Exploring a New Natural Treating Agent for Primary Hypertension: Recent Findings and Forthcoming Perspectives
Abstract
:1. Introduction
2. Physiological Regulation Mechanism in Blood-Pressure
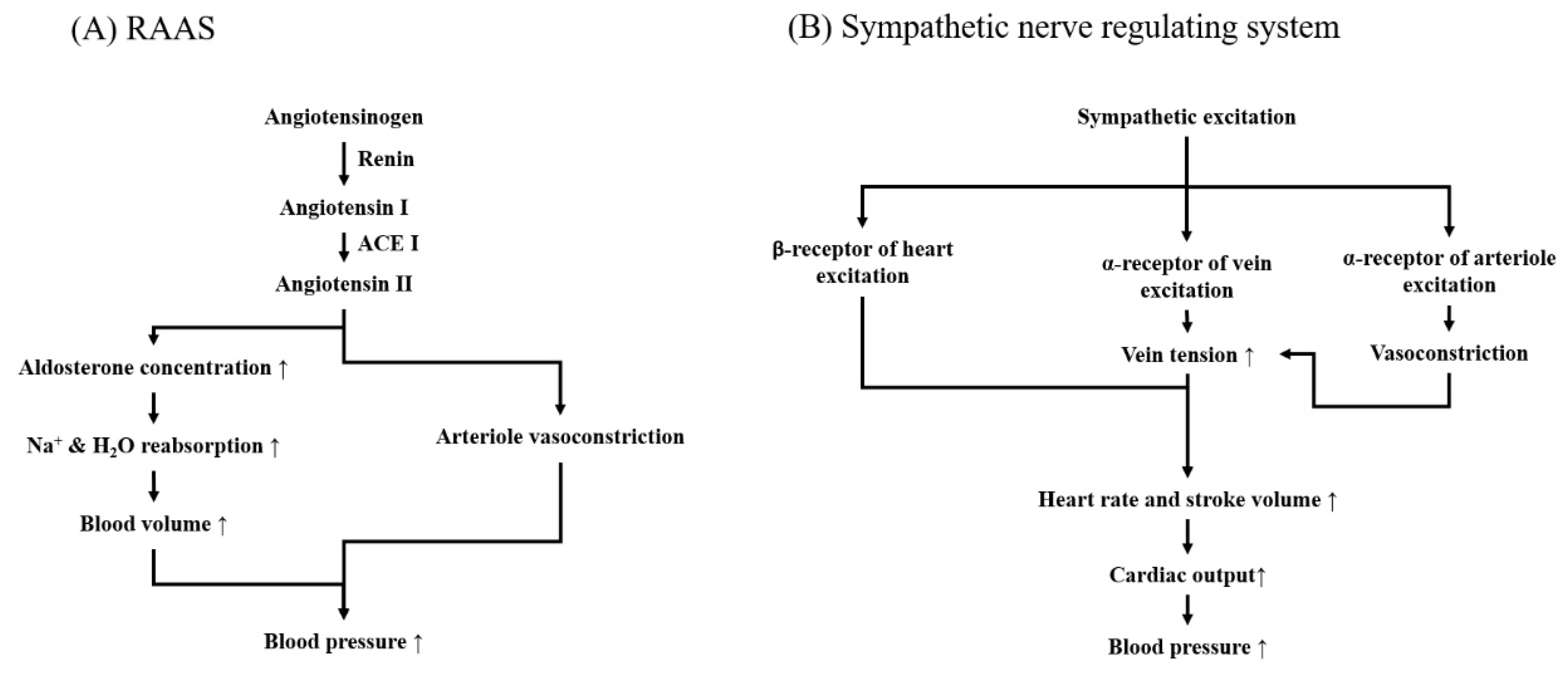
2.1. Renin-Angiotensin-Aldosterone System (RAAS)
2.1.1. Mechanical Action of RAAS in Blood-Pressure Controlling
2.1.2. Conventional RAAS Modulators in Hypertensive Medication
2.1.3. Methods for Screening RAAS Modulators
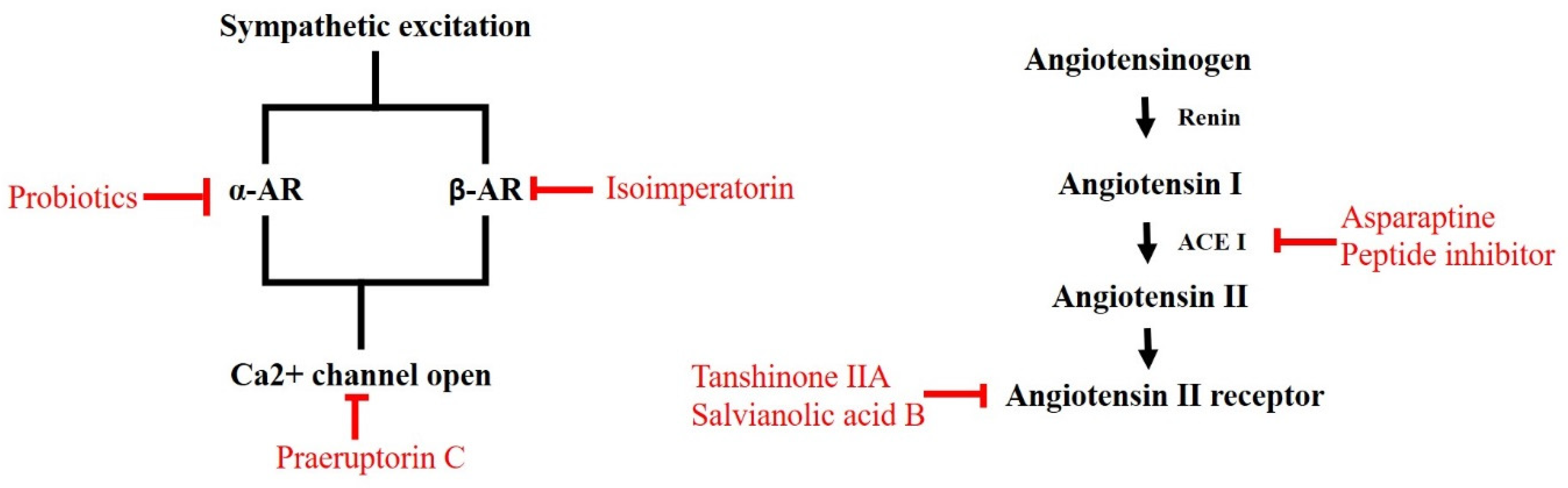
2.1.4. RAAS Modulators Identified from Nature
2.2. Neurogenic Hypertensive System
2.2.1. Action Mechanism of Neurogenic Hypertensive System in Blood Pressure Managing
2.2.2. Conventional Drugs for Modulating the Neurogenic Hypertensive System
2.2.3. Approaches to Screening Controllers for Neurogenic Hypertensive System
2.2.4. Natural ARs and Calcium Blockers
3. Conclusions and Remarks
Author Contributions
Funding
Conflicts of Interest
References
- James, P.A.; Oparil, S.; Carter, B.L.; Cushman, W.C.; Dennison-Himmelfarb, C.; Handler, J.; Lackland, D.T.; LeFevre, M.L.; MacKenzie, T.D.; Ogedegbe, O.; et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the eighth Joint National Committee (JNC 8). JAMA 2014, 311, 507–520. [Google Scholar] [CrossRef]
- Zhang, W.; Li, N. Prevalence, risk factors, and management of prehypertension. Int. J. Hypertens. 2011, 2011, 605359. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [CrossRef] [PubMed]
- Bolivar, J.J. Essential hypertension: An approach to its etiology and neurogenic pathophysiology. Int. J. Hypertens. 2013, 2013, 547809. [Google Scholar] [CrossRef] [PubMed]
- Puar, T.H.; Mok, Y.; Debajyoti, R.; Khoo, J.; How, C.H.; Ng, A.K. Secondary hypertension in adults. Singap. Med. J. 2016, 57, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.D.; Li, J.Y.; Yao, B.B.; Cai, X.B.; Shen, Q.J.; Xu, J. Are genetic polymorphisms in the renin-angiotensin-aldosterone system associated with essential hypertension? Evidence from genome-wide association studies. J. Hum. Hypertens. 2017, 31, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Alshaarawy, O.; Elbaz, H.A. Cannabis use and blood pressure levels: United States national health and nutrition examination survey, 2005–2012. J. Hypertens. 2016, 34, 1507–1512. [Google Scholar] [CrossRef]
- Andriolo, V.; Dietrich, S.; Knüppel, S.; Bernigau, W.; Boeing, H. Traditional risk factors for essential hypertension: Analysis of their specific combinations in the EPIC-Potsdam cohort. Sci. Rep. 2019, 9, 1501. [Google Scholar] [CrossRef]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global disparities of hypertension prevalence and control: A systematic analysis of population-based studies from 90 countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef]
- Lewington, S.; Lacey, B.; Clarke, R.; Guo, Y.; Kong, X.L.; Yang, L.; Chen, Y.; Bian, Z.; Chen, J.; Meng, J.; et al. The burden of hypertension and associated risk for cardiovascular mortality in China. JAMA Intern. Med. 2016, 176, 524–532. [Google Scholar] [CrossRef]
- Forouzanfar, M.H.; Liu, P.; Roth, G.A.; Ng, M.; Biryukov, S.; Marczak, L.; Alexander, L.; Estep, K.; Hassen Abate, K.; Akinyemiju, T.F.; et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA 2017, 317, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Hay, S.I.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; et al. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet 2017, 390, 1260–1344. [Google Scholar] [CrossRef]
- Muxfeldt, E.S.; de Souza, F.; Margallo, V.S.; Salles, G.F. Cardiovascular and renal complications in patients with resistant hypertension. Curr. Hypertens. Rep. 2014, 16, 471. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.A.; Power, M.C.; Gottesman, R.F. Defining the relationship between hypertension, cognitive decline, and dementia: A review. Curr. Hypertens. Rep. 2017, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Van den Born, B.H.; Lip, G.Y.H.; Brguljan-Hitij, J.; Cremer, A.; Segura, J.; Morales, E.; Mahfoud, F.; Amraoui, F.; Persu, A.; Kahan, T.; et al. ESC Council on hypertension position document on the management of hypertensive emergencies. Eur. Heart J. Cardiovasc. Pharmacother. 2019, 5, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Villalva, C.M.; Alvarez-Muino, X.L.L.; Mondelo, T.G.; Fachado, A.A.; Fernandez, J.C. Adherence to treatment in hypertension. Adv. Exp. Med. Biol. 2017, 956, 129–147. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Cooper-DeHoff, R.M.; Johnson, J.A. Hypertension pharmacogenomics: In search of personalized treatment approaches. Nat. Rev. Nephrol. 2016, 12, 110–122. [Google Scholar] [CrossRef]
- Nakaya, N.; Nakamura, T.; Tsuchiya, N.; Narita, A.; Tsuji, I.; Hozawa, A.; Tomita, H. Psychological distress and the risk of withdrawing from hypertension treatment after an earthquake disaster. Disaster Med. Public Health Prep. 2017, 11, 179–182. [Google Scholar] [CrossRef]
- Hu, H.H.; Li, G.; Arao, T. The association of family social support, depression, anxiety and self-efficacy with specific hypertension self-care behaviours in Chinese local community. J. Hum. Hypertens. 2015, 29, 198–203. [Google Scholar] [CrossRef]
- Osamor, P.E. Social support and management of hypertension in South-west Nigeria. Cardiovasc. J. Afr. 2015, 26, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.; Lee, S.Y.; Clark, P.C.; Perilla, J. Social ecology of adherence to hypertension treatment in latino migrant and seasonal farmworkers. J. Transcult. Nurs. 2016, 27, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Fongwa, M.N.; Dela Cruz, F.A.; Hays, R.D. African American women’s perceptions of the meaning of support groups for improving adherence to hypertension treatment: A conceptual model. Nurs. Open 2019, 6, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Noubiap, J.J.; Nansseu, J.R.; Nyaga, U.F.; Sime, P.S.; Francis, I.; Bigna, J.J. Global prevalence of resistant hypertension: A meta-analysis of data from 3.2 million patients. Heart 2019, 105, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Nadal, J.; Channavajjhala, S.K.; Jia, W.; Clayton, J.; Hall, I.P.; Glover, M. Clinical and molecular features of thiazide-induced hyponatremia. Curr. Hypertens. Rep. 2018, 20, 31. [Google Scholar] [CrossRef] [PubMed]
- Bandak, G.; Sang, Y.; Gasparini, A.; Chang, A.R.; Ballew, S.H.; Evans, M.; Arnlov, J.; Lund, L.H.; Inker, L.A.; Coresh, J.; et al. Hyperkalemia after initiating renin-angiotensin system blockade: The Stockholm creatinine measurements (SCREAM) project. J. Am. Heart Assoc. 2017, 6, e005428. [Google Scholar] [CrossRef]
- Hoover, T.; Lippmann, M.; Grouzmann, E.; Marceau, F.; Herscu, P. Angiotensin converting enzyme inhibitor induced angio-oedema: A review of the pathophysiology and risk factors. Clin. Exp. Allergy 2010, 40, 50–61. [Google Scholar] [CrossRef]
- Tedla, Y.G.; Bautista, L.E. drug side effect symptoms and adherence to antihypertensive medication. Am. J. Hypertens. 2016, 29, 772–779. [Google Scholar] [CrossRef]
- Lin, S.R.; Fu, Y.S.; Tsai, M.J.; Cheng, H.; Weng, C.F. Natural compounds from herbs that can potentially execute as autophagy inducers for cancer therapy. Int. J. Mol. Sci. 2017, 18, 1412. [Google Scholar] [CrossRef]
