. Tumor growth factor (TGF)-β is known to enhance fibrosis and is thought to stimulate CTGF gene expression [146]. Studies have shown that corneal fibroblasts, keratocytes, and inflammatory cells may produce IL-1α and/or IL-1β that may act in a paracrine fashion to regulate myofibroblast apoptosis. Cao et al. assessed 1176 genes and found the expression of 37 of these genes are upregulated and the expression of 27 genes are downregulated more than 5-fold in the healing corneas when compared with those in the normal, uninjured corneas. IL-1, laminin-5, and thrombospondin-1 have all been found to be induced in the corneas in response to excimer laser treatment. The upregulated genes include intercellular adhesion molecule (ICAM)-1, macrophage inflammatory proteins, suppressors of cytokine signaling proteins (SOCS), IL-10 receptor, and galectin-7. The downregulated genes include connexin-31, a gap junction protein; ZO1 and occludin, tight junction proteins; and Smad2, a key component in the TGF signaling pathway [147].
MMPs also regulate corneal avascularity. MMP-7 (matrilysin) appears to protect against neovascularization, whereas MMP-14 is upregulated in the cornea in response to injury [143]. Studies have reported that prostaglandin E2 (PGE2) synthesis is increased in injured rabbit corneal endothelial cells [143,148]. Studies have also revealed that the concentration of EGF in tears changes rapidly following a corneal injury. The concentration of EGF is lower in unstimulated tears and higher in stimulated tears [149]. However, after chronic tearing, which occurs in patients with persistent epithelial defects or corneal ulcers, the concentration of EGF in tears decreases. This decrease is likely caused by the exhaustion of the lacrimal gland reserves of secreted proteins [150].
Other growth factors, including TGF-β, IL-1, acidic fibroblast growth factor (aFGF), bFGF, and PDGF, have been detected in tears, and PDGF concentrations were reported to increase in the tears of patients following surgery, suggesting their role in corneal wound healing [151]. Moreover, studies have reported that epiregulin is upregulated in limbal epithelial basal cells when compared with central cornea cells in mice. This upregulation of epiregulin increases corneal epithelial cell proliferation in vitro by activating EGFR and increasing the expressions of HB-EGF and Amphiregulin [152,153]. These findings suggest that epiregulin plays a role in the maintenance and proliferative capacity of limbal basal cells [154].
After corneal epithelial wounding, human growth factor (HGF) expression is upregulated in keratocytes [155] and epithelial cells [156,157], which may contribute to the epithelial wound healing process. While the rapid overexpression of insulin-like growth factor (IGF)-I in wounded mice cornea stimulates limbal cell differentiation with no effects on limbal cell proliferation [154,158], urokinase-type plasminogen activator (uPA/PLAU) is upregulated in wounded corneal epithelial cells and may stimulate cell migration [159].
Finally, damage triggers a release of inflammatory cytokines, mainly IL-1 (α and β), from epithelial cells and/or tears [143] that cause rapid apoptosis through Fas/Fas ligand system and necrosis of anterior keratocytes that induce corneal cell turnover.

Table 5.
Markers of wound healing.
Table 5.
Markers of wound healing.
| Markers of Wound Healing | Clinical Significance | References |
|---|---|---|
| IL-1α, IL-1β | Paracrine regulation of myofibroblasts apoptosis | [143,145,147] |
| TNF-α | Triggers stromal keratocytes responses, including IL-1-mediated synthesis of Fas ligand | [144,145] |
| EGF | Reflects level of intrastromal inflammation. Responds to key inflammatory mediators including IL-1 and TNF. Observed as early as 2 months before rejection. Levels decreased as immunosuppressant treatment progresses. | [145] |
| PDGF | Sub-basal and endothelial immune cell density increases associated with graft rejection. Reflects levels of stromal inflammation by responding to inflammatory mediators. | [145,151] |
| aFGF, bFGF | Binds to VEGF-A; VEGF-C; VEGF-C and D, respectively. Can act as anti-angiogenic factors in the corneal epithelial cells. | [151] |
| uPA | Corneal epithelial cells migration and proliferation | [159] |
Abbreviation listed as followed. IL: Interleukin; TNF: Tumor necrosis factor; PDGF: platelet derived growth; EGF: epidermal growth factor; aFGF: acidic fibroblast growth factor; bFGF: basic fibroblast growth factor; uPA: urokinase-type plasminogen activator.
7. Future Directions
Various physical, cellular, and molecular biomarkers have been shown to influence the outcome of corneal grafts. However, it is important to identify the optimal biomarkers for prediction of graft outcomes. Omics analysis of host and corneal grafts has not been conducted and could reveal these key markers [160]. Extracting information from a large number of biomarkers is a challenge; however, well-trained clinicians may be able to glean diagnostic and prognostic information from a smaller number of markers. Mathematical technologies are being developed to estimate the patient state from a large set of biomarkers [161] or from a very short time-series of single biomarker [162,163]. Technologies like Bayesian belief networks and random survival forests constructed from conditional inference trees [162] have been recently developed to predict solid organ graft failure and can in principle be applied to corneal grafts [164]. Mathematical models can also help in understanding the mechanisms involved in corneal graft rejection and response to therapy [43]. These technologies may be used to predict graft survival and the progression of eye diseases in general.
Engineered corneas are emerging as promising alternatives to allografts. Advances in 3D bioprinting [165] and induced pluripotent stem cells (iPSC) technology [166] are enabling the construction of cornea mimics. Biomarkers that determine the survival of these engineered grafts still need to be identified and will expectedly depend on the engineering approach used. However, studies on allograft survival may guide the development of engineered grafts with improved survival.
