Mental Health and Metabolic Outcomes in Early Postpartum in Women with Prediabetes After Gestational Diabetes: A Secondary Analysis of the MELINDA Trial
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GDM | Gestational Diabetes Mellitus |
| T2DM | Type 2 Diabetes Mellitus |
| MELINDA | Mobile-Based Lifestyle Intervention in Women with Glucose Intolerance after Gestational Diabetes Mellitus |
| RCT | Randomized Controlled Trial |
| IADPSG | International Association of the Diabetes and Pregnancy Study Groups |
| ADA | American Diabetes Association |
| BMI | Body Mass Index |
| OGTT | Oral Glucose Tolerance Test |
| IDF | International Diabetes Federation |
| IFG | Impaired Fasting Glucose |
| IGT | Impaired Glucose Tolerance |
| NAM | National Academy of Medicine |
| IOM | Institute of Medicine |
| NGT | Normal glucose tolerance |
| MET | Metabolic Equivalent of Task |
| IPAQ | International Physical Activity Questionnaire |
| CES-D | Center for Epidemiologic Studies Depression Scale |
| FFQ | Food Frequency Questionnaire |
| STAI-6 | State-Trait Anxiety Inventory-6 |
| RPS-DD | Risk Perception Survey for Developing Diabetes |
| SF-36 | Quality-of-life questionnaire |
| HOMA-IR | Homeostasis Model of Assessment—Insulin Resistance |
| HOMA-B | Homeostasis Model of Assessment—Beta-cell Function |
| ISSI-2 | Insulin secretion-sensitivity index-2 |
| BP | Blood pressure |
| PPWR | Postpartum Weight Retention |
| HDL | High-Density Lipoprotein |
| LDL | Low-Density Lipoprotein |
| PCOS | Polycystic Ovary Syndrome |
References
- Modak, A.; Ronghe, V.; Gomase, K.P.; Mahakalkar, M.G.; Taksande, V. A Comprehensive Review of Motherhood and Mental Health: Postpartum Mood Disorders in Focus. Cureus 2023, 15, e46209. [Google Scholar] [CrossRef] [PubMed]
- Rallis, S.; Skouteris, H.; McCabe, M.; Milgrom, J. The transition to motherhood: Towards a broader understanding of perinatal distress. Women Birth 2014, 27, 68–71. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee; ElSayed, N.A.; Aleppo, G.; Bannuru, R.R.; Bruemmer, D.; Collins, B.S.; Ekhlaspour, L.; Hilliard, M.E.; Johnson, E.L.; Khunti, K.; et al. 1. Improving Care and Promoting Health in Populations: Stand. Care Diabetes—2024. Diabetes Care 2024, 47, S11–S19. [Google Scholar] [CrossRef]
- Benhalima, K.; Van Crombrugge, P.; Moyson, C.; Verhaeghe, J.; Vandeginste, S.; Verlaenen, H.; Vercammen, C.; Maes, T.; Dufraimont, E.; De Block, C.; et al. Prediction of Glucose Intolerance in Early Postpartum in Women with Gestational Diabetes Mellitus Based on the 2013 WHO Criteria. J. Clin. Med. 2019, 8, 383. [Google Scholar] [CrossRef]
- Ohene-Agyei, P.; Iqbal, A.; Harding, J.E.; Crowther, C.A.; Lin, L. Postnatal care after gestational diabetes—A systematic review of clinical practice guidelines. BMC Pregnancy Childbirth 2024, 24, 720. [Google Scholar] [CrossRef]
- Amer, S.A.; Zaitoun, N.A.; Abdelsalam, H.A.; Abbas, A.; Ramadan, M.S.; Ayal, H.M.; Ba-Gais, S.E.A.; Basha, N.M.; Allahham, A.; Agyenim, E.B.; et al. Exploring predictors and prevalence of postpartum depression among mothers: Multinational study. BMC Public Health 2024, 24, 1308. [Google Scholar] [CrossRef]
- Konjevod, M.; Gredicak, M.; Vuic, B.; Tudor, L.; Perkovic, M.N.; Milos, T.; Strac, D.S.; Pivac, N.; Erjavec, G.N. Overview of metabolomic aspects in postpartum depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 127, 110836. [Google Scholar] [CrossRef]
- Matthey, S. Are we overpathologising motherhood? J. Affect. Disord. 2010, 120, 263–266. [Google Scholar] [CrossRef]
- Webb, J.B.; Siega-Riz, A.M.; Dole, N. Psychosocial Determinants of Adequacy of Gestational Weight Gain. Obesity 2009, 17, 300–309. [Google Scholar] [CrossRef]
- Pavlik, L.B.; Rosculet, K. Maternal Obesity and Perinatal Depression: An Updated Literature Review. Cureus 2020, 12, e10736. [Google Scholar] [CrossRef]