- Rabkin, S.W. Considerations in understanding the coronary blood flow- left ventricular mass relationship in patients with hypertension. Curr. Cardiol. Rev. 2017, 13, 75–83. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. Regulation of nitric oxide production in the developmental programming of hypertension and kidney disease. Int. J. Mol. Sci. 2019, 20, 681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernstein, K.E.; Khan, Z.; Giani, J.F.; Cao, D.Y.; Bernstein, E.A.; Shen, X.Z. Angiotensin-converting enzyme in innate and adaptive immunity. Nat. Rev. Nephrol. 2018, 14, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Feraille, E.; Dizin, E. Coordinated control of ENaC and Na+,K+-ATPase in renal Collecting DUCT. J. Am. Soc. Nephrol. 2016, 27, 2554–2563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michel, M.C.; Brunner, H.R.; Foster, C.; Huo, Y. Angiotensin II type 1 receptor antagonists in animal models of vascular, cardiac, metabolic and renal disease. Pharmacol. Ther. 2016, 164, 1–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvador, G.L.; Marmentini, V.M.; Cosmo, W.R.; Junior, E.L. Angiotensin-converting enzyme inhibitors reduce mortality compared to angiotensin receptor blockers: Systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2017, 24, 1914–1924. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Haga, T.; Hosaka, M.; Obara, T.; Metoki, H.; Murakami, T.; Kikuya, M.; Inoue, R.; Asayama, K.; Mano, N.; et al. The velocity of antihypertensive effects of seven angiotensin II receptor blockers determined by home blood pressure measurements. J. Hypertens. 2016, 34, 1218–1223. [Google Scholar] [CrossRef]
- Roush, G.C.; Sica, D.A. Diuretics for Hypertension: A Review and Update. Am. J. Hypertens. 2016, 29, 1130–1137. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.Y.; Lee, Y.T.; Chan, Y.W.; Tse, G. Animal models for the study of primary and secondary hypertension in humans. Biomed. Rep. 2016, 5, 653–659. [Google Scholar] [CrossRef] [Green Version]
- Sadegh Vishkaei, M.; Ebrahimpour, A.; Abdul-Hamid, A.; Ismail, A.; Saari, N. Angiotensin-I converting enzyme (ACE) inhibitory and anti-hypertensive effect of protein hydrolysate from Actinopyga lecanora (Sea Cucumber) in rats. Mar. Drugs 2016, 14, 176. [Google Scholar] [CrossRef] [Green Version]
- Basting, T.; Lazartigues, E. DOCA-salt hypertension: An update. Curr. Hypertens. Rep. 2017, 19, 32. [Google Scholar] [CrossRef]
- Bressan, A.F.; Fonseca, G.A.; Tostes, R.C.; Webb, R.C.; Lima, V.V.; Giachini, F.R. Interleukin-10 negatively modulates extracellular signal-regulated kinases 1 and 2 in aorta from hypertensive mouse induced by angiotensin II infusion. Fundam. Clin. Pharmacol. 2019, 33, 31–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Howatt, D.A.; Balakrishnan, A.; Moorleghen, J.J.; Rateri, D.L.; Cassis, L.A.; Daugherty, A. Subcutaneous angiotensin II infusion using osmotic pumps induces aortic aneurysms in mice. J. Vis. Exp. 2015, 103, e53191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Huang, X.R.; Chen, H.Y.; Fung, E.; Liu, J.; Lan, H.Y. Deletion of angiotensin-converting enzyme-2 promotes hypertensive nephropathy by targeting Smad7 for ubiquitin degradation. Hypertension 2017, 70, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Morgan, H.L.; Butler, E.; Ritchie, S.; Herse, F.; Dechend, R.; Beattie, E.; McBride, M.W.; Graham, D. Modeling superimposed preeclampsia using ang II (Angiotensin II) infusion in pregnant stroke-prone spontaneously hypertensive rats. Hypertension 2018, 72, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Foulquier, S.; Namsolleck, P.; Van Hagen, B.T.; Milanova, I.; Post, M.J.; Blankesteijn, W.M.; Rutten, B.P.; Prickaerts, J.; Van Oostenbrugge, R.J.; Unger, T. Hypertension-induced cognitive impairment: Insights from prolonged angiotensin II infusion in mice. Hypertens. Res. 2018, 41, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, S.M.; Textor, S.C. Current concepts in the treatment of renovascular hypertension. Am. J. Hypertens. 2018, 31, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Grossman, R.C. Experimental models of renal disease and the cardiovascular system. Open Cardiovasc. Med. J. 2010, 4, 257–264. [Google Scholar] [CrossRef]
- Carrive, P. Orexin, stress and central cardiovascular control. A link with hypertension? Neurosci. Biobehav. Rev. 2017, 74, 376–392. [Google Scholar] [CrossRef]
- Mohammed-Ali, Z.; Carlisle, R.E.; Nademi, S.; Dickhout, J.G. Animal models of kidney disease. In Animal Models for the Study of Human Disease; Academic Press: Cambridge, MA, USA, 2017; pp. 379–417. [Google Scholar] [CrossRef]
- Dupont, S.; Maizel, J.; Mentaverri, R.; Chillon, J.M.; Six, I.; Giummelly, P.; Brazier, M.; Choukroun, G.; Tribouilloy, C.; Massy, Z.A.; et al. The onset of left ventricular diastolic dysfunction in SHR rats is not related to hypertrophy or hypertension. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1524–H1532. [Google Scholar] [CrossRef] [Green Version]
- Hall, J.E.; do Carmo, J.M.; da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity-induced hypertension: Interaction of neurohumoral and renal mechanisms. Circ. Res. 2015, 116, 991–1006. [Google Scholar] [CrossRef] [Green Version]
- Lawson, H.A. Chapter 11—Animal models of metabolic syndrome. In Animal Models for the Study of Human Disease; Conn, P.M., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 243–264. [Google Scholar] [CrossRef]
- Bazzano, L.A.; Green, T.; Harrison, T.N.; Reynolds, K. Dietary approaches to prevent hypertension. Curr. Hypertens. Rep. 2013, 15, 694–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.Y.; Liao, J.K. A mouse model of diet-induced obesity and insulin resistance. Methods Mol. Biol. 2012, 821, 421–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drenjancevic-Peric, I.; Jelakovic, B.; Lombard, J.H.; Kunert, M.P.; Kibel, A.; Gros, M. High-salt diet and hypertension: Focus on the renin-angiotensin system. Kidney Blood Press. Res. 2011, 34, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ivy, J.R.; Evans, L.C.; Moorhouse, R.; Richardson, R.V.; Al-Dujaili, E.A.S.; Flatman, P.W.; Kenyon, C.J.; Chapman, K.E.; Bailey, M.A. Renal and blood pressure response to a high-salt diet in mice with reduced global expression of the glucocorticoid receptor. Front. Physiol. 2018, 9, 848. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.S.; Lue, S.I.; Lin, S.Y.; Luo, C.L.; Chou, C.C.; Weng, C.F. Plantago asiatica seed extracts alleviated blood pressure in phase I(-)Spontaneous hypertension rats. Molecules 2019, 24, 1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banegas-Luna, A.J.; Ceron-Carrasco, J.P.; Perez-Sanchez, H. A review of ligand-based virtual screening web tools and screening algorithms in large molecular databases in the age of big data. Future Med. Chem. 2018, 10, 2641–2658. [Google Scholar] [CrossRef]
- Ke, Z.; Su, Z.; Zhang, X.; Cao, Z.; Ding, Y.; Cao, L.; Ding, G.; Wang, Z.; Liu, H.; Xiao, W. Discovery of a potent angiotensin converting enzyme inhibitor via virtual screening. Bioorg. Med. Chem. Lett. 2017, 27, 3688–3692. [Google Scholar] [CrossRef]
- Qiao, L.; Li, B.; Chen, Y.; Li, L.; Chen, X.; Wang, L.; Lu, F.; Luo, G.; Li, G.; Zhang, Y. Discovery of anti-hypertensive oligopeptides from adlay based on in silico proteolysis and virtual screening. Int. J. Mol. Sci. 2016, 17, 2099. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, I.; Yanuar, A.; Mulia, K.; Mun’im, A. Review of angiotensin-converting enzyme inhibitory assay: Rapid method in drug discovery of herbal plants. Pharmacogn. Rev. 2017, 11, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Contreras, R.; Vos, P.; Westerhoff, H.V.; Boogerd, F.C. Why in vivo may not equal in vitro—New effectors revealed by measurement of enzymatic activities under the same in vivo-like assay conditions. FEBS J. 2012, 279, 4145–4159. [Google Scholar] [CrossRef]
- Han, S.; Lv, Y.; Wei, F.; Fu, J.; Hu, Q.; Wang, S. Screening of bioactive components from traditional Chinese medicines using cell membrane chromatography coupled with mass spectrometry. Phytochem. Anal. 2018, 29, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Wang, S.; Zhang, T.; Ma, J.; Zhang, J.; Zhang, Y.; Lu, W.; He, H.; He, L. Recent advances in cell membrane chromatography for traditional Chinese medicines analysis. J. Pharm. Biomed. Anal. 2014, 101, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.R.; Yuan, Z.Y. Dopamine-mediated inhibition of renal Na+/K+-ATPase in HK-2 cells is reduced by ouabain. Clin. Exp. Pharmacol. Physiol. 2010, 37, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, Z.; Ma, C.; Zhan, F.; Wu, W.; Han, H.; Huang, Y.; Li, W.; Chen, D.; Peng, Y. Rho kinase pathway is likely responsible for the profibrotic actions of aldosterone in renal epithelial cells via inducing epithelial-mesenchymal transition and extracellular matrix excretion. Cell Biol. Int. 2013, 37, 725–730. [Google Scholar] [CrossRef]