Author Contributions
Conceptualization, A.M. and T.I.; Resources, T.I.; Writing—Original Draft Preparation, A.D.Z., S.-M.L., J.S., M.N., M.C., A.M. and T.I.; Writing—Review & Editing, A.M. and T.I.; Funding Acquisition, T.I. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Perez, V.L.; Foulsham, W.; Peterson, K.; Dana, R. Pathophysiology of Corneal Graft Rejection. In Foundations of Corneal Disease; Colby, K., Dana, R., Eds.; Springer: Cham, Germany, 2020; pp. 87–96. [Google Scholar]
- Armitage, W.J.; Goodchild, C.; Griffin, M.D.; Gunn, D.J.; Hjortdal, J.; Lohan, P.; Murphy, C.C.; Pleyer, U.; Ritter, T.; Tole, D.M.; et al. High-risk Corneal Transplantation: Recent Developments and Future Possibilities. Transplantation 2019, 103, 2468–2478. [Google Scholar] [CrossRef]
- The collaborative corneal transplantation studies (CCTS). Effectiveness of histocompatibility matching in high-risk corneal transplantation. The Collaborative Corneal Transplantation Studies Research Group. Arch. Ophthalmol. 1992, 110, 1392–1403. [Google Scholar] [CrossRef]
- Streilein, J.W.; Arancibia-Caracamo, C.; Osawa, H. The role of minor histocompatibility alloantigens in penetrating keratoplasty. Dev. Ophthalmol. 2003, 36, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Bartels, M.C.; Doxiadis, I.I.; Colen, T.P.; Beekhuis, W.H. Long-term outcome in high-risk corneal transplantation and the influence of HLA-A and HLA-B matching. Cornea 2003, 22, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Volker-Dieben, H.J.; Claas, F.H.; Schreuder, G.M.; Schipper, R.F.; Pels, E.; Persijn, G.G.; Smits, J.; D’Amaro, J. Beneficial effect of HLA-DR matching on the survival of corneal allografts. Transplantation 2000, 70, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Hamrah, P.; Zhang, Q.; Liu, Y.; Dana, M.R. Novel characterization of MHC class II-negative population of resident corneal Langerhans cell-type dendritic cells. Investig. Ophthalmol. Vis. Sci. 2002, 43, 639–646. [Google Scholar]
- Huq, S.; Liu, Y.; Benichou, G.; Dana, M.R. Relevance of the direct pathway of sensitization in corneal transplantation is dictated by the graft bed microenvironment. J. Immunol. 2004, 173, 4464–4469. [Google Scholar] [CrossRef]
- Kocaba, V.; Colica, C.; Rabilloud, M.; Burillon, C. Predicting Corneal Graft Rejection by Confocal Microscopy. Cornea 2015, 34 (Suppl. 10), S61–S64. [Google Scholar] [CrossRef]
- Chirapapaisan, C.; Abbouda, A.; Jamali, A.; Muller, R.T.; Cavalcanti, B.M.; Colon, C.; Witkin, D.; Sahin, A.; Dana, R.; Cruzat, A.; et al. In Vivo Confocal Microscopy Demonstrates Increased Immune Cell Densities in Corneal Graft Rejection Correlating with Signs and Symptoms. Am. J. Ophthalmol. 2019, 203, 26–36. [Google Scholar] [CrossRef]
- Niederer, R.L.; Sherwin, T.; McGhee, C.N. In vivo confocal microscopy of subepithelial infiltrates in human corneal transplant rejection. Cornea 2007, 26, 501–504. [Google Scholar] [CrossRef]
- Beauregard, C.; Huq, S.O.; Barabino, S.; Zhang, Q.; Kazlauskas, A.; Dana, M.R. Keratocyte apoptosis and failure of corneal allografts. Transplantation 2006, 81, 1577–1582. [Google Scholar] [CrossRef] [PubMed]
- Hovakimyan, M.; Falke, K.; Stahnke, T.; Guthoff, R.; Witt, M.; Wree, A.; Stachs, O. Morphological analysis of quiescent and activated keratocytes: A review of ex vivo and in vivo findings. Curr. Eye Res. 2014, 39, 1129–1144. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, A.; Elewahel, S.M.; Kaufman, H.E. Confocal microscopy of corneal wound healing after deep lamellar keratoplasty in rabbits. Arch. Ophthalmol. 2010, 128, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Nishida T, S.S.; Morishige, N. Cornea and sclera: Anatomy and physiology. In Cornea, 4th ed.; Mannis MJ, H.E., Ed.; Elsevier Mosby: London, UK, 2017; Volume 1, pp. 1–22. [Google Scholar]
- Inomata, T.; Hua, J.; Nakao, T.; Shiang, T.; Chiang, H.; Amouzegar, A.; Dana, R. Corneal Tissue From Dry Eye Donors Leads to Enhanced Graft Rejection. Cornea 2018, 37, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Kendall, R.L.; Thomas, K.A. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc. Natl. Acad. Sci. USA 1993, 90, 10705–10709. [Google Scholar] [CrossRef]
- Cursiefen, C.; Chen, L.; Saint-Geniez, M.; Hamrah, P.; Jin, Y.; Rashid, S.; Pytowski, B.; Persaud, K.; Wu, Y.; Streilein, J.W.; et al. Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision. Proc. Natl. Acad. Sci. USA 2006, 103, 11405–11410. [Google Scholar] [CrossRef]
- Hajrasouliha, A.R.; Funaki, T.; Sadrai, Z.; Hattori, T.; Chauhan, S.K.; Dana, R. Vascular endothelial growth factor-C promotes alloimmunity by amplifying antigen-presenting cell maturation and lymphangiogenesis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1244–1250. [Google Scholar] [CrossRef]
- Emami-Naeini, P.; Dohlman, T.H.; Omoto, M.; Hattori, T.; Chen, Y.; Lee, H.S.; Chauhan, S.K.; Dana, R. Soluble vascular endothelial growth factor receptor-3 suppresses allosensitization and promotes corneal allograft survival. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 1755–1762. [Google Scholar] [CrossRef]
- Chauhan, S.K.; Dohlman, T.H.; Dana, R. Corneal Lymphatics: Role in Ocular Inflammation as Inducer and Responder of Adaptive Immunity. J. Clin. Cell Immunol 2014, 5. [Google Scholar] [CrossRef]