- Minschart, C.; De Weerdt, K.; Elegeert, A.; Van Crombrugge, P.; Moyson, C.; Verhaeghe, J.; Vandeginste, S.; Verlaenen, H.; Vercammen, C.; Maes, T.; et al. Antenatal Depression and Risk of Gestational Diabetes, Adverse Pregnancy Outcomes, and Postpartum Quality of Life. J. Clin. Endocrinol. Metab. 2021, 106, e3110–e3124. [Google Scholar] [CrossRef] [PubMed]
- Demissie, Z.; Siega-Riz, A.M.; Evenson, K.R.; Herring, A.H.; Dole, N.; Gaynes, B.N. Associations between physical activity and postpartum depressive symptoms. J. Womens Health 2011, 20, 1025–1034. [Google Scholar] [CrossRef]
- Nicklas, J.M.; Miller, L.J.; Zera, C.A.; Davis, R.B.; Levkoff, S.E.; Seely, E.W. Factors Associated with Depressive Symptoms in the Early Postpartum Period Among Women with Recent Gestational Diabetes Mellitus. Matern. Child Health J. 2013, 17, 1665–1672. [Google Scholar] [CrossRef]
- Nakamura, A.; van der Waerden, J.; Melchior, M.; Bolze, C.; El-Khoury, F.; Pryor, L. Physical activity during pregnancy and postpartum depression: Systematic review and meta-analysis. J. Affect. Disord. 2019, 246, 29–41. [Google Scholar] [CrossRef]
- Ruohomäki, A.; Toffol, E.; Upadhyaya, S.; Keski-Nisula, L.; Pekkanen, J.; Lampi, J.; Voutilainen, S.; Tuomainen, T.-P.; Heinonen, S.; Kumpulainen, K.; et al. The association between gestational diabetes mellitus and postpartum depressive symptomatology: A prospective cohort study. J. Affect. Disord. 2018, 241, 263–268. [Google Scholar] [CrossRef]
- Minschart, C.; Myngheer, N.; Maes, T.; De Block, C.; Van Pottelbergh, I.; Abrams, P.; Vinck, W.; Leuridan, L.; Driessens, S.; Mathieu, C.; et al. Effectiveness of a blended mobile-based lifestyle intervention in women with glucose intolerance after a recent history of gestational diabetes (MELINDA): A 1-year, prospective, multicentre, randomised controlled trial. eClinicalMedicine 2024, 70, 102523. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2010, 33, S62–S69. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Verbeke, J.; Boedt, T.; Matthys, C. Development and validity of a short web-based semi-quantitative Food Frequency Questionnaire applicable in both clinical and research setting: An evolution over time. Front. Nutr. 2023, 10, 1073559. [Google Scholar] [CrossRef]
- Harrison, C.L.; Thompson, R.G.; Teede, H.J.; Lombard, C.B. Measuring physical activity during pregnancy. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 19. [Google Scholar] [CrossRef]
- Petrou, S.; Morrell, J.; Spiby, H. Assessing the empirical validity of alternative multi-attribute utility measures in the maternity context. Health Qual. Life Outcomes 2009, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.M.; Yaktine, A.L. Weight Gain During Pregnancy; National Academies Press: Washington, DC, USA, 2009. [Google Scholar] [CrossRef]
- Woody, C.A.; Ferrari, A.J.; Siskind, D.J.; Whiteford, H.A.; Harris, M.G. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J. Affect. Disord. 2017, 219, 86–92. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, M.W.; McCabe, J.E. Postpartum Depression: Current Status and Future Directions. Annu. Rev. Clin. Psychol. 2013, 9, 379–407. [Google Scholar] [CrossRef]
- Arafa, A.; Dong, J.-Y. Gestational diabetes and risk of postpartum depressive symptoms: A meta-analysis of cohort studies. J. Affect. Disord. 2019, 253, 312–316. [Google Scholar] [CrossRef]
- Wilson, C.A.; Newham, J.; Rankin, J.; Ismail, K.; Simonoff, E.; Reynolds, R.M.; Stoll, N.; Howard, L.M. Is there an increased risk of perinatal mental disorder in women with gestational diabetes? A systematic review and meta-analysis. Diabet. Med. 2020, 37, 602–622. [Google Scholar] [CrossRef]
- Sumlin, L.L.; Garcia, T.J.; Brown, S.A.; Winter, M.A.; García, A.A.; Brown, A.; Cuevas, H.E. Depression and adherence to lifestyle changes in type 2 diabetes: A systematic review. Diabetes Educ. 2014, 40, 731–744. [Google Scholar] [CrossRef]