- Saleem, M.; Pokkunuri, I.; Asghar, M. Superoxide increases angiotensin II AT1 receptor function in human kidney-2 cells. FEBS Open Bio 2016, 6, 1273–1284. [Google Scholar] [CrossRef]
- Kitada, K.; Nakano, D.; Hitomi, H.; Kobori, H.; Deguchi, K.; Mori, H.; Masaki, T.; Nishiyama, A. Aldosterone induces p21-regulated apoptosis via increased synthesis and secretion of tumour necrosis factor-alpha in human proximal tubular cells. Clin. Exp. Pharmacol. Physiol. 2012, 39, 858–863. [Google Scholar] [CrossRef]
- Hou, E.; Sun, N.; Zhang, F.; Zhao, C.; Usa, K.; Liang, M.; Tian, Z. Malate and aspartate increase l-arginine and nitric oxide and attenuate hypertension. Cell Rep. 2017, 19, 1631–1639. [Google Scholar] [CrossRef] [Green Version]
- Norlander, A.E.; Saleh, M.A.; Kamat, N.V.; Ko, B.; Gnecco, J.; Zhu, L.; Dale, B.L.; Iwakura, Y.; Hoover, R.S.; McDonough, A.A.; et al. Interleukin-17A regulates renal sodium transporters and renal injury in angiotensin II-induced hypertension. Hypertension 2016, 68, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Mahapatra, D.K.; Bharti, S.K. Therapeutic potential of chalcones as cardiovascular agents. Life Sci. 2016, 148, 154–172. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A privileged structure in medicinal chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D. Overview on the antihypertensive and anti-obesity effects of secondary metabolites from seaweeds. Mar. Drugs 2018, 16, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhat, Z.F.; Kumar, S.; Bhat, H.F. Antihypertensive peptides of animal origin: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.Y.; Kim, D.S.; Choi, G.; Kim, S.H.; Kim, H.K. Dohaekseunggi-tang extract inhibits obesity, hyperlipidemia, and hypertension in high-fat diet-induced obese mice. BMC Complement. Altern. Med. 2014, 14, 372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.Z.; Jiang, Y.G.; Zuo, Y.; Li, A.X. Establishment of the method for screening the potential targets and effective components of huatuo reconstruction pill. Interdiscip. Sci. 2014, 6, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Basi, Z.; Turkoglu, V. Purification of angiotensin-converting enzyme from human plasma and investigation of the effect of some active ingredients isolated from Nigella sativa L. extract on the enzyme activity. Biomed. Chromatogr. 2018, 32, e4175. [Google Scholar] [CrossRef] [PubMed]
- Kolsi, R.B.A.; Salah, H.B.; Saidi, S.A.; Allouche, N.; Belghith, H.; Belghith, K. Evaluation of nutritional value, characteristics, functional properties of Cymodocea nodosa and its benefits on health diseases. Lipids Health Dis. 2017, 16, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oboh, G.; Ademiluyi, A.O.; Oyeleye, S.I.; Olasehinde, T.A.; Boligon, A.A. Modulation of some markers of erectile dysfunction and malonaldehyde levels in isolated rat penile tissue with unripe and ripe plantain peels: Identification of the constituents of the plants using HPLC. Pharm. Biol. 2017, 55, 1920–1926. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.Y.; Kumar, V. Mechanism of antihypertensive effect of Mucuna pruriens L. seed extract and its isolated compounds. J. Complement. Integr. Med. 2017, 14. [Google Scholar] [CrossRef]
- Syama, H.P.; Arya, A.D.; Dhanya, R.; Nisha, P.; Sundaresan, A.; Jacob, E.; Jayamurthy, P. Quantification of phenolics in Syzygium cumini seed and their modulatory role on tertiary butyl-hydrogen peroxide-induced oxidative stress in H9c2 cell lines and key enzymes in cardioprotection. J. Food Sci. Technol. 2017, 54, 2115–2125. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Neto, A.I.; Baptista, J. Angiotensin I-converting enzyme (ACE) inhibitory activity of Fucus spiralis macroalgae and influence of the extracts storage temperature—A short report. J. Pharm. Biomed. Anal. 2016, 131, 503–507. [Google Scholar] [CrossRef]
- Gamboa-Gomez, C.I.; Gonzalez-Laredo, R.F.; Gallegos-Infante, J.A.; Perez, M.D.; Moreno-Jimenez, M.R.; Flores-Rueda, A.G.; Rocha-Guzman, N.E. Antioxidant and angiotensin-converting enzyme inhibitory activity of eucalyptus camaldulensis and Litsea glaucescens infusions fermented with kombucha consortium. Food Technol. Biotechnol. 2016, 54, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Penas, E.; Limon, R.I.; Martinez-Villaluenga, C.; Restani, P.; Pihlanto, A.; Frias, J. Impact of elicitation on antioxidant and potential antihypertensive properties of Lentil Sprouts. Plant Foods Hum. Nutr. 2015, 70, 401–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Shah, N.P. Effects of Pleurotus eryngii polysaccharides on bacterial growth, texture properties, proteolytic capacity, and angiotensin-I-converting enzyme-inhibitory activities of fermented milk. J. Dairy Sci. 2015, 98, 2949–2961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limon, R.I.; Penas, E.; Torino, M.I.; Martinez-Villaluenga, C.; Duenas, M.; Frias, J. Fermentation enhances the content of bioactive compounds in kidney bean extracts. Food Chem. 2015, 172, 343–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sila, A.; Bayar, N.; Ghazala, I.; Bougatef, A.; Ellouz-Ghorbel, R.; Ellouz-Chaabouni, S. Water-soluble polysaccharides from agro-industrial by-products: Functional and biological properties. Int. J. Biol. Macromol. 2014, 69, 236–243. [Google Scholar] [CrossRef]
- Kancabas, A.; Karakaya, S. Angiotensin-converting enzyme (ACE)-inhibitory activity of boza, a traditional fermented beverage. J. Sci. Food Agric. 2013, 93, 641–645. [Google Scholar] [CrossRef]
- Bakri, M.; Yi, Y.; Chen, L.D.; Aisa, H.A.; Wang, M.H. Alkaloids of Nitraria sibirica Pall. decrease hypertension and albuminuria in angiotensin II-salt hypertension. Chin. J. Nat. Med. 2014, 12, 266–272. [Google Scholar] [CrossRef]
- Ramesar, S.; Baijnath, H.; Govender, T.; Mackraj, I. Angiotensin I-converting enzyme inhibitor activity of nutritive plants in KwaZulu-Natal. J. Med. Food 2008, 11, 331–336. [Google Scholar] [CrossRef]
- Kang, D.G.; Oh, H.; Cho, D.K.; Kwon, E.K.; Han, J.H.; Lee, H.S. Effects of bulb of Fritillaria ussuriensis maxim. on angiotensin converting enzyme and vascular release of NO/cGMP in rats. J. Ethnopharmacol. 2002, 81, 49–55. [Google Scholar] [CrossRef]
- Lau, Y.S.; Kwan, C.Y.; Ku, T.C.; Hsieh, W.T.; Wang, H.D.; Nishibe, S.; Dharmani, M.; Mustafa, M.R. Apocynum venetum leaf extract, an antihypertensive herb, inhibits rat aortic contraction induced by angiotensin II: A nitric oxide and superoxide connection. J. Ethnopharmacol. 2012, 143, 565–571. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Guo, F.F.; Wu, H.W.; Yu, Y.Y.; Wei, J.Y.; Wang, S.F.; Zhang, Y.X.; Xian, M.H.; Wu, Q.H.; Zhao, B.C.; et al. DanHong injection targets endothelin receptor type B and angiotensin II receptor type 1 in protection against cardiac hypertrophy. Oncotarget 2017, 8, 103393–103409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Qian, X.; Sun, X.; Lin, C.; Jing, Y.; Yao, Y.; Ma, Z.; Kuai, M.; Lu, Y.; Kong, X.; et al. Liuwei Dihuang, a traditional Chinese medicinal formula, inhibits proliferation and migration of vascular smooth muscle cells via modulation of estrogen receptors. Int. J. Mol. Med. 2018, 42, 31–40. [Google Scholar] [CrossRef] [PubMed]