- Albuquerque, R.J.; Hayashi, T.; Cho, W.G.; Kleinman, M.E.; Dridi, S.; Takeda, A.; Baffi, J.Z.; Yamada, K.; Kaneko, H.; Green, M.G.; et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat. Med. 2009, 15, 1023–1030. [Google Scholar] [CrossRef]
- Zhu, S.; Dekaris, I.; Duncker, G.; Dana, M.R. Early expression of proinflammatory cytokines interleukin-1 and tumor necrosis factor-alpha after corneal transplantation. J. Interferon Cytokine Res. 1999, 19, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.W.; Liu, J.J.; Lee, J.S.; Mohan, R.R.; Mohan, R.R.; Woods, D.J.; He, Y.G.; Wilson, S.E. Proinflammatory chemokine induction in keratocytes and inflammatory cell infiltration into the cornea. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2795–2803. [Google Scholar]
- Nishida, T. Commanding roles of keratocytes in health and disease. Cornea 2010, 29 (Suppl. 1), S3–S6. [Google Scholar] [CrossRef]
- King, W.J.; Comer, R.M.; Hudde, T.; Larkin, D.F.; George, A.J. Cytokine and chemokine expression kinetics after corneal transplantation. Transplantation 2000, 70, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Maier, P.; Heizmann, U.; Bohringer, D.; Kern, Y.; Reinhard, T. Predicting the risk for corneal graft rejection by aqueous humor analysis. Mol. Vis. 2011, 17, 1016–1023. [Google Scholar] [PubMed]
- Johnson, L.A.; Jackson, D.G. Inflammation-induced secretion of CCL21 in lymphatic endothelium is a key regulator of integrin-mediated dendritic cell transmigration. Int. Immunol 2010, 22, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Yamagami, S.; Hamrah, P.; Zhang, Q.; Liu, Y.; Huq, S.; Dana, M.R. Early ocular chemokine gene expression and leukocyte infiltration after high-risk corneal transplantation. Mol. Vis. 2005, 11, 632–640. [Google Scholar]
- Jin, Y.; Shen, L.; Chong, E.M.; Hamrah, P.; Zhang, Q.; Chen, L.; Dana, M.R. The chemokine receptor CCR7 mediates corneal antigen-presenting cell trafficking. Mol. Vis. 2007, 13, 626–634. [Google Scholar]
- Yamaguchi, T.; Higa, K.; Suzuki, T.; Nakayama, N.; Yagi-Yaguchi, Y.; Dogru, M.; Satake, Y.; Shimazaki, J. Elevated Cytokine Levels in the Aqueous Humor of Eyes with Bullous Keratopathy and Low Endothelial Cell Density. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5954–5962. [Google Scholar] [CrossRef]
- Yoon, C.H.; Choi, S.H.; Lee, H.J.; Kang, H.J.; Kim, M.K. Predictive biomarkers for graft rejection in pig-to-non-human primate corneal xenotransplantation. Xenotransplantation 2019, 26, e12515. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, J.; Qian, T.; Dai, Y.; Mashaghi, A.; Xu, J.; Hong, J. IFN-gamma Regulates the Expression of MICA in Human Corneal Epithelium Through miRNA4448 and NFkappaB. Front. Immunol. 2018, 9, 1530. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.N.; Yamada, J.; Streilein, J.W.; Dana, M.R. ICAM-1 deficiency suppresses host allosensitization and rejection of MHC-disparate corneal transplants. Transplantation 2000, 69, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Grimaldo, S.; Yuen, D.; Ecoiffier, T.; Chen, L. Very late antigen-1 mediates corneal lymphangiogenesis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4808–4812. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.N.; Dana, M.R. Expression of cell adhesion molecules on limbal and neovascular endothelium in corneal inflammatory neovascularization. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1427–1434. [Google Scholar]
- Goldberg, M.F.; Ferguson, T.A.; Pepose, J.S. Detection of cellular adhesion molecules in inflamed human corneas. Ophthalmol. Ogy 1994, 101, 161–168. [Google Scholar] [CrossRef]
- Hamrah, P.; Liu, Y.; Zhang, Q.; Dana, M.R. Alterations in corneal stromal dendritic cell phenotype and distribution in inflammation. Arch. Ophthalmol. 2003, 121, 1132–1140. [Google Scholar] [CrossRef]
- Hamrah, P.; Liu, Y.; Zhang, Q.; Dana, M.R. The corneal stroma is endowed with a significant number of resident dendritic cells. Investig. Ophthalmol. Vis. Sci. 2003, 44, 581–589. [Google Scholar] [CrossRef]
- Inomata, T.; Hua, J.; Di Zazzo, A.; Dana, R. Impaired Function of Peripherally Induced Regulatory T Cells in Hosts at High Risk of Graft Rejection. Sci. Rep. 2016, 6, 39924. [Google Scholar] [CrossRef]
- Bestard, O.; Cruzado, J.M.; Rama, I.; Torras, J.; Goma, M.; Seron, D.; Moreso, F.; Gil-Vernet, S.; Grinyo, J.M. Presence of FoxP3+ regulatory T Cells predicts outcome of subclinical rejection of renal allografts. J. Am. Soc. Nephrol. J. Asn 2008, 19, 2020–2026. [Google Scholar] [CrossRef]
- Amouzegar, A.; Chauhan, S.K.; Dana, R. Alloimmunity and Tolerance in Corneal Transplantation. J. Immunol. 2016, 196, 3983–3991. [Google Scholar] [CrossRef]
- Azimzade, Y.; Hong, J.; Mashaghi, A. Immunophysical analysis of corneal neovascularization: Mechanistic insights and implications for pharmacotherapy. Sci. Rep. 2017, 7, 12220. [Google Scholar] [CrossRef]
- Hill, J.C. Systemic cyclosporine in high-risk keratoplasty: Long-term results. Eye 1995, 9 Pt 4, 422–428. [Google Scholar] [CrossRef]
- Poon, A.C.; Forbes, J.E.; Dart, J.K.; Subramaniam, S.; Bunce, C.; Madison, P.; Ficker, L.A.; Tuft, S.J.; Gartry, D.S.; Buckley, R.J. Systemic cyclosporin A in high risk penetrating keratoplasties: A case-control study. Br. J. Ophthalmol. 2001, 85, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Rumelt, S.; Bersudsky, V.; Blum-Hareuveni, T.; Rehany, U. Systemic cyclosporin A in high failure risk, repeated corneal transplantation. Br J. Ophthalmol. 2002, 86, 988–992. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, E.; Papaliodis, G.N.; Lobo, A.M.; Sobrin, L. Side-effects of anti-inflammatory therapy in uveitis. Semin. Ophthalmol. 2014, 29, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Jalbert, I.; Stapleton, F.; Papas, E.; Sweeney, D.F.; Coroneo, M. In vivo confocal microscopy of the human cornea. Br. J. Ophthalmol. 2003, 87, 225–236. [Google Scholar] [CrossRef]