- Hoogendoorn, C.J.; Krause-Steinrauf, H.; Uschner, D.; Wen, H.; Presley, C.A.; Legowski, E.A.; Naik, A.D.; Golden, S.H.; Arends, V.L.; Brown-Friday, J.; et al. Emotional Distress Predicts Reduced Type 2 Diabetes Treatment Adherence in the Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE). Diabetes Care 2024, 47, 629–637. [Google Scholar] [CrossRef]
- Dennis, C.-L.; Hodnett, E.D. Psychosocial and psychological interventions for treating postpartum depression. Cochrane Database Syst. Rev. 2007. [Google Scholar] [CrossRef]
- Van Uytsel, H.; Ameye, L.; Devlieger, R.; Bijlholt, M.; Van der Gucht, K.; Jacquemyn, Y.; Bogaerts, A. Effect of the INTER-ACT lifestyle intervention on maternal mental health during the first year after childbirth: A randomized controlled trial. PLoS ONE 2023, 18, e0284770. [Google Scholar] [CrossRef]
- Van Uytsel, H.; Ameye, L.; Devlieger, R.; Bijlholt, M.; Jacquemyn, Y.; Catry, V.; Schreurs, A.; Bogaerts, A. Mental health after childbirth and the impact on postpartum weight retention and body composition. Data from the INTER-ACT randomized controlled trial. Clin. Obes. 2022, 12, e12550. [Google Scholar] [CrossRef]
- Shovers, S.M.; Bachman, S.S.; Popek, L.; Turchi, R.M. Maternal postpartum depression: Risk factors, impacts, and interventions for the NICU and beyond. Curr. Opin. Pediatr. 2021, 33, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, J.; Shuai, H.; Cai, Z.; Fu, X.; Liu, Y.; Xiao, X.; Zhang, W.; Krabbendam, E.; Liu, S.; et al. Mapping global prevalence of depression among postpartum women. Transl. Psychiatry 2021, 11, 543. [Google Scholar] [CrossRef]
- Stewart, D.E.; Vigod, S.N. Postpartum Depression: Pathophysiology, Treatment, and Emerging Therapeutics. Annu. Rev. Med. 2019, 70, 183–196. [Google Scholar] [CrossRef]
- Gavin, N.I.; Gaynes, B.N.; Lohr, K.N.; Meltzer-Brody, S.; Gartlehner, G.; Swinson, T. Perinatal Depression. Obstet. Gynecol. 2005, 106 Pt 1, 1071–1083. [Google Scholar] [CrossRef]
- Matsumura, K.; Hamazaki, K.; Tsuchida, A.; Kasamatsu, H.; Inadera, H. Education level and risk of postpartum depression: Results from the Japan Environment and Children’s Study (JECS). BMC Psychiatry 2019, 19, 419. [Google Scholar] [CrossRef]
- Moya, E.; Mzembe, G.; Mwambinga, M.; Truwah, Z.; Harding, R.; Ataide, R.; Larson, L.M.; Fisher, J.; Braat, S.; Pasricha, S.; et al. Prevalence of early postpartum depression and associated risk factors among selected women in southern Malawi: A nested observational study. BMC Pregnancy Childbirth 2023, 23, 229. [Google Scholar] [CrossRef]
- Bradshaw, H.; Riddle, J.N.; Salimgaraev, R.; Zhaunova, L.; Payne, J.L. Risk factors associated with postpartum depressive symptoms: A multinational study. J. Affect. Disord. 2022, 301, 345–351. [Google Scholar] [CrossRef]
- Martín-Gómez, C.; Moreno-Peral, P.; Bellón, J.A.; Conejo-Cerón, S.; Campos-Paino, H.; Gómez-Gómez, I.; Rigabert, A.; Benítez, I.; Motrico, E. Effectiveness of psychological interventions in preventing postpartum depression in non-depressed women: A systematic review and meta-analysis of randomized controlled trials. Psychol. Med. 2022, 52, 1001–1013. [Google Scholar] [CrossRef]
- Beck, C.T. Predictors of Postpartum Depression. Nurs. Res. 2001, 50, 275–285. [Google Scholar] [CrossRef]
- Guintivano, J.; Manuck, T.; Meltzer-Brody, S. Predictors of Postpartum Depression: A Comprehensive Review of the Last Decade of Evidence. Clin. Obstet. Gynecol. 2018, 61, 591–603. [Google Scholar] [CrossRef]
- Pan, A.; Keum, N.; Okereke, O.I.; Sun, Q.; Kivimaki, M.; Rubin, R.R.; Hu, F.B. Bidirectional Association Between Depression and Metabolic Syndrome. Diabetes Care 2012, 35, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Jurgens, S.M.; Prieto, S.; Hayes, J.P. Inflammatory biomarkers link perceived stress with metabolic dysregulation. Brain Behav. Immun. Health 2023, 34, 100696. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef]
- Epel, E.S.; McEwen, B.; Seeman, T.; Matthews, K.; Castellazzo, G.; Brownell, K.D.; Bell, J.; Ickovics, J.R. Stress and Body Shape: Stress-Induced Cortisol Secretion Is Consistently Greater Among Women with Central Fat. Psychosom. Med. 2000, 62, 623–632. [Google Scholar] [CrossRef]