- He, Y.M.; Yang, H.J.; Yang, Q.; Cui, J.G.; Wang, T.Z.; Chen, Y.; Wang, P.W.; Zhang, T.; Wang, W.J. Yiqi Huaju formula, a Chinese herbal medicine, reduces arterial pressure in saltsensitive hypertension by inhibiting reninangiotensin system activation. Mol. Med. Rep. 2015, 12, 5321–5327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.Q.; Liu, W.; Cai, X.Y.; Xu, Q.; Shi, H.; Wang, W.; Wu, C.Y.; Li, J.; Wang, R.H.; Jiang, H.T. The regulatory mechanism of songling xuemaikang capsule on PPARgamma in spontaneously hypertensive rats: An experimental study. Zhongguo Zhong Xi Yi Jie He Za Zhi 2013, 33, 1236–1241. [Google Scholar]
- Su, P.S.; Doerksen, R.J.; Chen, S.H.; Sung, W.C.; Juan, C.C.; Rawendra, R.D.; Chen, C.R.; Li, J.W.; Aisha; Huang, T.C.; et al. Screening and profiling stilbene-type natural products with angiotensin-converting enzyme inhibitory activity from Ampelopsis brevipedunculata var. hancei (Planch.) Rehder. J. Pharm. Biomed. Anal. 2015, 108, 70–77. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Yang, Z.; Nishizawa, T.; Mori, T.; Saito, K. Top-down targeted metabolomics reveals a sulfur-containing metabolite with inhibitory activity against angiotensin-converting enzyme in Asparagus officinalis. J. Nat. Prod. 2015, 78, 1179–1183. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M.; O’Shea, N.; Gallagher, E.; Lafarga, T. Predicted release and analysis of novel ACE-I, renin, and DPP-IV inhibitory peptides from common oat (Avena sativa) protein hydrolysates using in silico analysis. Foods 2017, 6, 108. [Google Scholar] [CrossRef] [Green Version]
- Morais, K.L.; Ianzer, D.; Miranda, J.R.; Melo, R.L.; Guerreiro, J.R.; Santos, R.A.; Ulrich, H.; Lameu, C. Proline rich-oligopeptides: Diverse mechanisms for antihypertensive action. Peptides 2013, 48, 124–133. [Google Scholar] [CrossRef]
- Siemerink, M.; Schebb, N.H.; Liesener, A.; Perchuc, A.M.; Schoni, R.; Wilmer, M.; Hayen, H.; Karst, U.; Vogel, M. Development of a fast liquid chromatography/mass spectrometry screening method for angiotensin-converting enzyme (ACE) inhibitors in complex natural mixtures like snake venom. Rapid Commun. Mass Spectrom. 2010, 24, 687–697. [Google Scholar] [CrossRef]
- Nawaz, K.A.A.; David, S.M.; Murugesh, E.; Thandeeswaran, M.; Kiran, K.G.; Mahendran, R.; Palaniswamy, M.; Angayarkanni, J. Identification and in silico characterization of a novel peptide inhibitor of angiotensin converting enzyme from pigeon pea (Cajanus cajan). Phytomedicine 2017, 36, 1–7. [Google Scholar] [CrossRef]
- Liu, J.C.; Hsu, F.L.; Tsai, J.C.; Chan, P.; Liu, J.Y.; Thomas, G.N.; Tomlinson, B.; Lo, M.Y.; Lin, J.Y. Antihypertensive effects of tannins isolated from traditional Chinese herbs as non-specific inhibitors of angiontensin converting enzyme. Life Sci. 2003, 73, 1543–1555. [Google Scholar] [CrossRef]
- Vijayakumar, B.; Parasuraman, S.; Raveendran, R.; Velmurugan, D. Identification of natural inhibitors against angiotensin I converting enzyme for cardiac safety using induced fit docking and MM-GBSA studies. Pharmacogn. Mag. 2014, 10, S639–S644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, D.G.; Lee, Y.S.; Kim, H.J.; Lee, Y.M.; Lee, H.S. Angiotensin converting enzyme inhibitory phenylpropanoid glycosides from Clerodendron trichotomum. J. Ethnopharmacol. 2003, 89, 151–154. [Google Scholar] [CrossRef]
- Li, B.; Qiao, L.; Li, L.; Zhang, Y.; Li, K.; Wang, L.; Qiao, Y. A novel antihypertensive derived from adlay (Coix larchryma-jobi L. var. ma-yuen Stapf) glutelin. Molecules 2017, 22, 123. [Google Scholar] [CrossRef] [Green Version]
- Parichatikanond, W.; Pinthong, D.; Mangmool, S. Blockade of the renin-angiotensin system with delphinidin, cyanin, and quercetin. Planta Med. 2012, 78, 1626–1632. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.Q.; Luo, J.G.; Han, C.; Xu, J.F.; Kong, L.Y. Bioassay-guided preparative separation of angiotensin-converting enzyme inhibitory C-flavone glycosides from Desmodium styracifolium by recycling complexation high-speed counter-current chromatography. J. Pharm. Biomed. Anal. 2015, 102, 276–281. [Google Scholar] [CrossRef]
- Nagai, T.; Nagashima, T. Functional properties of dioscorin, a soluble viscous protein from Japanese yam (Dioscorea opposita thunb.) tuber mucilage Tororo. Z. Naturforsch. C. J. Biosci. 2006, 61, 792–798. [Google Scholar] [CrossRef]
- Eckert, E.; Zambrowicz, A.; Pokora, M.; Setner, B.; Dabrowska, A.; Szoltysik, M.; Szewczuk, Z.; Polanowski, A.; Trziszka, T.; Chrzanowska, J. Egg-yolk protein by-product as a source of ACE-inhibitory peptides obtained with using unconventional proteinase from Asian pumpkin (Cucurbita ficifolia). J. Proteom. 2014, 110, 107–116. [Google Scholar] [CrossRef]
- Yan, J.; Shi, X.; Donkor, P.O.; Zhu, H.; Gao, X.; Ding, L.; Qiu, F. Nine pairs of megastigmane enantiomers from the leaves of Eucommia ulmoides oliver. J. Nat. Med. 2017, 71, 780–790. [Google Scholar] [CrossRef]
- Jenis, J.; Kim, J.Y.; Uddin, Z.; Song, Y.H.; Lee, H.H.; Park, K.H. Phytochemical profile and angiotensin I converting enzyme (ACE) inhibitory activity of Limonium michelsonii Lincz. J. Nat. Med. 2017, 71, 650–658. [Google Scholar] [CrossRef]
- Geng, F.; He, Y.; Yang, L.; Wang, Z. A rapid assay for angiotensin-converting enzyme activity using ultra-performance liquid chromatography-mass spectrometry. Biomed. Chromatogr. 2010, 24, 312–317. [Google Scholar] [CrossRef] [PubMed]
- San Pablo-Osorio, B.; Mojica, L.; Urias-Silvas, J.E. Chia Seed (Salvia hispanica L.) pepsin hydrolysates inhibit angiotensin-converting enzyme by interacting with its catalytic site. J. Food Sci. 2019, 84, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Li, P.; Fu, B.; Chuo, W.; Gao, K.; Zhang, W.; Wang, J.; Chen, J.; Wang, W. Systems-biology dissection of mechanisms and chemical basis of herbal formula in treating chronic myocardial ischemia. Pharmacol. Res. 2016, 114, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.P.; Xu, D.Y.; Deng, Y.L.; Zhang, Y. Screening of angiotensin converting enzyme inhibitors from Salvia miltiorrhizae. Zhongguo Zhong Yao Za Zhi 2004, 29, 359–362. [Google Scholar]
- Maneesh, A.; Chakraborty, K. Previously undescribed antioxidative O-heterocyclic angiotensin converting enzyme inhibitors from the intertidal seaweed Sargassum wightii as potential antihypertensives. Food Res. Int. 2018, 113, 474–486. [Google Scholar] [CrossRef]
- Xing, Y.; Liao, J.; Tang, Y.; Zhang, P.; Tan, C.; Ni, H.; Wu, X.; Li, N.; Jia, X. ACE and platelet aggregation inhibitors from Tamarix hohenackeri Bunge (host plant of Herba Cistanches) growing in Xinjiang. Pharmacogn. Mag. 2014, 10, 111–117. [Google Scholar] [CrossRef]
- Hou, D.; Yousaf, L.; Xue, Y.; Hu, J.; Wu, J.; Hu, X.; Feng, N.; Shen, Q. Mung bean (Vigna radiata L.): Bioactive polyphenols, polysaccharides, peptides, and health benefits. Nutrients 2019, 11, 1238. [Google Scholar] [CrossRef] [Green Version]
- Makino, B.; Kobayashi, M.; Kimura, K.; Ishimatsu, M.; Sakakibara, I.; Higuchi, M.; Kubo, M.; Sasaki, H.; Okada, M. Local variation in the content of angiotensin II and arginine vasopressin receptor antagonistic terpenoids in the rhizomes of Alisma orientale. Planta Med. 2002, 68, 226–231. [Google Scholar] [CrossRef]
- Lu, Y.; Li, S.; Wu, H.; Bian, Z.; Xu, J.; Gu, C.; Chen, X.; Yang, D. Beneficial effects of astragaloside IV against angiotensin II-induced mitochondrial dysfunction in rat vascular smooth muscle cells. Int. J. Mol. Med. 2015, 36, 1223–1232. [Google Scholar] [CrossRef] [Green Version]