- Ing, J.J.; Ing, H.H.; Nelson, L.R.; Hodge, D.O.; Bourne, W.M. Ten-year postoperative results of penetrating keratoplasty. Ophthalmol. Ogy 1998, 105, 1855–1865. [Google Scholar] [CrossRef]
- Feizi, S. Corneal endothelial cell dysfunction: Etiologies and management. Adv Ophthalmol. 2018, 10, 2515841418815802. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Sakaguchi, N.; Asano, M.; Itoh, M.; Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995, 155, 1151–1164. [Google Scholar]
- Chauhan, S.K.; Saban, D.R.; Lee, H.K.; Dana, R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J. Immunol. 2009, 182, 148–153. [Google Scholar] [CrossRef]
- McBride, B.W.; McGill, J.I.; Smith, J.L. MHC class I and class II antigen expression in normal human corneas and in corneas from cases of herpetic keratitis. Immunology 1988, 65, 583–587. [Google Scholar] [PubMed]
- Whitsett, C.F.; Stulting, R.D. The distribution of HLA antigens on human corneal tissue. Investig. Ophthalmol. Vis. Sci. 1984, 25, 519–524. [Google Scholar]
- Chong, E.M.; Dana, M.R. Graft failure IV. Immunologic mechanisms of corneal transplant rejection. Int Ophthalmol. 2008, 28, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Lagali, N.S.; Badian, R.A.; Liu, X.; Feldreich, T.R.; Arnlov, J.; Utheim, T.P.; Dahlin, L.B.; Rolandsson, O. Dendritic cell maturation in the corneal epithelium with onset of type 2 diabetes is associated with tumor necrosis factor receptor superfamily member 9. Sci. Rep. 2018, 8, 14248. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Jin, Y.; Chen, Y.; Inomata, T.; Lee, H.; Chauhan, S.K.; Petasis, N.A.; Serhan, C.N.; Dana, R. The resolvin D1 analogue controls maturation of dendritic cells and suppresses alloimmunity in corneal transplantation. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5944–5951. [Google Scholar] [CrossRef]
- Hua, J.; Stevenson, W.; Dohlman, T.H.; Inomata, T.; Tahvildari, M.; Calcagno, N.; Pirmadjid, N.; Sadrai, Z.; Chauhan, S.K.; Dana, R. Graft Site Microenvironment Determines Dendritic Cell Trafficking Through the CCR7-CCL19/21 Axis. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1457–1467. [Google Scholar] [CrossRef]
- Hatou, S.; Yoshida, S.; Higa, K.; Miyashita, H.; Inagaki, E.; Okano, H.; Tsubota, K.; Shimmura, S. Functional corneal endothelium derived from corneal stroma stem cells of neural crest origin by retinoic acid and Wnt/beta-catenin signaling. Stem. Cells Dev. 2013, 22, 828–839. [Google Scholar] [CrossRef]
- Tuft, S.J.; Coster, D.J. The corneal endothelium. Eye 1990, 4 Pt 3, 389–424. [Google Scholar] [CrossRef]
- Yamamoto, A.; Tanaka, H.; Toda, M.; Sotozono, C.; Hamuro, J.; Kinoshita, S.; Ueno, M.; Tanaka, M. A physical biomarker of the quality of cultured corneal endothelial cells and of the long-term prognosis of corneal restoration in patients. Nat. Biomed. Eng. 2019. [Google Scholar] [CrossRef]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. Jama Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef]
- Bourne, W.M. Biology of the corneal endothelium in health and disease. Eye 2003, 17, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Sayegh RR, B.B.; Lass, J.H. Specular Microscopy. In Cornea, 4th ed.; Mannis MJ, H.E., Ed.; Elsevier Mosby: London, UK, 2017; Volume 1, pp. 160–179. [Google Scholar]
- Nishimura, J.K.; Hodge, D.O.; Bourne, W.M. Initial endothelial cell density and chronic endothelial cell loss rate in corneal transplants with late endothelial failure. Ophthalmol. Ogy 1999, 106, 1962–1965. [Google Scholar] [CrossRef]
- Lass, J.H.; Sugar, A.; Benetz, B.A.; Beck, R.W.; Dontchev, M.; Gal, R.L.; Kollman, C.; Gross, R.; Heck, E.; Holland, E.J.; et al. Endothelial cell density to predict endothelial graft failure after penetrating keratoplasty. Arch. Ophthalmol. 2010, 128, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Benetz, B.A.; Lass, J.H.; Gal, R.L.; Sugar, A.; Menegay, H.; Dontchev, M.; Kollman, C.; Beck, R.W.; Mannis, M.J.; Holland, E.J.; et al. Endothelial morphometric measures to predict endothelial graft failure after penetrating keratoplasty. Jama Ophthalmol. 2013, 131, 601–608. [Google Scholar] [CrossRef]
- Rock, T.; Landenberger, J.; Bramkamp, M.; Bartz-Schmidt, K.U.; Rock, D. The Evolution of Corneal Transplantation. Ann. Transpl. 2017, 22, 749–754. [Google Scholar] [CrossRef]
- Patel, S.V. Graft survival and endothelial outcomes in the new era of endothelial keratoplasty. Exp. Eye Res. 2012, 95, 40–47. [Google Scholar] [CrossRef]
- Ishii, N.; Yamaguchi, T.; Yazu, H.; Satake, Y.; Yoshida, A.; Shimazaki, J. Factors associated with graft survival and endothelial cell density after Descemet’s stripping automated endothelial keratoplasty. Sci. Rep. 2016, 6, 25276. [Google Scholar] [CrossRef]
- Oellerich, S.; Ham, L.; Frank, L.E.; Gorges, S.; Bourgonje, V.J.A.; Baydoun, L.; van Dijk, K.; Melles, G.R.J. Parameters associated with endothelial cell density variability after Descemet membrane endothelial keratoplasty. Am. J. Ophthalmol. 2019. [Google Scholar] [CrossRef]