- Slyepchenko, A.; Maes, M.; Köhler, C.A.; Anderson, G.; Quevedo, J.; Alves, G.S.; Berk, M.; Fernandes, B.S.; Carvalho, A.F. T helper 17 cells may drive neuroprogression in major depressive disorder: Proposal of an integrative model. Neurosci. Biobehav. Rev. 2016, 64, 83–100. [Google Scholar] [CrossRef]
- Shan, D.; Wang, A.; Yi, K. MTNR1B rs1387153 Polymorphism and Risk of Gestational Diabetes Mellitus: Meta-Analysis and Trial Sequential Analysis. Public Health Genom. 2023, 26, 201–211. [Google Scholar] [CrossRef]
- Han, W.; Wang, J.; Yan, X.; Liu, C.; Huang, J.; Zhang, L.; Zhang, Y.; Zhao, Y.; Hou, Y.; Zheng, W.; et al. Butyrate and iso-butyrate: A new perspective on nutrition prevention of gestational diabetes mellitus. Nutr. Diabetes 2024, 14, 24. [Google Scholar] [CrossRef]
- de Melo, I.M.F.; Ferreira, C.G.M.; Alves, É.R.; D’assunção, C.G.; Neto, C.J.C.L.; de Albuquerque, Y.M.L.; Teixeira, V.W.; Teixeira, Á.A.C. Melatonin Administration Prevents Placental and Fetal Changes Induced by Gestational Diabetes. Reprod. Sci. 2022, 29, 1111–1123. [Google Scholar] [CrossRef]
- Sadat, Z.; Abedzadeh-Kalahroudi, M.; Atrian, M.K.; Karimian, Z.; Sooki, Z. The Impact of Postpartum Depression on Quality of Life in Women After Child’s Birth. Iran. Red Crescent Med. J. 2014, 16, e14995. [Google Scholar] [CrossRef]
- van der Zee-van den Berg, A.I.; Boere-Boonekamp, M.M.; Groothuis-Oudshoorn, C.G.M.; Reijneveld, S.A. Postpartum depression and anxiety: A community-based study on risk factors before, during and after pregnancy. J. Affect. Disord. 2021, 286, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, T.; Bondza, E.; Lethin, C. Evidence of the Importance of Dietary Habits Regarding Depressive Symptoms and Depression. Int. J. Environ. Res. Public Health 2020, 17, 1616. [Google Scholar] [CrossRef] [PubMed]
- Medawar, E.; Huhn, S.; Villringer, A.; Veronica Witte, A. The effects of plant-based diets on the body and the brain: A systematic review. Transl. Psychiatry 2019, 9, 226. [Google Scholar] [CrossRef]
- Almarshad, M.I.; Algonaiman, R.; Alharbi, H.F.; Almujaydil, M.S.; Barakat, H. Relationship between Ultra-Processed Food Consumption and Risk of Diabetes Mellitus: A Mini-Review. Nutrients 2022, 14, 2366. [Google Scholar] [CrossRef]
- Sánchez-Villegas, A.; Martínez-González, M.A.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Covas, M.I.; Arós, F.; Romaguera, D.; Gómez-Gracia, E.; Lapetra, J.; et al. Mediterranean dietary pattern and depression: The PREDIMED randomized trial. BMC Med. 2013, 11, 208. [Google Scholar] [CrossRef]
- Kirkbride, J.B.; Anglin, D.M.; Colman, I.; Dykxhoorn, J.; Jones, P.B.; Patalay, P.; Pitman, A.; Soneson, E.; Steare, T.; Wright, T.; et al. The social determinants of mental health and disorder: Evidence, prevention and recommendations. World Psychiatry 2024, 23, 58–90. [Google Scholar] [CrossRef]
- Patel, V.; Saxena, S.; Lund, C.; Thornicroft, G.; Baingana, F.; Bolton, P.; Chisholm, D.; Collins, P.Y.; Cooper, J.L.; Eaton, J.; et al. The Lancet Commission on global mental health and sustainable development. Lancet 2018, 392, 1553–1598. [Google Scholar] [CrossRef]
- Bhati, S.; Richards, K. A Systematic Review of the Relationship Between Postpartum Sleep Disturbance and Postpartum Depression. J. Obstet. Gynecol. Neonatal Nurs. 2015, 44, 350–357. [Google Scholar] [CrossRef]
- Park, E.M.; Meltzer-Brody, S.; Stickgold, R. Poor sleep maintenance and subjective sleep quality are associated with postpartum maternal depression symptom severity. Arch. Womens Ment. Health 2013, 16, 539–547. [Google Scholar] [CrossRef]
- Xie, H.; Cong, S.; Wang, R.; Sun, X.; Han, J.; Ni, S.; Zhang, A. Effect of eHealth interventions on perinatal depression: A meta-analysis. J. Affect. Disord. 2024, 354, 160–172. [Google Scholar] [CrossRef]