- Jananie, R.K.; Priya, V.; Vijayalakshmi, K. Secondary metabolites of Cynodon dactylon as an antagonist to angiotensin II type1 receptor: Novel in silico drug targeting approach for diabetic retinopathy. J. Pharmacol. Pharmacother. 2012, 3, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Ling, W.C.; Liu, J.; Lau, C.W.; Murugan, D.D.; Mustafa, M.R.; Huang, Y. Treatment with salvianolic acid B restores endothelial function in angiotensin II-induced hypertensive mice. Biochem. Pharmacol. 2017, 136, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Dong, M.; Luo, Y.; Zhao, F.; Li, Y. Danshensu prevents hypoxic pulmonary hypertension in rats by inhibiting the proliferation of pulmonary artery smooth muscle cells via TGF-beta-smad3-associated pathway. Eur. J. Pharmacol. 2018, 820, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Robles-Vera, I.; Toral, M.; Romero, M.; Jimenez, R.; Sanchez, M.; Perez-Vizcaino, F.; Duarte, J. Antihypertensive effects of probiotics. Curr. Hypertens. Rep. 2017, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Tabeshpour, J.; Razavi, B.M.; Hosseinzadeh, H. Effects of Avocado (Persea americana) on metabolic syndrome: A comprehensive systematic review. Phytother. Res. 2017, 31, 819–837. [Google Scholar] [CrossRef]
- Osinska, A.N.; Begier-Krasinska, B.; Rzymski, P.; Krasinska, A.; Tykarski, A.; Krasinski, Z. The influence of adding tomato extract and acetylsalicylic acid to hypotensive therapy on the daily blood pressure profiles of patients with arterial hypertension and high cardiovascular risk. Kardiochir. Torakochirurgia Pol. 2017, 14, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Krasinska, B.; Osinska, A.; Osinski, M.; Krasinska, A.; Rzymski, P.; Tykarski, A.; Krasinski, Z. Standardised tomato extract as an alternative to acetylsalicylic acid in patients with primary hypertension and high cardiovascular risk—A randomised, controlled trial. Arch. Med. Sci. 2018, 14, 773–780. [Google Scholar] [CrossRef]
- Krasinska, B.; Osinska, A.; Krasinska, A.; Osinski, M.; Rzymski, P.; Tykarski, A.; Krasinski, Z. Favourable hypotensive effect after standardised tomato extract treatment in hypertensive subjects at high cardiovascular risk: A randomised controlled trial. Kardiol. Pol. 2018, 76, 388–395. [Google Scholar] [CrossRef] [Green Version]
- Michalickova, D.; Belovic, M.; Ilic, N.; Kotur-Stevuljevic, J.; Slanar, O.; Sobajic, S. Comparison of polyphenol-enriched tomato juice and standard tomato juice for cardiovascular benefits in subjects with stage 1 hypertension: A randomized controlled study. Plant Foods Hum. Nutr. 2019, 74, 122–127. [Google Scholar] [CrossRef]
- Wolak, T.; Sharoni, Y.; Levy, J.; Linnewiel-Hermoni, K.; Stepensky, D.; Paran, E. Effect of tomato nutrient complex on blood pressure: A double blind, randomized dose(-)response study. Nutrients 2019, 11, 950. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.I.; Noori, S.; Mahboob, T. Efficacy of lycopene on modulation of renal antioxidant enzymes, ACE and ACE gene expression in hyperlipidaemic rats. J. Renin Angiotensin Aldosterone Syst. 2016, 17, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Di Lorenzo, A.; Curti, V.; Tenore, G.C.; Nabavi, S.M.; Daglia, M. Effects of tea and coffee consumption on cardiovascular diseases and relative risk factors: An update. Curr. Pharm. Des. 2017, 23, 2474–2487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Coca, A.; Casa, D.J.; Antonio, J.; Green, J.M.; Bishop, P.A. Caffeine and diuresis during rest and exercise: A meta-analysis. J. Sci. Med. Sport 2015, 18, 569–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abeywickrama, K.R.; Ratnasooriya, W.D.; Amarakoon, A.M. Oral diuretic activity of hot water infusion of Sri Lankan black tea (Camellia sinensis L.) in rats. Pharmacogn. Mag. 2010, 6, 271–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancini, E.; Beglinger, C.; Drewe, J.; Zanchi, D.; Lang, U.E.; Borgwardt, S. Green tea effects on cognition, mood and human brain function: A systematic review. Phytomedicine 2017, 34, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Guessous, I.; Eap, C.B.; Bochud, M. Blood pressure in relation to coffee and caffeine consumption. Curr. Hypertens. Rep. 2014, 16, 468. [Google Scholar] [CrossRef]
- Ye, N.S. A minireview of analytical methods for the geographical origin analysis of teas (Camellia sinensis). Crit. Rev. Food Sci. Nutr. 2012, 52, 775–780. [Google Scholar] [CrossRef]
- Baspinar, B.; Eskici, G.; Ozcelik, A.O. How coffee affects metabolic syndrome and its components. Food Funct. 2017, 8, 2089–2101. [Google Scholar] [CrossRef]
- Agunloye, O.M.; Oboh, G.; Ademiluyi, A.O.; Ademosun, A.O.; Akindahunsi, A.A.; Oyagbemi, A.A.; Omobowale, T.O.; Ajibade, T.O.; Adedapo, A.A. Cardio-protective and antioxidant properties of caffeic acid and chlorogenic acid: Mechanistic role of angiotensin converting enzyme, cholinesterase and arginase activities in cyclosporine induced hypertensive rats. Biomed. Pharmacother. 2019, 109, 450–458. [Google Scholar] [CrossRef]
- Hussein, R.M.; Elsirafy, O.M.; Wahba, Y.S.; Kawy, H.S.; Hasanin, A.H.; Hamam, G.G. Theophylline, an old drug with multi-faceted effects: Its potential benefits in immunological liver injury in rats. Life Sci. 2015, 136, 100–107. [Google Scholar] [CrossRef]
- Legeay, S.; Rodier, M.; Fillon, L.; Faure, S.; Clere, N. Epigallocatechin gallate: A review of its beneficial properties to prevent metabolic syndrome. Nutrients 2015, 7, 5443–5468. [Google Scholar] [CrossRef] [Green Version]
- Ngueta, G. Caffeine and caffeine metabolites in relation to hypertension in U.S. adults. Eur. J. Clin. Nutr. 2019, 1. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Cui, L.; Zhu, J.; Wang, K.; Sun, N.; Sun, C. Coffee consumption and risk of hypertension: A systematic review and dose-response meta-analysis of cohort studies. J. Hum. Hypertens. 2018, 32, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Chei, C.L.; Loh, J.K.; Soh, A.; Yuan, J.M.; Koh, W.P. Coffee, tea, caffeine, and risk of hypertension: The Singapore Chinese health study. Eur. J. Nutr. 2018, 57, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Bes-Rastrollo, M.; Galvano, F.; Martinez-Gonzalez, M.A. Long-term coffee consumption is associated with decreased incidence of new-onset hypertension: A dose-response meta-analysis. Nutrients 2017, 9, 890. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.M.; Martinez-Gonzalez, M.A.; Gea, A.; Ramallal, R.; Ruiz-Canela, M.; Toledo, E. Coffee consumption and risk of hypertension in the SUN project. Clin. Nutr. 2019, 38, 389–397. [Google Scholar] [CrossRef]
- Rhee, J.J.; Qin, F.; Hedlin, H.K.; Chang, T.I.; Bird, C.E.; Zaslavsky, O.; Manson, J.E.; Stefanick, M.L.; Winkelmayer, W.C. Coffee and caffeine consumption and the risk of hypertension in postmenopausal women. Am. J. Clin. Nutr. 2016, 103, 210–217. [Google Scholar] [CrossRef] [Green Version]
- Tobe, S.W. beta-adrenergic receptor blockers in hypertension. Can. J. Cardiol. 2014, 30, S1–S2. [Google Scholar] [CrossRef] [Green Version]
- Heran, B.S.; Galm, B.P.; Wright, J.M. Blood pressure lowering efficacy of alpha blockers for primary hypertension. Cochrane Database Syst. Rev. 2012, CD004643. [Google Scholar] [CrossRef]
- Wiysonge, C.S.; Bradley, H.A.; Volmink, J.; Mayosi, B.M.; Opie, L.H. Beta-blockers for hypertension. Cochrane Database Syst. Rev. 2017, 1, CD002003. [Google Scholar] [CrossRef] [Green Version]
- Godfraind, T. Discovery and development of calcium channel blockers. Front. Pharmacol. 2017, 8, 286. [Google Scholar] [CrossRef] [Green Version]
- Voora, R.; Hinderliter, A.L. Modulation of sympathetic overactivity to treat resistant hypertension. Curr. Hypertens. Rep. 2018, 20, 92. [Google Scholar] [CrossRef] [PubMed]