- Ivarsdottir, E.V.; Benonisdottir, S.; Thorleifsson, G.; Sulem, P.; Oddsson, A.; Styrkarsdottir, U.; Kristmundsdottir, S.; Arnadottir, G.A.; Thorgeirsson, G.; Jonsdottir, I.; et al. Sequence variation at ANAPC1 accounts for 24% of the variability in corneal endothelial cell density. Nat. Commun. 2019, 10, 1284. [Google Scholar] [CrossRef]
- Joyce, N.C.; Zhu, C.C. Human corneal endothelial cell proliferation: Potential for use in regenerative medicine. Cornea 2004, 23, S8–S19. [Google Scholar] [CrossRef]
- Shen, L.; Sun, P.; Zhang, C.; Yang, L.; Du, L.; Wu, X. Therapy of corneal endothelial dysfunction with corneal endothelial cell-like cells derived from skin-derived precursors. Sci. Rep. 2017, 7, 13400. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, E.; Hatou, S.; Higa, K.; Yoshida, S.; Shibata, S.; Okano, H.; Tsubota, K.; Shimmura, S. Skin-Derived Precursors as a Source of Progenitors for Corneal Endothelial Regeneration. Stem. Cells Transl. Med. 2017, 6, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Koizumi, N.; Ueno, M.; Okumura, N.; Imai, K.; Tanaka, H.; Yamamoto, Y.; Nakamura, T.; Inatomi, T.; Bush, J.; et al. Injection of Cultured Cells with a ROCK Inhibitor for Bullous Keratopathy. N. Engl. J. Med. 2018, 378, 995–1003. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Shima, N.; Kimoto, M.; Ebihara, N.; Murakami, A.; Yamagami, S. Markers for distinguishing cultured human corneal endothelial cells from corneal stromal myofibroblasts. Curr. Eye Res. 2015, 40, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Dorfmueller, S.; Tan, H.C.; Ngoh, Z.X.; Toh, K.Y.; Peh, G.; Ang, H.P.; Seah, X.Y.; Chin, A.; Choo, A.; Mehta, J.S.; et al. Isolation of a recombinant antibody specific for a surface marker of the corneal endothelium by phage display. Sci. Rep. 2016, 6, 21661. [Google Scholar] [CrossRef] [PubMed]
- Bartakova, A.; Alvarez-Delfin, K.; Weisman, A.D.; Salero, E.; Raffa, G.A.; Merkhofer, R.M., Jr.; Kunzevitzky, N.J.; Goldberg, J.L. Novel Identity and Functional Markers for Human Corneal Endothelial Cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2749–2762. [Google Scholar] [CrossRef]
- Hamuro, J.; Toda, M.; Asada, K.; Hiraga, A.; Schlotzer-Schrehardt, U.; Montoya, M.; Sotozono, C.; Ueno, M.; Kinoshita, S. Cell Homogeneity Indispensable for Regenerative Medicine by Cultured Human Corneal Endothelial Cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4749–4761. [Google Scholar] [CrossRef]
- Cheong, Y.K.; Ngoh, Z.X.; Peh, G.S.; Ang, H.P.; Seah, X.Y.; Chng, Z.; Colman, A.; Mehta, J.S.; Sun, W. Identification of cell surface markers glypican-4 and CD200 that differentiate human corneal endothelium from stromal fibroblasts. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4538–4547. [Google Scholar] [CrossRef]
- Yoshihara, M.; Ohmiya, H.; Hara, S.; Kawasaki, S.; Consortium, F.; Hayashizaki, Y.; Itoh, M.; Kawaji, H.; Tsujikawa, M.; Nishida, K. Discovery of molecular markers to discriminate corneal endothelial cells in the human body. PLoS ONE 2015, 10, e0117581. [Google Scholar] [CrossRef]
- Inomata, T.; Mashaghi, A.; Di Zazzo, A.; Lee, S.M.; Chiang, H.; Dana, R. Kinetics of Angiogenic Responses in Corneal Transplantation. Cornea 2017, 36, 491–496. [Google Scholar] [CrossRef]
- Di Zazzo, A.; Kheirkhah, A.; Abud, T.B.; Goyal, S.; Dana, R. Management of high-risk corneal transplantation. Surv. Ophthalmol. 2017, 62, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, N.S.; Amgad, M.; Zayed, A.A.; Salem, H.; Elkhanany, A.E.; Hussein, H.; Abd El-Baky, N. Clinical correlates of common corneal neovascular diseases: A literature review. Int. J. Ophthalmol. 2015, 8, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care (New Rochelle) 2014, 3, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Mizia-Malarz, A.; Sobol, G.; Wos, H. Proangiogenic factors: Vascular-endothelial growth factor (VEGF) and basic fibroblast growth factor--the characteristics and function. Przegl Lek 2008, 65, 353–357. [Google Scholar] [PubMed]
- Niu, G.; Chen, X. Why integrin as a primary target for imaging and therapy. Theranostics 2011, 1, 30–47. [Google Scholar] [CrossRef]
- Voiculescu, O.B.; Voinea, L.M.; Alexandrescu, C. Corneal neovascularization and biological therapy. J. Med. Life 2015, 8, 444–448. [Google Scholar]
- Cauchi, P.A.; Ang, G.S.; Azuara-Blanco, A.; Burr, J.M. A systematic literature review of surgical interventions for limbal stem cell deficiency in humans. Am. J. Ophthalmol. 2008, 146, 251–259. [Google Scholar] [CrossRef]
- Cursiefen, C.; Chen, L.; Borges, L.P.; Jackson, D.; Cao, J.; Radziejewski, C.; D’Amore, P.A.; Dana, M.R.; Wiegand, S.J.; Streilein, J.W. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Investig. 2004, 113, 1040–1050. [Google Scholar] [CrossRef]
- Anshu, A.; Price, M.O.; Price, F.W., Jr. Risk of corneal transplant rejection significantly reduced with Descemet’s membrane endothelial keratoplasty. Ophthalmol. Ogy 2012, 119, 536–540. [Google Scholar] [CrossRef]
- Goel, S.; Duda, D.G.; Xu, L.; Munn, L.L.; Boucher, Y.; Fukumura, D.; Jain, R.K. Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev. 2011, 91, 1071–1121. [Google Scholar] [CrossRef]