- Lewkowitz, A.K.; Whelan, A.R.; Ayala, N.K.; Hardi, A.; Stoll, C.; Battle, C.L.; Tuuli, M.G.; Ranney, M.L.; Miller, E.S. The effect of digital health interventions on postpartum depression or anxiety: A systematic review and meta-analysis of randomized controlled trials. Am. J. Obstet. Gynecol. 2024, 230, 12–43. [Google Scholar] [CrossRef]

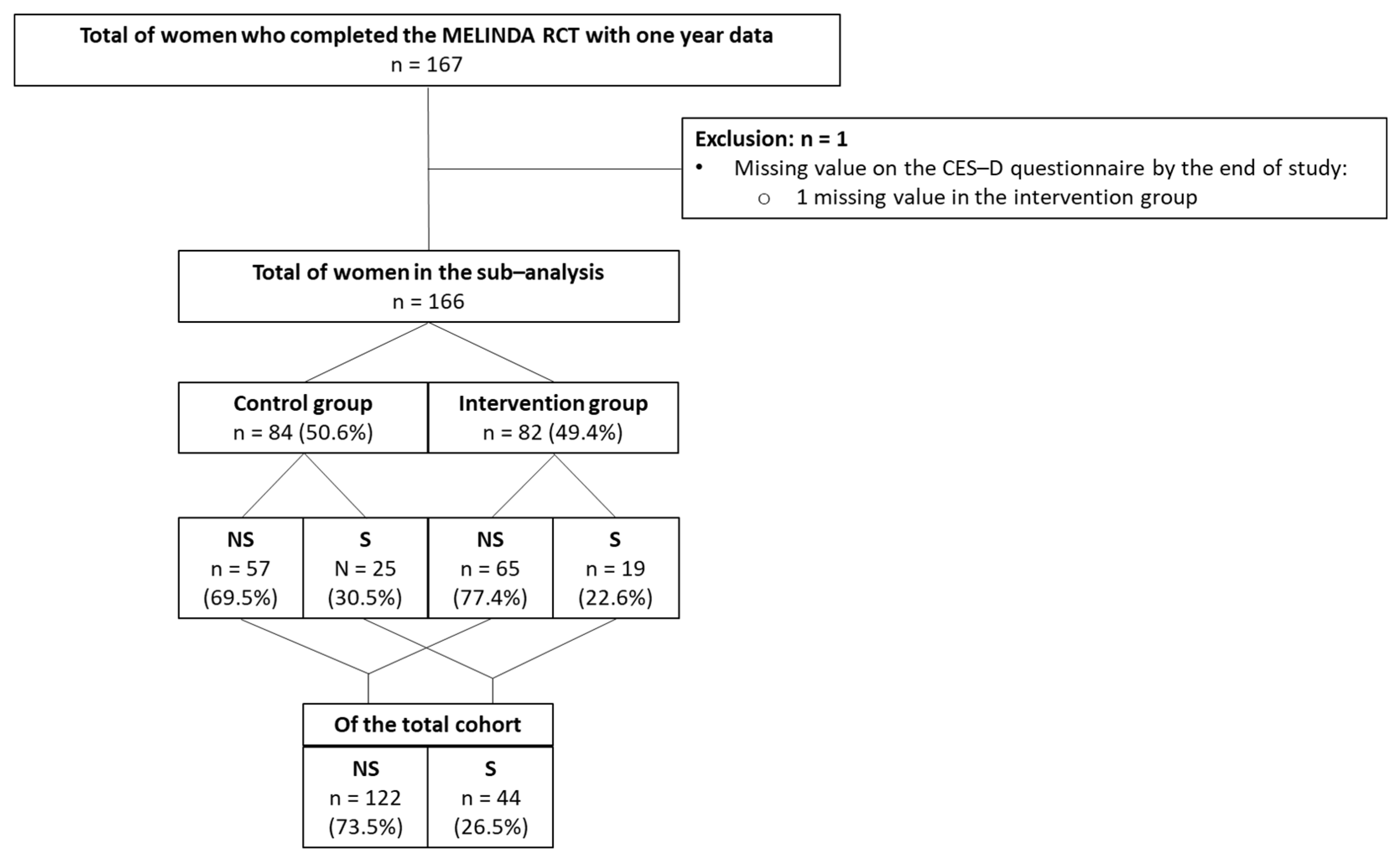
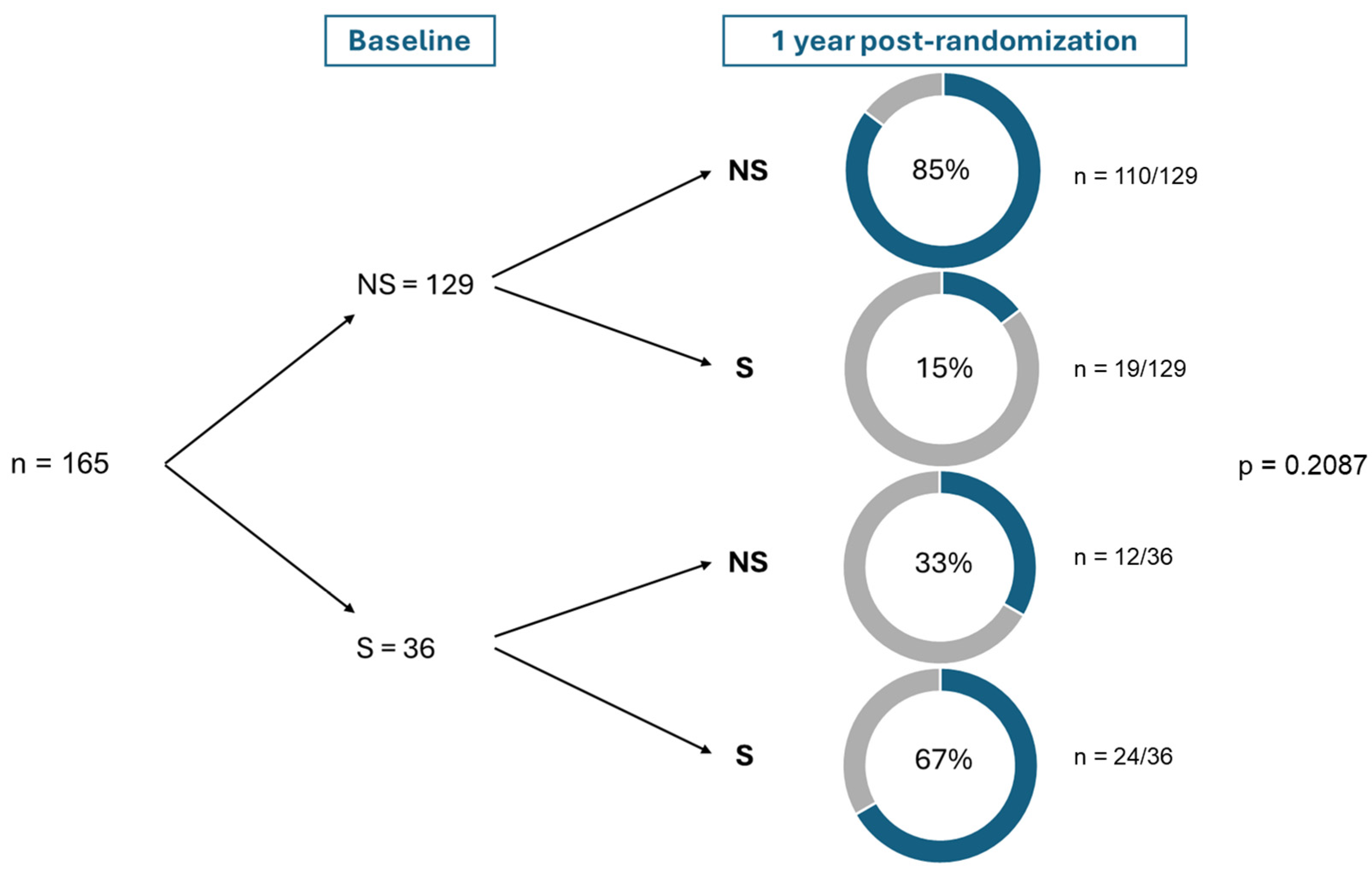
| Group with no Symptoms of Depression (<16 on CES-D Questionnaire) N = 122 (73.5%) | Group with Symptoms of Depression (≥16 on CES-D Questionnaire) N = 44 (26.5%) | p-Value | |
|---|---|---|---|
| General characteristics | |||
| Age (years) baseline | 32.5 ± 3.78 | 30.9 ± 4.93 | 0.033 |
| % Non-Caucasian | 16.39 (20) | 18.18 (8) | 0.816 |
| Highest education % None/primary school % Until age of 15 years % High school % Higher education (bachelor/master) | 0.00 (0) 5.74 (7) 13.93 (17) 80.33 (98) | 2.27 (1) 11.36 (5) 25.00 (11) 61.36 (27) | 0.028 |
| % Paid professional activity | 88.52 (108) | 79.55 (35) | 0.201 |
| Monthly net income family %Low income < €1500 %€1500–5000 % >€5000 | 2.46 (3) 86.89 (106) 10.66 (13) | 6.98 (3) 88.37 (38) 4.65 (2) | 0.227 |
| % Living without partner | 15.57 (19) | 22.73 (10) | 0.354 |
| % Currently smoking | 3.28 (4) | 11.36 (5) | 0.056 |
| % Multiparity | 54.10 (66) | 47.73(21) | 0.487 |
| % History of GDM in previous pregnancy | 21.25 (17) | 23.08 (6) | 1.000 |
| % History of PCOS | 5.98 (7) | 2.44 (1) | 0.681 |
| % History of miscarriage | 36.07 (44) | 29.55 (13) | 0.465 |
| Pre-pregnancy BMI (kg/m2) | 26.7 ± 4.93 | 28.0 ± 6.66 | 0.389 |
| % First degree family history of T2DM | 28.45 (33) | 33.33 (14) | 0.560 |
| % Insulin use in pregnancy | 31.15 (38) | 36.36 (16) | 0.575 |
| Gestational weight gain | |||
| Total Weight gain (first visit till delivery) (Kg) | 8.3 ± 5.54 | 8.1 ± 6.25 | 0.808 |
| % Excessive weight gain | 17.12 (19) | 28.57 (12) | 0.122 |
| % Inadequate weight gain | 50.45 (56) | 52.38 (22) | 0.858 |
| Baseline (6–16 weeks postpartum) | |||
| % Breastfeeding | 78.69 (96) | 74.42 (32) | 0.671 |
| Timing OGTT (months) | 2.9 ± 0.60 | 2.8 ± 0.76 | 0.185 |
| FPG (mmol/L) 30 min glucose OGTT (mmol/L) 1-h glucose OGTT (mmol/L) 2-h glucose OGTT (mmol/L) | 5.2 ± 0.57 9.0 ± 1.31 9.5 ± 1.97 8.2 ± 1.33 | 5.3 ± 0.62 9.0 ± 1.42 9.4 ± 2.00 7.9 ± 1.65 | 0.185 0.733 0.878 0.475 |
| % Metabolic syndrome | 21.85 (26) | 38.64 (17) | 0.044 |
| BMI (kg/m2) | 26.9 ± 4.98 | 28.5 ± 6.81 | 0.285 |
| % Overweight (BMI 25.0–29.9) % Obese (BMI ≥ 30) | 30.33 (37) 28.69 (35) | 34.09 (15) 31.82 (14) | 0.719 |
| Mean systolic blood pressure (mmHg) | 117.6 ± 11.85 | 119.8 ± 12.33 | 0.137 |
| Mean diastolic blood pressure (mmHg) | 74.5 ± 9.12 | 76.8 ± 9.14 | 0.204 |
| % Hypertension (systolic BP ≥ 140 and/or diastolic BP ≥ 90 mmHg) | 5.74 (7) | 15.91 (7) | 0.055 |
| % Waist circumference >80 cm | 80.67 (96) | 79.55 (35) | 1.000 |
| % Waist circumference > 88 cm | 51.26 (61) | 65.91 (29) | 0.112 |
| % PPWR > 0 kg % PPWR > 5 kg | 54.10 (66) 10.66 (13) | 56.82 (25) 22.73 (10) | 0.860 0.072 |
| HbA1c (%) | 5.4 ± 0.31 | 5.4 ± 0.37 | 0.736 |
| HbA1c (mmol/mol) | 35.6 ± 3.41 | 35.4 ± 4.03 | 0.736 |
| Fasting total cholesterol (mmol/L) Fasting HDL-cholesterol (mmol/L) Fasting LDL-cholesterol (mmol/L) Triglycerides (mmol/L) | 4.8 ± 0.81 1.6 ± 0.36 2.8 ± 0.76 1.1 ± 0.69 | 5.0 ± 1.06 1.5 ± 0.56 3.2 ± 0.82 1.2 ± 0.72 | 0.071 0.305 0.009 0.267 |
| Matsuda insulin sensitivity | 3.8 ± 2.12 | 3.8 ± 2.58 | 0.385 |
| HOMA-IR | 2.4 ± 1.48 | 3.0 ± 2.42 | 0.381 |
| HOMA-B | 120.9 ± 66.70 | 127.3 ± 66.78 | 0.674 |
| ISSI-2 | 1.4 ± 0.39 | 1.5 ± 0.45 | 0.935 |
| Insulinogenic index/HOMA-IR | 0.2 ± 0.16 | 0.2 ± 0.14 | 0.730 |
| FFQ Total fruit (g) Total vegetables (g) Total meat (g) Total fish (g) Total discretionary foods (g) Daily protein intake (g) Daily fat intake (g) Daily carbohydrate intake (g) Daily fiber intake (g) Daily water intake (ml) Dietary Health Index (%) | 133.9 ± 104.08 204.5 ± 100.61 116.7 ± 59.45 24.3 ± 31.69 326.9 ± 290.20 66.1 ± 21.76 55.0 ± 18.47 188.8 ± 57.44 18.4 ± 6.19 2132.5 ± 531.46 75.9 ± 11.04 | 120.3 ± 92.63 184.1 ± 102.48 116.4 ± 56.14 20.9 ± 19.40 274.9 ± 234.04 62.2 ± 17.86 54.1 ± 17.74 174.1 ± 51.86 17.0 ± 5.58 2000.1 ± 512.84 75.5 ± 10.37 | 0.488 0.232 0.856 0.741 0.424 0.659 0.936 0.170 0.289 0.141 0.782 |