- Saladini, F.; Palatini, P. Isolated systolic hypertension in young individuals: Pathophysiological mechanisms, prognostic significance, and clinical implications. High Blood Press. Cardiovasc. Prev. 2017, 24, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Cruickshank, J.M. The role of beta-blockers in the treatment of hypertension. Adv. Exp. Med. Biol. 2017, 956, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.M.; Musini, V.M.; Gill, R. First-line drugs for hypertension. Cochrane Database Syst. Rev. 2018, 4, CD001841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Torre, A.; Giupponi, G.; Duffy, D.; Conca, A.; Cai, T.; Scardigli, A. Sexual dysfunction related to drugs: A critical review. Part V: Alpha-blocker and 5-ARI drugs. Pharmacopsychiatry 2016, 49, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharp, R.P.; Gales, B.J. Nebivolol versus other beta blockers in patients with hypertension and erectile dysfunction. Ther. Adv. Urol. 2017, 9, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Yue, Y.; Xue, H.; Wang, X.; Yang, Q.; Song, Y.; Li, X. High-expression β(1) adrenergic receptor/cell membrane chromatography method based on a target receptor to screen active ingredients from traditional Chinese medicines. J. Sep. Sci. 2014, 37, 244–249. [Google Scholar] [CrossRef]
- Tubio, M.R.; Fernandez, N.; Fitzsimons, C.P.; Copsel, S.; Santiago, S.; Shayo, C.; Davio, C.; Monczor, F. Expression of a G protein-coupled receptor (GPCR) leads to attenuation of signaling by other GPCRs: Experimental evidence for a spontaneous GPCR constitutive inactive form. J. Biol. Chem. 2010, 285, 14990–14998. [Google Scholar] [CrossRef] [Green Version]
- Valero, M.S.; Olivan-Viguera, A.; Garrido, I.; Langa, E.; Berzosa, C.; Lopez, V.; Gomez-Rincon, C.; Murillo, M.D.; Kohler, R. Rock Tea extract (Jasonia glutinosa) relaxes rat aortic smooth muscle by inhibition of L-type Ca2+ channels. J. Physiol. Biochem. 2015, 71, 785–793. [Google Scholar] [CrossRef]
- Parichatikanond, W.; Nishimura, A.; Nishida, M.; Mangmool, S. Prolonged stimulation of beta2-adrenergic receptor with beta2-agonists impairs insulin actions in H9c2 cells. J. Pharmacol. Sci. 2018, 138, 184–191. [Google Scholar] [CrossRef]
- Ren, G.; Qiao, H.X.; Yang, J.; Zhou, C.X. Protective effects of steroids from Allium chinense against H2O2-induced oxidative stress in rat cardiac H9C2 cells. Phytother. Res. 2010, 24, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.C.; Li, Y.; Cheng, Y.Z.; Lee, W.J.; Cheng, J.T.; Cheng, K.C. Molecular mechanisms regarding potassium bromateinduced cardiac hypertrophy without apoptosis in H9c2 cells. Mol. Med. Rep. 2018, 18, 4700–4708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, F.J.; Ho, T.J.; Cheng, C.F.; Shiao, Y.T.; Chien, W.K.; Chen, J.H.; Liu, X.; Tsang, H.; Lin, T.H.; Liao, C.C.; et al. Characteristics of Chinese herbal medicine usage in ischemic heart disease patients among type 2 diabetes and their protection against hydrogen peroxide-mediated apoptosis in H9C2 cardiomyoblasts. Oncotarget 2017, 8, 15470–15489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do, K.H.; Kim, M.S.; Kim, J.H.; Rhim, B.Y.; Lee, W.S.; Kim, C.D.; Bae, S.S. Angiotensin II-induced aortic ring constriction is mediated by phosphatidylinositol 3-kinase/L-type calcium channel signaling pathway. Exp. Mol. Med. 2009, 41, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Mao, L.M.; Qi, X.W.; Hao, J.H.; Liu, H.F.; Xu, Q.H.; Bu, P.L. In vitro, ex vivo and in vivo anti-hypertensive activity of Chrysophyllum cainito L. extract. Int. J. Clin. Exp. Med. 2015, 8, 17912–17921. [Google Scholar]
- Sviglerova, J.; Kuncova, J.; Stengl, M. Chapter 9—Cardiovascular models: Heart secondarily affected by disease. In Animal Models for the Study of Human Disease; Conn, P.M., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 195–220. [Google Scholar] [CrossRef]
- Redina, O.E.; Markel, A.L. Stress, genes, and hypertension. Contribution of the ISIAH rat strain study. Curr. Hypertens. Rep. 2018, 20, 66. [Google Scholar] [CrossRef]
- Leong, X.F.; Ng, C.Y.; Jaarin, K. Animal models in cardiovascular research: Hypertension and atherosclerosis. Biomed. Res. Int. 2015, 2015, 528757. [Google Scholar] [CrossRef] [Green Version]
- Davern, P.J.; Jackson, K.L.; Nguyen-Huu, T.P.; La Greca, L.; Head, G.A. Cardiovascular responses to aversive and nonaversive stressors in Schlager genetically hypertensive mice. Am. J. Hypertens. 2010, 23, 838–844. [Google Scholar] [CrossRef] [Green Version]
- Palma-Rigo, K.; Jackson, K.L.; Davern, P.J.; Nguyen-Huu, T.P.; Elghozi, J.L.; Head, G.A. Renin-angiotensin and sympathetic nervous system contribution to high blood pressure in Schlager mice. J. Hypertens. 2011, 29, 2156–2166. [Google Scholar] [CrossRef]
- Jackson, K.L.; Dampney, B.W.; Moretti, J.L.; Stevenson, E.R.; Davern, P.J.; Carrive, P.; Head, G.A. Contribution of orexin to the neurogenic hypertension in BPH/2J mice. Hypertension 2016, 67, 959–969. [Google Scholar] [CrossRef]
- The UniProt, C. UniProt: The universal protein knowledgebase. Nucleic Acids Res. 2017, 45, D158–D169. [Google Scholar] [CrossRef]
- Chan, H.C.; Filipek, S.; Yuan, S. The Principles of Ligand Specificity on beta-2-adrenergic receptor. Sci. Rep. 2016, 6, 34736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talebianpoor, M.S.; Mirkhani, H. The effect of tempol administration on the aortic contractile responses in rat preeclampsia model. ISRN Pharmacol. 2012, 2012, 187208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ettinger, G.; Burton, J.P.; Gloor, G.B.; Reid, G. Lactobacillus rhamnosus GR-1 attenuates induction of hypertrophy in cardiomyocytes but not through secreted protein MSP-1 (p75). PLoS ONE 2017, 12, e0168622. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Cai, Y. Gut microbiota and hypertension: From pathogenesis to new therapeutic strategies. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Li, C.; Huang, J.; Wei, F.; Zhang, Y.; Wang, S. Cell membrane chromatography coupled with UHPLC-ESI-MS/MS method to screen target components from Peucedanum praeruptorum Dunn acting on alpha1A adrenergic receptor. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1011, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.S.; Loh, Y.C.; Ng, C.H.; Ch’ng, Y.S.; Asmawi, M.Z.; Ahmad, M.; Yam, M.F. Anti-hypertensive and vasodilatory effects of amended Banxia Baizhu Tianma Tang. Biomed. Pharmacother. 2018, 97, 985–994. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, X.; Jing, H.; Zhang, J.; Miao, Y.; Zhai, X.; Chen, S. Using open-tubular capillary electrochromatography with part-coating column for binding constants determination of beta2 -adrenergic receptor with seven drugs. Electrophoresis 2019, 40, 289–295. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, X.; Jing, H.; Miao, Y.; Zhao, L.; Han, Y.; Cui, C. Research on drug-receptor interactions and prediction of drug activity via oriented immobilized receptor capillary electrophoresis. Electrophoresis 2015, 36, 2433–2441. [Google Scholar] [CrossRef]
- Feng, T.S.; Yuan, Z.Y.; Yang, R.Q.; Zhao, S.; Lei, F.; Xiao, X.Y.; Xing, D.M.; Wang, W.H.; Ding, Y.; Du, L.J. Purgative components in rhubarbs: Adrenergic receptor inhibitors linked with glucose carriers. Fitoterapia 2013, 91, 236–246. [Google Scholar] [CrossRef]
- Endale, M.; Song, J.C.; Rhee, M.H.; Liu, K.H.; Kim, T.K.; Kwon, J.G.; Park, K.S.; Chung, K.M.; Kim, T.W. Inhibitory effect of Suaeda asparagoides (Miq.) extract on the motility of rat gastric antrum is mediated by beta-adrenoceptor. Lab. Anim. Res. 2011, 27, 317–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saqib, F.; Janbaz, K.H. Rationalizing ethnopharmacological uses of Alternanthera sessilis: A folk medicinal plant of Pakistan to manage diarrhea, asthma and hypertension. J. Ethnopharmacol. 2016, 182, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.J.; Gilani, A.H. Blood pressure lowering effect of the extract of aerial parts of Capparis aphylla is mediated through endothelium-dependent and independent mechanisms. Clin. Exp. Hypertens. 2011, 33, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.H.; Zhao, J.; Jin, H.T.; Cao, Y.; Ming, T.; Zhang, L.L.; Hu, M.Y.; Hamlati, H.; Pang, S.B.; Ma, X.P. Vasorelaxant effects of the extracts and some flavonoids from the buds of Coreopsis tinctoria. Pharm. Biol. 2013, 51, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Mustafa, M.R.; Khan, A.U.; Murugan, D.D. Hypotensive effect of Gentiana floribunda is mediated through Ca++ antagonism pathway. BMC Complement. Altern. Med. 2012, 12, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.; Khan, A.U.; Najeeb ur, R.; Zafar, M.A.; Hazrat, A.; Gilani, A.H. Cardiovascular effects of Juniperus excelsa are mediated through multiple pathways. Clin. Exp. Hypertens. 2012, 34, 209–216. [Google Scholar] [CrossRef]