- Dawson, D.W.; Volpert, O.V.; Gillis, P.; Crawford, S.E.; Xu, H.; Benedict, W.; Bouck, N.P. Pigment epithelium-derived factor: A potent inhibitor of angiogenesis. Science 1999, 285, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Hornig, C.; Weich, H.A. Soluble VEGF receptors. Angiogenesis 1999, 3, 33–39. [Google Scholar] [CrossRef]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dabrosin, C.; Yin, X.; Fuster, M.M.; Arreola, A.; Rathmell, W.K.; Generali, D.; Nagaraju, G.P.; El-Rayes, B.; Ribatti, D.; et al. Broad targeting of angiogenesis for cancer prevention and therapy. Semin. Cancer Biol. 2015, 35, S224–S243. [Google Scholar] [CrossRef] [PubMed]
- Ellenberg, D.; Azar, D.T.; Hallak, J.A.; Tobaigy, F.; Han, K.Y.; Jain, S.; Zhou, Z.; Chang, J.H. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog. Retin Eye Res. 2010, 29, 208–248. [Google Scholar] [CrossRef]
- Han, K.Y.; Chang, J.H.; Dugas-Ford, J.; Alexander, J.S.; Azar, D.T. Involvement of lysosomal degradation in VEGF-C-induced down-regulation of VEGFR-3. Febs Lett. 2014, 588, 4357–4363. [Google Scholar] [CrossRef]
- Otrock, Z.K.; Makarem, J.A.; Shamseddine, A.I. Vascular endothelial growth factor family of ligands and receptors: Review. Blood Cells Mol. Dis. 2007, 38, 258–268. [Google Scholar] [CrossRef]
- Zhong, W.; Montana, M.; Santosa, S.M.; Isjwara, I.D.; Huang, Y.H.; Han, K.Y.; O’Neil, C.; Wang, A.; Cortina, M.S.; de la Cruz, J.; et al. Angiogenesis and lymphangiogenesis in corneal transplantation-A review. Surv. Ophthalmol. 2018, 63, 453–479. [Google Scholar] [CrossRef]
- Chen, L.; Huq, S.; Gardner, H.; de Fougerolles, A.R.; Barabino, S.; Dana, M.R. Very late antigen 1 blockade markedly promotes survival of corneal allografts. Arch. Ophthalmol. 2007, 125, 783–788. [Google Scholar] [CrossRef][Green Version]
- Yamagami, S.; Dana, M.R. The critical role of lymph nodes in corneal alloimmunization and graft rejection. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1293–1298. [Google Scholar]
- Patel, S.P.; Dana, R. Corneal lymphangiogenesis: Implications in immunity. Semin. Ophthalmol. 2009, 24, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Betsholtz, C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev. 2004, 15, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Hellstrom, M.; Gerhardt, H.; Kalen, M.; Li, X.; Eriksson, U.; Wolburg, H.; Betsholtz, C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J. Cell Biol. 2001, 153, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.; Giacomini, C.; Bignami, F.; Moi, D.; Ranghetti, A.; Doglioni, C.; Naldini, L.; Rama, P.; Mazzieri, R. Angiopoietin 2 expression in the cornea and its control of corneal neovascularisation. Br. J. Ophthalmol. 2016, 100, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Ambati, B.K.; Nozaki, M.; Singh, N.; Takeda, A.; Jani, P.D.; Suthar, T.; Albuquerque, R.J.; Richter, E.; Sakurai, E.; Newcomb, M.T.; et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature 2006, 443, 993–997. [Google Scholar] [CrossRef]
- Diamond, M.A.; Chan, S.W.S.; Zhou, X.; Glinka, Y.; Girard, E.; Yucel, Y.; Gupta, N. Lymphatic vessels identified in failed corneal transplants with neovascularisation. Br. J. Ophthalmol. 2019, 103, 421–427. [Google Scholar] [CrossRef]
- Baluk, P.; McDonald, D.M. Markers for microscopic imaging of lymphangiogenesis and angiogenesis. Ann. N. Y. Acad. Sci. 2008, 1131, 1–12. [Google Scholar] [CrossRef]
- Kong, L.L.; Yang, N.Z.; Shi, L.H.; Zhao, G.H.; Zhou, W.; Ding, Q.; Wang, M.H.; Zhang, Y.S. The optimum marker for the detection of lymphatic vessels. Mol. Clin. Oncol. 2017, 7, 515–520. [Google Scholar] [CrossRef]
- Schroedl, F.; Kaser-Eichberger, A.; Schlereth, S.L.; Bock, F.; Regenfuss, B.; Reitsamer, H.A.; Lutty, G.A.; Maruyama, K.; Chen, L.; Lutjen-Drecoll, E.; et al. Consensus statement on the immunohistochemical detection of ocular lymphatic vessels. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6440–6442. [Google Scholar] [CrossRef]
- Randleman, J.B.; Woodward, M.; Lynn, M.J.; Stulting, R.D. Risk assessment for ectasia after corneal refractive surgery. Ophthalmol. Ogy 2008, 115, 37–50. [Google Scholar] [CrossRef]
- Stewart, W.C.; Jenkins, J.N.; Stewart, J.A. Corneal thickness after refractive surgery. Ophthalmol. Ogy 2005, 112, 1637. [Google Scholar] [CrossRef]
- Jonas, J.B.; Holbach, L. Central corneal thickness and thickness of the lamina cribrosa in human eyes. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Verdier, D.D.; Sugar, A.; Baratz, K.; Beck, R.; Dontchev, M.; Dunn, S.; Gal, R.L.; Holland, E.J.; Kollman, C.; Lass, J.H.; et al. Corneal thickness as a predictor of corneal transplant outcome. Cornea 2013, 32, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee for the Cornea Donor Study Research, G.; Sugar, A.; Gal, R.L.; Kollman, C.; Raghinaru, D.; Dontchev, M.; Croasdale, C.R.; Feder, R.S.; Holland, E.J.; Lass, J.H.; et al. Factors associated with corneal graft survival in the cornea donor study. Jama Ophthalmol. 2015, 133, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Su, D.H.; Wong, T.Y.; Wong, W.L.; Saw, S.M.; Tan, D.T.; Shen, S.Y.; Loon, S.C.; Foster, P.J.; Aung, T.; Singapore Malay Eye Study, G. Diabetes, hyperglycemia, and central corneal thickness: The Singapore Malay Eye Study. Ophthalmol. Ogy 2008, 115, 964–968. [Google Scholar] [CrossRef]
- Wang, D.; Huang, W.; Li, Y.; Zheng, Y.; Foster, P.J.; Congdon, N.; He, M. Intraocular pressure, central corneal thickness, and glaucoma in chinese adults: The liwan eye study. Am. J. Ophthalmol. 2011, 152, 454–462. [Google Scholar] [CrossRef]