| IPAQ/METs category at time of OGTT % Low % Moderate % High | 11.97 (14) 43.59 (51) 44.44 (52) | 17.07 (7) 39.02 (16) 43.90 (18) | 0.656 |
| SF36 Physical functioning Role physical Role emotional Emotional Wellbeing Social functioning Pain General health Vitality | 88.8 ± 15.87 80.8 ± 23.52 87.6 ± 17.48 78.9 ± 12.54 90.1 ±14.98 83.0 ± 20.92 74.5 ± 15.58 62.9 ± 15.53 | 84.2 ± 20.33 69.6 ± 31.53 66.7 ± 27.24 65.6 ± 16.09 71.4 ± 27.92 74.2 ± 24.22 61.1 ± 19.89 52.08 ± 19.60 | 0.097 0.049 <0.001 <0.001 <0.001 0.027 <0.001 0.002 |
| STAI-6 | 11.2 ± 2.60 | 14.5 ± 3.58 | <0.001 |
| One year post-randomization | |||
| % Breastfeeding | 76.23 (93) | 68.18 (30) | 0.319 |
| Timing OGTT (months) | 14.9 ± 0.80 | 14.9 ± 0.91 | 0.788 |
| FPG (mmol/L) 30 min glucose OGTT (mmol/L) 1-h glucose OGTT (mmol/L) 2-h glucose OGTT (mmol/L) | 5.3 ± 0.65 8.7 ± 1.49 8.8 ± 2.26 7.2 ± 2.03 | 5.5 ± 0.65 9.0 ± 1.75 9.2 ± 2.55 7.6 ± 2.11 | 0.063 0.286 0.379 0.307 |
| % IFG % IGT % IFG + IGT | 26.15 (17) 52.31 (34) 21.54 (14) | 26.92 (7) 30.77 (8) 42.31 (11) | 0.098 |
| % T2DM | 3.28 (4) | 9.09 (4) | 0.210 |
| % Metabolic syndrome | 22.31 (27) | 45.45 (20) | 0.006 |
| BMI (kg/m2) | 26.3 ± 5.25 | 27.8 ± 7.23 | 0.403 |
| % Overweight (BMI 25.0–29.9) % Obese (BMI ≥ 30) | 27.05 (33) 26.23 (32) | 31.82 (14) 27.27 (12) | 0.749 |
| Mean systolic blood pressure (mmHg) | 117.7 ± 12.65 | 121.9 ± 12.44 | 0.080 |
| Mean diastolic blood pressure (mmHg) | 76.4 ± 10.18 | 78.4 ± 10.52 | 0.212 |
| % Hypertension (systolic BP ≥ 140 and/or diastolic BP ≥ 90 mmHg) | 12.30 (15) | 20.45 (9) | 0.214 |
| % Waist circumference >80 cm | 62.81 (76) | 72.73 (32) | 0.270 |
| % Waist circumference > 88 cm | 42.98 (52) | 47.73 (21) | 0.600 |
| % PPWR > 0 kg % PPWR > 5 kg | 45.90 (56) 10.66 (13) | 40.91 (18) 11.36 (5) | 0.600 |
| HbA1c (%) | 5.3 ± 0.37 | 5.4 ± 0.32 | 0.344 |
| HbA1c (mmol/mol) | 34.7 ± 4.03 | 35.2 ± 3.44 | 0.344 |
| Fasting total cholesterol (mmol/L) Fasting HDL-cholesterol (mmol/L) Fasting LDL-cholesterol (mmol/L) Triglycerides (mmol/L) | 4.9 ± 0.97 1.5 ± 0.40 2.8 ± 0.79 1.2 ± 0.71 | 5.2 ± 1.55 1.4 ± 0.40 3.0 ± 0.97 1.4 ± 0.69 | 0.520 0.057 0.593 0.005 |
| Matsuda insulin sensitivity | 4.0 ± 2.21 | 3.5 ± 2.03 | 0.260 |
| HOMA-IR | 2.5 ± 1.78 | 3.7 ± 3.79 | 0.178 |
| HOMA-B | 118.8 ± 52.54 | 131.8 ± 75.19 | 0.550 |
| ISSI-2 | 1.7 ± 0.69 | 1.5 ± 0.58 | 0.117 |
| Insulinogenic index/HOMA-IR | 0.2 ± 0.20 | 0.2 ± 0.11 | 0.225 |
| FFQ Total fruit (g) Total vegetables (g) Total meat (g) Total fish (g) Total discretionary foods (g) Daily protein intake (g) Daily fat intake (g) Daily carbohydrate intake (g) Daily fiber intake (g) Daily water intake (ml) Dietary Health Index (%) | 136.7 ± 92.54 209.5 ± 93.67 105.8 ± 49.92 20.3 ± 19.69 268.6 ± 242.78 58.3 ± 16.08 48.4 ± 15.82 161.1 ± 50.43 16.7 ± 5.15 2008.1 ± 555.40 77.5 ± 9.76 | 104.2 ± 89.21 187.4 ± 107.21 87.6 ± 53.61 22.6 ± 21.13 239.1 ± 208.66 53.4 ± 16.28 45.8 ± 18.64 152.1 ± 46.32 15.0 ± 4.94 1773.9 ± 585.64 75.4 ± 9.12 | 0.019 0.168 0.009 0.455 0.667 0.055 0.188 0.414 0.051 0.014 0.097 |
| IPAQ/METs category at time of OGTT % Low % Moderate % High | 8.26 (9) 41.28 (45) 50.46 (55) | 8.11 (3) 32.43 (12) 59.46 (22) | 0.620 |
| SF36 Physical functioning Role physical Role emotional Vitality Mental health Social functioning Pain General health | 91.4 ± 11.01 75.9 ± 16.70 86.4 ± 16.81 83.3 ± 12.22 74.6 ± 11.77 63.3 ± 16.99 77.2 ± 15.57 56.7 ± 13.15 | 83.3 ± 20.80 59.2 ± 23.64 55.3 ± 22.67 59.5 ± 15.40 50.5 ± 12.38 36.7 ± 21.12 61.6 ± 19.16 40.1 ± 14.04 | 0.001 <0.001 <0.001 <0.001 <0.001 <0.001 <0.001 <0.001 |
| STAI-6 | 11.2 ± 2.59 | 16.2 ± 3.63 | <0.001 |