- Shah, A.J.; Rasheed, M.; Jabeen, Q.; Ahmed, A.; Tareen, R.B.; Gilani, A.H.; Nadir, M.; Ahmad, V.U. Chemical analysis and calcium channel blocking activity of the essential oil of Perovskia abrotanoides. Nat. Prod. Commun. 2013, 8, 1633–1636. [Google Scholar] [CrossRef] [Green Version]
- El-Mosallamy, A.E.; Sleem, A.A.; Abdel-Salam, O.M.; Shaffie, N.; Kenawy, S.A. Antihypertensive and cardioprotective effects of pumpkin seed oil. J. Med. Food 2012, 15, 180–189. [Google Scholar] [CrossRef]
- Liu, S.J.; Liu, Y.; Zhang, X.; Yang, Z.C.; Liu, W.Z.; Tan, Y.Z. Effect of TGRJ on blood press, NO and Ang II in renal hypertensive rats. Zhong Yao Cai 2012, 35, 953–957. [Google Scholar]
- Yang, Q.; Li, Y.; Weng, X.; Chen, Y.; Ruan, C.; Zhu, X. Effect of Tiangou Jiangya capsule on rabbit aortic strip contraction. Zhongguo Zhong Yao Za Zhi 2011, 36, 3349–3352. [Google Scholar]
- Siddiqi, H.S.; Mehmood, M.H.; Rehman, N.U.; Gilani, A.H. Studies on the antihypertensive and antidyslipidemic activities of Viola odorata leaves extract. Lipids Health Dis. 2012, 11, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, E.; Inagaki, Y.; Kawabata, J. Higenamine 4′-O-beta-d-glucoside in the lotus plumule induces glucose uptake of L6 cells through beta2-adrenergic receptor. Bioorg. Med. Chem. 2015, 23, 3317–3321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, N.H.; Mukherjee, S.; Min, T.; Kang, S.C.; Yun, J.W. Trans-anethole ameliorates obesity via induction of browning in white adipocytes and activation of brown adipocytes. Biochimie 2018, 151, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Carreyre, H.; Carre, G.; Ouedraogo, M.; Vandebrouck, C.; Bescond, J.; Supuran, C.T.; Thibaudeau, S. Bioactive natural product and superacid chemistry for lead compound identification: A case study of selective hCA III and L-Type Ca2+ current inhibitors for hypotensive agent discovery. Molecules 2017, 22, 915. [Google Scholar] [CrossRef] [Green Version]
- Cuong, N.M.; Khanh, P.N.; Huyen, P.T.; Duc, H.V.; Huong, T.T.; Ha, V.T.; Durante, M.; Sgaragli, G.; Fusi, F. Vascular L-type Ca2+ channel blocking activity of sulfur-containing indole alkaloids from Glycosmis petelotii. J. Nat. Prod. 2014, 77, 1586–1593. [Google Scholar] [CrossRef]
- Wenjie, W.; Houqing, L.; Liming, S.; Ping, Z.; Gengyun, S. Effects of praeruptorin C on blood pressure and expression of phospholamban in spontaneously hypertensive rats. Phytomedicine 2014, 21, 195–198. [Google Scholar] [CrossRef]
- Lim, K.M.; Kwon, J.H.; Kim, K.; Noh, J.Y.; Kang, S.; Park, J.M.; Lee, M.Y.; Bae, O.N.; Chung, J.H. Emodin inhibits tonic tension through suppressing PKCdelta-mediated inhibition of myosin phosphatase in rat isolated thoracic aorta. Br. J. Pharmacol. 2014, 171, 4300–4310. [Google Scholar] [CrossRef] [Green Version]
- Park, B.G.; Shin, W.S.; Oh, S.; Park, G.M.; Kim, N.I.; Lee, S. A novel antihypertension agent, sargachromenol D from marine brown algae, Sargassum siliquastrum, exerts dual action as an L-type Ca2+ channel blocker and endothelin A/B2 receptor antagonist. Bioorg. Med. Chem. 2017, 25, 4649–4655. [Google Scholar] [CrossRef]
- Huang, Y.L.; Cui, S.Y.; Cui, X.Y.; Cao, Q.; Ding, H.; Song, J.Z.; Hu, X.; Ye, H.; Yu, B.; Sheng, Z.F.; et al. Tetrandrine, an alkaloid from S. tetrandra exhibits anti-hypertensive and sleep-enhancing effects in SHR via different mechanisms. Phytomedicine 2016, 23, 1821–1829. [Google Scholar] [CrossRef]
- Onakpoya, I.J.; Heneghan, C.J. Effect of the natural sweetener, steviol glycoside, on cardiovascular risk factors: A systematic review and meta-analysis of randomised clinical trials. Eur. J. Prev. Cardiol. 2015, 22, 1575–1587. [Google Scholar] [CrossRef]
- Petit, G.; Berra, E.; Georges, C.M.G.; Capron, A.; Huang, Q.F.; Lopez-Sublet, M.; Rabbia, F.; Staessen, J.A.; Wallemacq, P.; de Timary, P.; et al. Impact of psychological profile on drug adherence and drug resistance in patients with apparently treatment-resistant hypertension. Blood Press. 2018, 27, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Bae, E.H.; Ma, S.K.; Kim, S.W. Altered nitric oxide system in cardiovascular and renal diseases. Chonnam Med. J. 2016, 52, 81–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rattmann, Y.D.; Anselm, E.; Kim, J.H.; Dal-Ros, S.; Auger, C.; Miguel, O.G.; Santos, A.R.; Chataigneau, T.; Schini-Kerth, V.B. Natural product extract of Dicksonia sellowiana induces endothelium-dependent relaxations by a redox-sensitive Src- and Akt-dependent activation of eNOS in porcine coronary arteries. J. Vasc. Res. 2012, 49, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.H.; Lee, S.J.; Choi, Y.W.; Park, S.Y.; Kim, C.D. Preventive effect of gomisin J from Schisandra chinensis on angiotensin II-induced hypertension via an increased nitric oxide bioavailability. Hypertens. Res. 2015, 38, 169–177. [Google Scholar] [CrossRef]
- Abdallah, H.M.; El-Bassossy, H.M.; Mohamed, G.A.; El-Halawany, A.M.; Alshali, K.Z.; Banjar, Z.M. Phenolics from Garcinia mangostana alleviate exaggerated vasoconstriction in metabolic syndrome through direct vasodilatation and nitric oxide generation. BMC Complement. Altern. Med. 2016, 16, 359. [Google Scholar] [CrossRef]
- Je, H.D.; Kim, H.D.; La, H.O. The inhibitory effect of apigenin on the agonist-induced regulation of vascular contractility via calcium desensitization-related pathways. Biomol. Ther. 2014, 22, 100–105. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Wang, X.; Dai, Y.; Kong, L.; Wang, F.; Xu, H.; Lu, D.; Song, J.; Hou, Z. (+/−)-Praeruptorin A enantiomers exert distinct relaxant effects on isolated rat aorta rings dependent on endothelium and nitric oxide synthesis. Chem. Biol. Interact. 2010, 186, 239–246. [Google Scholar] [CrossRef]
- Chen, C.Y.; Ho, L.T.; Yang, F.Y.; Juan, C.C.; Au, L.C. Prunellae spica extract contains antagonists for human endothelin receptors. Am. J. Chin. Med. 2013, 41, 85–98. [Google Scholar] [CrossRef]
- Rayner, B.; Ramesar, R. The importance of G protein-coupled receptor kinase 4 (GRK4) in pathogenesis of salt sensitivity, salt sensitive hypertension and response to antihypertensive treatment. Int. J. Mol. Sci. 2015, 16, 5741–5749. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Zubcevic, J. Gut-brain axis in regulation of blood pressure. Front. Physiol. 2017, 8, 845. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, A.; Forman, J.P. Vitamin D and hypertension: Current evidence and future directions. Hypertension 2010, 56, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Magee, K.L.; Colon-Perez, L.M.; Larkin, R.; Liao, Y.S.; Balazic, E.; Cowart, J.R.; Arocha, R.; Redler, T.; Febo, M.; et al. Impaired butyrate absorption in the proximal colon, low serum butyrate and diminished central effects of butyrate on blood pressure in spontaneously hypertensive rats. Acta Physiol. 2019, 226, e13256. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, Q.; Lu, A.; Liu, X.; Zhang, L.; Xu, C.; Liu, X.; Li, H.; Yang, T. Sodium butyrate suppresses angiotensin II-induced hypertension by inhibition of renal (pro)renin receptor and intrarenal renin-angiotensin system. J. Hypertens. 2017, 35, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Zuo, K.; Li, J.; Xu, Q.; Hu, C.; Gao, Y.; Chen, M.; Hu, R.; Liu, Y.; Chi, H.; Yin, Q.; et al. Dysbiotic gut microbes may contribute to hypertension by limiting vitamin D production. Clin. Cardiol. 2019, 42, 710–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mushtaq, N.; Hussain, S.; Zhang, S.; Yuan, L.; Li, H.; Ullah, S.; Wang, Y.; Xu, J. Molecular characterization of alterations in the intestinal microbiota of patients with grade 3 hypertension. Int. J. Mol. Med. 2019, 44, 513–522. [Google Scholar] [CrossRef] [Green Version]