- Maurice, D.M. The location of the fluid pump in the cornea. J. Physiol 1972, 221, 43–54. [Google Scholar] [CrossRef]
- Levenson, J.E. Corneal edema: Cause and treatment. Surv. Ophthalmol. 1975, 20, 190–204. [Google Scholar] [CrossRef]
- Kopplin, L.J.; Przepyszny, K.; Schmotzer, B.; Rudo, K.; Babineau, D.C.; Patel, S.V.; Verdier, D.D.; Jurkunas, U.; Iyengar, S.K.; Lass, J.H.; et al. Relationship of Fuchs endothelial corneal dystrophy severity to central corneal thickness. Arch. Ophthalmol. 2012, 130, 433–439. [Google Scholar] [CrossRef]
- Lu, Y.; Dimasi, D.P.; Hysi, P.G.; Hewitt, A.W.; Burdon, K.P.; Toh, T.; Ruddle, J.B.; Li, Y.J.; Mitchell, P.; Healey, P.R.; et al. Common genetic variants near the Brittle Cornea Syndrome locus ZNF469 influence the blinding disease risk factor central corneal thickness. PLoS Genet. 2010, 6, e1000947. [Google Scholar] [CrossRef]
- Vitart, V.; Bencic, G.; Hayward, C.; Skunca Herman, J.; Huffman, J.; Campbell, S.; Bucan, K.; Navarro, P.; Gunjaca, G.; Marin, J.; et al. New loci associated with central cornea thickness include COL5A1, AKAP13 and AVGR8. Hum. Mol. Genet. 2010, 19, 4304–4311. [Google Scholar] [CrossRef] [PubMed]
- Vithana, E.N.; Aung, T.; Khor, C.C.; Cornes, B.K.; Tay, W.T.; Sim, X.; Lavanya, R.; Wu, R.; Zheng, Y.; Hibberd, M.L.; et al. Collagen-related genes influence the glaucoma risk factor, central corneal thickness. Hum. Mol. Genet. 2011, 20, 649–658. [Google Scholar] [CrossRef]
- Nicholls, S.; Pong-Wong, R.; Mitchard, L.; Harley, R.; Archibald, A.; Dick, A.; Bailey, M. Genome-Wide Analysis in Swine Associates Corneal Graft Rejection with Donor-Recipient Mismatches in Three Novel Histocompatibility Regions and One Locus Homologous to the Mouse H-3 Locus. PLoS ONE 2016, 11, e0152155. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, C.; Roberts, C.J.; Mahmoud, A.M.; Colin, J.; Maurice-Tison, S.; Kerautret, J. Screening of forme fruste keratoconus with the ocular response analyzer. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2403–2410. [Google Scholar] [CrossRef] [PubMed]
- Dimasi, D.P.; Burdon, K.P.; Craig, J.E. The genetics of central corneal thickness. Br. J. Ophthalmol. 2010, 94, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Moestrup, B. Tenuity of cornea with Ehlers-Danlos syndrome. Acta Ophthalmol. (Copenh) 1969, 47, 704–708. [Google Scholar] [CrossRef]
- Pesudovs, K. Orbscan mapping in Ehlers-Danlos syndrome. J. Cataract Refract. Surg. 2004, 30, 1795–1798. [Google Scholar] [CrossRef]
- Blumcke, S.; Niedorf, H.R.; Thiel, H.J.; Langness, U. Histochemical and fine structural studies on the cornea with osteogenesis imperfecta congenita. Virchows Arch. B Cell Pathol. 1972, 11, 124–132. [Google Scholar] [CrossRef]
- Surquin, M.; Le Moine, A.; Flamand, V.; Nagy, N.; Rombaut, K.; Demoor, F.X.; Stordeur, P.; Salmon, I.; Guery, J.C.; Goldman, M.; et al. Skin graft rejection elicited by beta 2-microglobulin as a minor transplantation antigen involves multiple effector pathways: Role of Fas-Fas ligand interactions and Th2-dependent graft eosinophil infiltrates. J. Immunol. 2002, 169, 500–506. [Google Scholar] [CrossRef]
- Bohringer, D.; Spierings, E.; Enczmann, J.; Bohringer, S.; Sundmacher, R.; Goulmy, E.; Reinhard, T. Matching of the minor histocompatibility antigen HLA-A1/H-Y may improve prognosis in corneal transplantation. Transplantation 2006, 82, 1037–1041. [Google Scholar] [CrossRef]
- Qazi, Y.; Hamrah, P. Gene therapy in corneal transplantation. Semin. Ophthalmol. 2013, 28, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.S.; Lee, J.; Ferrara, N. Targeting the tumour vasculature: Insights from physiological angiogenesis. Nat. Rev. Cancer 2010, 10, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Chazaud, B. Macrophages: Supportive cells for tissue repair and regeneration. Immunobiology 2014, 219, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, O.; Sumioka, T.; Saika, S. The role of extracellular matrix in corneal wound healing. Cornea 2013;32(suppl): s43-s45-retracted. Cornea 2014, 33, 100. [Google Scholar] [CrossRef]
- Chung, E.S.; Chauhan, S.K.; Jin, Y.; Nakao, S.; Hafezi-Moghadam, A.; van Rooijen, N.; Zhang, Q.; Chen, L.; Dana, R. Contribution of macrophages to angiogenesis induced by vascular endothelial growth factor receptor-3-specific ligands. Am. J. Pathol. 2009, 175, 1984–1992. [Google Scholar] [CrossRef]
- Davis, G.E.; Senger, D.R. Endothelial extracellular matrix: Biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ. Res. 2005, 97, 1093–1107. [Google Scholar] [CrossRef]
- Greenhalgh, D.G. The role of apoptosis in wound healing. Int. J. Biochem. Cell Biol. 1998, 30, 1019–1030. [Google Scholar] [CrossRef]
- Boosani, C.S.; Sudhakar, Y.A. Proteolytically Derived Endogenous Angioinhibitors Originating from the Extracellular Matrix. Pharmaceuticals 2011, 4, 1551–1577. [Google Scholar] [CrossRef]
- Jackson, J.R.; Seed, M.P.; Kircher, C.H.; Willoughby, D.A.; Winkler, J.D. The codependence of angiogenesis and chronic inflammation. Faseb J. 1997, 11, 457–465. [Google Scholar] [CrossRef]
- Maycock, N.J.; Marshall, J. Genomics of corneal wound healing: A review of the literature. Acta Ophthalmol. 2014, 92, e170–e184. [Google Scholar] [CrossRef]
- Wilson, S.E.; Liu, J.J.; Mohan, R.R. Stromal-epithelial interactions in the cornea. Prog. Retin. Eye Res. 1999, 18, 293–309. [Google Scholar] [CrossRef]