| Group with no Symptoms of Depression (<16 on CES-D Questionnaire) N = 57 (69.5%) | Group with Symptoms of Depression (≥16 on CES-D Questionnaire) N = 25 (30.5%) | p-Value | |
|---|---|---|---|
| General characteristics | |||
| Age (years) baseline | 32.1 ± 3.57 | 29.7 ± 4.85 | 0.018 |
| % Non-Caucasian | 14.04 (8) | 12.00 (3) | 1.000 |
| Highest education % Until age of 15 years % High school % Higher education (bachelor/master) | 7.02 (4) 15.79 (9) 77.19 (44) | 12.00 (3) 24.00 (6) 64.00 (16) | 0.431 |
| % Paid professional activity | 91.23 (52) | 84.00 (21) | 0.445 |
| Monthly net income family %Low income < €1500 %€1500–5000 % >€5000 | 0.00 (0) 87.72 (50) 12.28 (7) | 4.00 (1) 92.00 (23) 4.00 (1) | 0.199 |
| % Living without partner | 14.04 (8) | 24.00 (6) | 0.341 |
| % Currently smoking | 0.00 (0) | 12.00 (3) | 0.026 |
| % Multiparity | 49.12 (28) | 48.00 (12) | 1.000 |
| % History of GDM in previous pregnancy | 27.27 (9) | 7.14 (1) | 0.242 |
| % History of PCOS | 9.09 (5) | 0.00(0) | 0.316 |
| % History of miscarriage | 26.32 (15) | 24.00 (6) | 1.000 |
| Pre-pregnancy BMI (kg/m2) | 26.0 ± 5.19 | 27.4 ± 5.31 | 0.194 |
| % First degree family history of T2DM | 27.27 (15) | 21.74 (5) | 0.778 |
| % Insulin use in pregnancy | 26.32 (15) | 32.00 (8) | 0.604 |
| Gestational weight gain | |||
| Total Weight gain (first visit till delivery) (Kg) | 8.11 ± 4.561 | 9.74 ± 5.730 | 0.241 |
| % Excessive weight gain | 17.31 (9) | 41.67 (10) | 0.044 |
| % Inadequate weight gain | 53.85 (28) | 37.50 (9) | 0.222 |
| Baseline (6–16 weeks postpartum) | |||
| % Breastfeeding | 73.68 (42) | 68.00 (17) | 0.604 |
| Timing OGTT (months) | 2.8 ± 0.63 | 2.9 ± 0.87 | 0.910 |
| FPG (mmol/L) 30 min glucose OGTT (mmol/L) 1-h glucose OGTT (mmol/L) 2-h glucose OGTT (mmol/L) | 5.2 ±0.56 9.1 ±1.25 9.7 ±2.04 8.4 ±1.34 | 5.3 ±0.62 8.9 ±1.46 8.9 ±2.14 7.8 ±1.85 | 0.506 0.658 0.201 0.238 |
| % Metabolic syndrome | 21.05 (12) | 32.00 (8) | 0.402 |
| BMI (kg/m2) | 26.1 ± 5.26 | 28.1 ± 5.54 | 0.110 |
| % Overweight (BMI 25.0–29.9) % Obese (BMI ≥ 30) | 22.81 (13) 24.56 (14) | 56.00 (14) 20.00 (5) | 0.011 |
| Mean systolic blood pressure (mmHg) | 116.5 ± 11.16 | 118.4 ± 12.80 | 0.414 |
| Mean diastolic blood pressure (mmHg) | 74.5 ± 7.02 | 76.2 ± 9.31 | 0.455 |
| % Hypertension (systolic BP ≥ 140 and/or diastolic BP ≥ 90 mmHg) | 3.51 (2) | 12.00 (3) | 0.163 |
| % Waist circumference >80 cm | 73.68 (42) | 84.00 (21) | 0.400 |
| % Waist circumference > 88 cm | 40.35 (23) | 64.00 (16) | 0.058 |
| % PPWR > 0 kg % PPWR > 5 kg | 50.88 (29) 7.02 (4) | 64.00 (16) 28.00 (7) | 0.338 0.029 |
| HbA1c (%) | 5.4 ± 0.25 | 5.3 ± 0.27 | 0.183 |
| HbA1c (mmol/mol) | 35.8 ± 2.68 | 34.8 ± 2.90 | 0.183 |
| Fasting total cholesterol (mmol/L) Fasting HDL-cholesterol (mmol/L) Fasting LDL-cholesterol (mmol/L) Triglycerides (mmol/L) | 4.7 ± 0.75 1.6 ± 0.32 2.7 ± 0.65 1.1 ± 0.61 | 4.9 ± 1.14 1.6 ± 0.67 3.2 ± 0.82 1.2 ± 0.85 | 0.160 0.553 0.026 0.553 |
| Matsuda insulin sensitivity | 3.9 ± 2.14 | 4.1 ± 2.77 | 0.769 |
| HOMA-IR | 2.3 ± 1.62 | 2.7 ± 2.03 | 0.526 |
| HOMA-B | 118.0 ± 59.46 | 124.9 ± 60.94 | 0.613 |
| ISSI-2 | 1.5 ± 0.45 | 1.6 ± 0.49 | 0.761 |
| Insulinogenic index/HOMA-IR | 0.3 ± 0.20 | 0.2 ± 0.14 | 0.526 |
| FFQ Total fruit (g) Total vegetables (g) Total meat (g) Total fish (g) Total discretionary foods (g) Daily protein intake (g) Daily fat intake (g) Daily carbohydrate intake (g) Daily fiber intake (g) Daily water intake (ml) Dietary Health Index (%) | 138.0 ± 100.19 218.9 ± 99.50 120.3 ± 60.01 26.7 ± 41.51 335.5 ± 309.23 66.4 ± 22.48 52.9 ± 19.45 186.3 ± 53.51 18.5 ± 5.78 2144.0 ± 510.70 76.2 ± 9.82 | 113.4 ± 89.06 178.9 ± 107.46 119.9 ± 55.68 24.4 ± 23.87 321.2 ± 255.40 61.3 ± 17.80 54.6 ±16.99 175.1 ± 53.81 16.9 ± 5.85 1996.5 ± 508.38 73.8 ± 9.89 | 0.308 0.096 0.774 0.828 0.968 0.559 0.319 0.344 0.370 0.251 0.311 |
| IPAQ/METs category at time of OGTT % Low % Moderate % High | 10.71 (6) 42.86 (24) 46.43 (26) | 16.00 (4) 44.00 (11) 40.00 (10) | 0.710 |