- Pimenta, F.S.; Luaces-Regueira, M.; Ton, A.M.; Campagnaro, B.P.; Campos-Toimil, M.; Pereira, T.M.; Vasquez, E.C. Mechanisms of action of kefir in chronic cardiovascular and metabolic diseases. Cell. Physiol. Biochem. 2018, 48, 1901–1914. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, J.; Tian, C.; Chen, X.; Li, H.; Wei, X.; Lin, W.; Zheng, N.; Jiang, A.; Feng, R.; et al. Targeting the gut microbiota to investigate the mechanism of lactulose in negating the effects of a high-salt diet on hypertension. Mol. Nutr. Food Res. 2019, 63, e1800941. [Google Scholar] [CrossRef]
- Yang, M.; Lao, L. Emerging applications of metabolomics in traditional chinese medicine treating hypertension: Biomarkers, pathways and more. Front. Pharmacol. 2019, 10, 158. [Google Scholar] [CrossRef] [Green Version]
| Affect Organ | Complications |
|---|---|
| Cardiovascular system | Cardiac hypertrophy, heart failure, angina pectoris, coronary heart disease, myocardial infarction. |
| Artery | Atherosclerosis, aneurysms. |
| Brain | Stroke (ischemic or hemorrhagic), hypertensive encephalopathy, cognitive decline, dementia. |
| Eye | Retinopathy. |
| Kidney | Hypertensive nephropathy, chronic kidney disease. |
| Source | Compound/Extractions | Evaluated Method | Reference |
|---|---|---|---|
| Crude extract/decoction for modulating ACE | |||
| Dohaekseunggi-tang | in vivo | [75] | |
| Huatuo reconstruction pill | in silico | [76] | |
| Nigella sativa | enzymatic | [77] | |
| Cymodocea Nodosa | + | [78] | |
| Plantago major | Seeds extract | + | [79] |
| Mucuna pruriens | + | [80] | |
| Syzygium cumini | Seeds extract | in vitro | [81] |
| Fucus spiralis | enzymatic | [82] | |
| Eucalyptus camaldulensis Litsea glaucescens | Ferment | + | [83] |
| Lens culinaris | Sprouted extract | + | [84] |
| Pleurotus eryngii | polysaccharide | + | [85] |
| Phaseolus vulgaris | Ferment | + | [86] |
| Prunus amygdalus Pistacia vera | juice byproduct | + | [87] |
| Boza | protein extract | + | [88] |
| Nitraria sibirica | alkaloids | in vivo | [89] |
| Amaranthus dubius Amaranthus hybridus Asystasia gangetica Galinsoga parviflora Justicia flava Oxygonum sinuatum Physalis viscosa | enzymatic | [90] | |
| Tulbaghia violacea | in vivo | [90] | |
| Bulbus Fritillaria | in vivo | [91] | |
| Crude extracts or decoction for AGT1R | |||
| Apocynum venetum leaf extract | in vitro | [92] | |
| DanHong injection | + | [93] | |
| Liuwei Dihuang formula | + | [94] | |
| Yiqi Huaju formula | in vivo | [95] | |
| Songling xuemaikang capsule | + | [96] | |
| ACE inhibitors | |||
| Ampelopsis Brevipedunculata | (+)-Hopeaphenol; (+)-Vitisin A | enzymatic | [97] |
| Asparagus officinalis | Asparaptine | + | [98] |
| Avena sativa | WWK, WCY, FLLA | + | [99] |
| Bothrops jararaca | EWPRQIPP, EARPPHPPIPP, EWGRPPGPPIPP, EGGWPRPGP(Glu)IPP, | in vitro | [100] |
| Bothrops moojeni | (Pyro)EKWPPGGKVPP, (Pyro)EKPRPGPEIPP, (Pyro)ENWPWPGPEIPP | enzymatic | [101] |
| Cajanus cajan | VVSLSIPR | + | [102] |
| Camellia sinensis | Epigallocatechin gallate | + | [59] |
| Chinese Herb | Pentagalloylglucose, Isochlorogenic acid B, Methyl 3,4-di-O-caffeoylquinate, (−)-Epigallocatechin gallate, Epigallocatechin-3-O-Methylgallate | in vivo | [103] |
| Cleistanthus collinus | Cleistanthins A, B | in silico | [104] |
| Clerodendron trichotomum | Acteoside, Isomartynoside, Leucosceptoside A, Martynoside | enzymatic | [105] |
| Coptis chinensis | Berberine | in vitro | [91] |
| Coix larchryma-jobi | GAAGGAF, NPATY | in vivo | [60,106] |
| Delphinium sp. | Cyanin, Delphinidin | enzymatic | [107] |
| Desmodium styracifolium | Carlinoside, Schaftoside, Vicenin 1–3 | + | [108] |
| Dioscorea opposita Thunb. | Diascorin | + | [109] |
| Egg York | Lapslpgkpkpd | + | [110] |
| Eucommia ulmoides | Megastigmane Enantiomers | in silico | [111] |
| Glycyrrhiza glabra | Licochalcone A | enzymatic | [59] |
| Glycyrrhiza uralensis | Echinatin | + | [59] |
| Limonium michelsonii | Isolates | + | [112] |
| Mucuna Pruriens | Genistein | + | [80] |
| Multisource | Caffeic Acid, Caffeoyl Acetate, Chlorogenic Acid, Ferulic Acid | + | [113] |
| Salvia hispanica L. | LIVSPLAGRL | + | [114] |
| Salvia miltiorrhizae | Salvianolic Acid B | + | [115,116] |
| Lithospermic Acid B | + | [105] | |
| Sargassum wightii | O-Heterocyclic Analogues | in silico | [117] |
| Tamarix hohenackeri | Chrysoeriol, Quercetin, Isoferulic acid, Methyl-4-O-methylgallate, Gallic acid, Methyl gallate | enzymatic | [118] |
| Toona sinensis | Quercetin, Resveratrol | + | [107] |
| Vigna radiata | KDYRL, VTPALR, KLPAGTLF | + | [119] |
| Xestospongia Cf. Vansoesti | Salsolinol | + | [115] |
| AGT1R inhibitors | |||
| Alisma orientale | 23-O-acetylalisol B, Alismol, Alisols A, Alisols B | enzymatic | [120] |
| Astragalus membranaceus | Astragaloside IV | in vitro | [121] |
| Cynodon dactylon | Linoleoylchloride, Diazoprogesteron, Didodecyl Phthalate | enzymatic | [122] |
| Eucommia ulmoides | Megastigmane Enantiomers | in silico | [111] |
| Salvia miltiorrhizae | Salvianolic acid B | in vivo | [123] |
| + | Tanshinone IIA | in vivo | [124] |
| Source | Compounds | Test Method | Reference |
|---|---|---|---|
| Crude extract/decoction | |||
| β-adrenergic receptor inhibitor | |||
| Banxia Baizhu Tianma Tang | in vitro | [180] | |
| Paeoniae Rubra | in vitro | [181,182] | |
| Rhubarbs | in silico | [183] | |
| Suaeda asparagoides | in vivo | [184] | |
| Ca2+ channel inhibitor | |||
| Alternanthera sessilis | in vitro | [185] | |
| Capparis aphylla | in vivo | [186] | |
| Coreopsis tinctoria | in vitro | [187] | |
| Gentiana floribunda | in vivo | [188] | |
| Jasonia glutinosa | in vitro | [161] | |
| Juniperus excelsa | in vitro | [189] | |
| Perovskia abrotanoides essential oil | in vitro | [190] | |
| Pumpkin seed oil | in vivo | [191] | |
| Ranunculus japoniucus | in vivo | [192] | |
| Tiangou Jiangya | in vitro | [193] | |
| Viola odorata | in vivo | [194] | |
| Pure compounds | |||
| β-adrenergic receptor inhibitor | |||
| Nelumbo nucifera | Higenamine 4′-O-β-d-glucoside | in vitro | [195] |
| Notopterygium incisum | Isoimperatorin | enzymatic | [159] |
| Pimpinella anisum | Trans-anethole | in silico | [196] |
| Ca2+ channel inhibitor | |||
| Agelanthus dodoneifolius | Dodoneine | in vitro | [197] |
| Glycosmis petelotii | N-demethylglypetelotine, Glypetelotine | in vitro | [198] |
| Peucedanum praeruptorum | Praeruptorin C | in vivo | [199] |
| Polygonum multiflorum | Emodin | in vitro | [200] |
| Sargassum siliquastrum | Sargachromenol D | in vitro | [201] |
| Stephaniae tetrandrae | Tetrandrine, Fangchinoline | in vivo | [202] |
| Stevia rebaudiana | Stevioside | in vivo | [203] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-R.; Lin, S.-Y.; Chen, C.-C.; Fu, Y.-S.; Weng, C.-F. Exploring a New Natural Treating Agent for Primary Hypertension: Recent Findings and Forthcoming Perspectives. J. Clin. Med. 2019, 8, 2003. https://doi.org/10.3390/jcm8112003
Lin S-R, Lin S-Y, Chen C-C, Fu Y-S, Weng C-F. Exploring a New Natural Treating Agent for Primary Hypertension: Recent Findings and Forthcoming Perspectives. Journal of Clinical Medicine. 2019; 8(11):2003. https://doi.org/10.3390/jcm8112003
Chicago/Turabian StyleLin, Shian-Ren, Shiuan-Yea Lin, Ching-Cheng Chen, Yaw-Syan Fu, and Ching-Feng Weng. 2019. "Exploring a New Natural Treating Agent for Primary Hypertension: Recent Findings and Forthcoming Perspectives" Journal of Clinical Medicine 8, no. 11: 2003. https://doi.org/10.3390/jcm8112003