- Tuominen, I.S.; Tervo, T.M.; Teppo, A.M.; Valle, T.U.; Gronhagen-Riska, C.; Vesaluoma, M.H. Human tear fluid PDGF-BB, TNF-alpha and TGF-beta1 vs corneal haze and regeneration of corneal epithelium and subbasal nerve plexus after PRK. Exp. Eye Res. 2001, 72, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, K.; Senn, B.C.; Reiser, P.; Pech, M.; Flammer, J.; Meyer, P. Connective tissue growth factor in retrocorneal membranes and corneal scars. Ophthalmol. Ogica 2000, 214, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Flanders, K.C.; Bertolette, D.; Lyakh, L.A.; Wurthner, J.U.; Parks, W.T.; Letterio, J.J.; Ruscetti, F.W.; Roberts, A.B. Levels of phospho-Smad2/3 are sensors of the interplay between effects of TGF-beta and retinoic acid on monocytic and granulocytic differentiation of HL-60 cells. Blood 2003, 101, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Jumblatt, M.M.; Willer, S.S. Corneal endothelial repair. Regulation of prostaglandin E2 synthesis. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1294–1301. [Google Scholar]
- van Setten, G.B.; Tervo, T.; Viinikka, L.; Perheentupa, J.; Tarkkanen, A. Epidermal growth factor in human tear fluid: A minireview. Int. Ophthalmol. 1991, 15, 359–362. [Google Scholar] [CrossRef]
- Lim, M.; Goldstein, M.H.; Tuli, S.; Schultz, G.S. Growth factor, cytokine and protease interactions during corneal wound healing. Ocul. Surf. 2003, 1, 53–65. [Google Scholar] [CrossRef]
- Vesaluoma, M.; Teppo, A.M.; Gronhagen-Riska, C.; Tervo, T. Platelet-derived growth factor-BB (PDGF-BB) in tear fluid: A potential modulator of corneal wound healing following photorefractive keratectomy. Curr. Eye Res. 1997, 16, 825–831. [Google Scholar] [CrossRef]
- Morita, S.; Shirakata, Y.; Shiraishi, A.; Kadota, Y.; Hashimoto, K.; Higashiyama, S.; Ohashi, Y. Human corneal epithelial cell proliferation by epiregulin and its cross-induction by other EGF family members. Mol. Vis. 2007, 13, 2119–2128. [Google Scholar]
- Zhou, M.; Li, X.M.; Lavker, R.M. Transcriptional profiling of enriched populations of stem cells versus transient amplifying cells. A comparison of limbal and corneal epithelial basal cells. J. Biol. Chem. 2006, 281, 19600–19609. [Google Scholar] [CrossRef]
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Q.; Tseng, S.C. Differential regulation of cytokine and receptor transcript expression in human corneal and limbal fibroblasts by epidermal growth factor, transforming growth factor-alpha, platelet-derived growth factor B, and interleukin-1 beta. Investig. Ophthalmol. Vis. Sci. 1996, 37, 2068–2080. [Google Scholar]
- Kakazu, A.; Sharma, G.; Bazan, H.E. Association of protein tyrosine phosphatases (PTPs)-1B with c-Met receptor and modulation of corneal epithelial wound healing. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2927–2935. [Google Scholar] [CrossRef] [PubMed]
- Saghizadeh, M.; Kramerov, A.A.; Yu, F.S.; Castro, M.G.; Ljubimov, A.V. Normalization of wound healing and diabetic markers in organ cultured human diabetic corneas by adenoviral delivery of c-Met gene. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Trosan, P.; Svobodova, E.; Chudickova, M.; Krulova, M.; Zajicova, A.; Holan, V. The key role of insulin-like growth factor I in limbal stem cell differentiation and the corneal wound-healing process. Stem. Cells Dev. 2012, 21, 3341–3350. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Yano, W.; Kondo, S.; Hattori, Y.; Yamada, N.; Yanai, R.; Nishida, T. Up-regulation of urokinase-type plasminogen activator in corneal epithelial cells induced by wounding. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3332–3338. [Google Scholar] [CrossRef]
- Paladini, S.V.; Pinto, G.H.; Bueno, R.H.; Calloni, R.; Recamonde-Mendoza, M. Identification of Candidate Biomarkers for Transplant Rejection from Transcriptome Data: A Systematic Review. Mol. Diagn. Ther. 2019, 23, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Rajkomar, A.; Dean, J.; Kohane, I. Machine Learning in Medicine. N. Engl. J. Med. 2019, 380, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Mark, E.; Goldsman, D.; Gurbaxani, B.; Keskinocak, P.; Sokol, J. Using machine learning and an ensemble of methods to predict kidney transplant survival. PLoS ONE 2019, 14, e0209068. [Google Scholar] [CrossRef] [PubMed]
- Morino, K.; Hirata, Y.; Tomioka, R.; Kashima, H.; Yamanishi, K.; Hayashi, N.; Egawa, S.; Aihara, K. Predicting disease progression from short biomarker series using expert advice algorithm. Sci. Rep. 2015, 5, 8953. [Google Scholar] [CrossRef]
- Senanayake, S.; White, N.; Graves, N.; Healy, H.; Baboolal, K.; Kularatna, S. Machine learning in predicting graft failure following kidney transplantation: A systematic review of published predictive models. Int. J. Med. Inf. 2019, 130, 103957. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jang, J.; Park, J.; Lee, K.P.; Lee, S.; Lee, D.M.; Kim, K.H.; Kim, H.K.; Cho, D.W. Shear-induced alignment of collagen fibrils using 3D cell printing for corneal stroma tissue engineering. Biofabrication 2019, 11, 035017. [Google Scholar] [CrossRef] [PubMed]
- Cyranoski, D. Woman is First to Receive Cornea Made from ‘Reprogrammed’ Stem Cells. Available online: https://www.nature.com/articles/d41586-019-02597-2 (accessed on 6 December 2019).
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).