| SF36 Physical functioning Role physical Role emotional Vitality Mental health Social functioning Pain General health | 91.8 ± 7.71 84.9 ± 19.62 88.0 ± 17.96 64.6 ± 14.91 79.0 ± 14.87 91.0 ± 11.75 86.2 ± 19.33 76.8 ± 14.31 | 84.6 ± 20.00 71.3 ± 29.15 69.3 ± 22.91 53.3 ± 18.05 67.0 ± 14.43 74.0 ± 27.24 73.4 ± 23.24 63.3 ± 19.60 | 0.247 0.040 <0.001 0.010 <0.001 0.003 0.002 0.010 |
| STAI-6 | 11.2 ± 2.91 | 14.3 ± 3.02 | <0.001 |
| One year post-randomization | |||
| % Breastfeeding | 70.18 (40) | 64.00 (16) | 0.613 |
| Timing OGTT (months) | 14.8 ± 0.71 | 14.9 ± 0.98 | 0.935 |
| FPG (mmol/L) 30 min glucose OGTT (mmol/L) 1-h glucose OGTT (mmol/L) 2-h glucose OGTT (mmol/L) | 5.2 ± 0.46 8.7 ± 1.34 9.1 ± 1.93 7.1 ± 1.75 | 5.3 ± 0.59 8.7 ± 1.86 8.9 ± 3.12 7.4 ± 2.55 | 0.579 0.786 0.487 0.932 |
| % IFG % IGT % IFG + IGT | 22.58 (7) 58.06 (18) 19.35 (6) | 25.00 (3) 33.33 (4) 41.67 (5) | 0.273 |
| % T2DM | 0.00 (0) | 12.00 (3) | 0.026 |
| % Metabolic syndrome | 19.30 (11) | 44.00 (11) | 0.030 |
| BMI (kg/m2) | 25.3 ± 5.43 | 27.0 ± 5.65 | 0.194 |
| % Overweight (BMI 25.0–29.9) % Obese (BMI ≥ 30) | 17.54 (10) 24.56 (14) | 56.00 (14) 12.00 (3) | 0.003 |
| Mean systolic blood pressure (mmHg) | 117.7 ± 12.52 | 120.4 ± 12.93 | 0.487 |
| Mean diastolic blood pressure (mmHg) | 77.7 ± 11.11 | 76.9 ± 11.69 | 0.661 |
| % Hypertension (systolic BP ≥ 140 and/or diastolic BP ≥ 90 mmHg) | 15.79 (9) | 16.00 (4) | 1.000 |
| % Waist circumference >80 cm | 49.12 (28) | 80.00 (20) | 0.014 |
| % Waist circumference > 88 cm | 35.09 (20) | 44.00 (11) | 0.468 |
| % PPWR > 0 kg % PPWR > 5 kg | 38.60 (22) 3.51 (2) | 44.00 (11) 16.00 (4) | 0.807 0.067 |
| HbA1c (%) | 5.3 ± 0. 26 | 5.33 ± 0.27 | 0.982 |
| HbA1c (mmol/mol) | 34.5 ± 2.80 | 34.8 ± 2.92 | 0.982 |
| Fasting total cholesterol (mmol/L) Fasting HDL-cholesterol (mmol/L) Fasting LDL-cholesterol (mmol/L) Triglycerides (mmol/L) | 4.8 ± 1.07 1.6 ± 0.36 2.8 ± 0.84 1.1 ± 0.46 | 5.1 ± 1.68 1.4 ± 0.44 2.8 ± 0.71 1.3 ± 0.67 | 0.827 0.123 0.945 0.186 |
| Matsuda insulin sensitivity | 4.0 ± 2.03 | 3.9 ± 1.97 | 0.978 |
| HOMA-IR | 2.3 ± 1.47 | 3.1 ± 2.51 | 0.838 |
| HOMA-B | 119.1 ± 51.99 | 126.3 ± 67.02 | 0.875 |
| ISSI-2 | 1.7 ± 0.64 | 1.6 ± 0.65 | 0.658 |
| Insulinogenic index/HOMA-IR | 0.2 ± 0.14 | 0.2 ± 0.13 | 0.527 |
| FFQ Total fruit (g) Total vegetables (g) Total meat (g) Total fish (g) Total discretionary foods (g) Daily protein intake (g) Daily fat intake (g) Daily carbohydrate intake (g) Daily fiber intake (g) Daily water intake (ml) Dietary Health Index (%) | 136.3 ± 90.29 208.2 ± 88.18 108.0 ± 45.49 21.2 ± 19.27 217.6 ± 200.50 58.1 ± 14.09 46.6 ± 15.57 152.0 ± 41.16 16.4 ± 4.79 1966.7 ± 506.31 79.0 ± 7.80 | 102.4 ± 106.03 193.9 ± 104.35 78.6 ± 42.12 27.1 ± 25.72 251.0 ± 207.61 51.1 ± 16.22 45.0 ± 14.81 152.7 ± 4.48 15.2 ± 5.12 1849.7 ± 566.24 76.8 ± 8.06 | 0.031 0.489 0.003 0.444 0.426 0.043 0.680 0.717 0.253 0.349 0.290 |
| IPAQ/METs category at time of OGTT % Low % Moderate % High | 1.89 (1) 43.40 (23) 54.72 (29) | 5.00 (1) 35.00 (7) 60.00 (12) | 0.539 |
| SF36 Physical functioning Role physical Role emotional Vitality Mental health Social functioning Pain General health | 92.6 ± 7.33 78.5 ± 15.22 87.6 ± 15.60 83.3 ± 12.29 76.1 ± 11.25 64.7 ± 15.87 79.0 ± 15.26 58.4 ± 11.69 | 85.4 ± 16.33 56.5 ± 23.49 54.0 ± 21.93 61.0 ± 14.35 50.0 ± 12.20 36.5 ± 19.74 64.0 ± 17.32 38.2 ± 16.32 | 0.003 <0.001 <0.001 <0.001 <0.001 <0.001 <0.001 <0.001 |
| STAI-6 | 10.8 ± 2.47 | 16.1 ± 3.40 | <0.001 |
| Group with no Symptoms of Depression (<16 on CES-D Questionnaire) N = 65 (77.4%) | Group with Symptoms of Depression (≥16 on CES-D Questionnaire) N = 19 (22.6%) | p-Value | |
|---|---|---|---|
| General characteristics | |||
| Age (years) baseline | 32.9 ± 3.94 | 32.5 ± 4.68 | 0.715 |
| % Non-Caucasian | 18.46 (12) | 26.32 (5) | 0.520 |
| Highest education % None/primary school % Until age of 15 years % High school % Higher education (bachelor/master) | 0.00 (0) 4.62 (3) 12.31 (8) 83.08 (56) | 5.26 (1) 10.53 (2) 26.32 (5) 57.89 (11) | 0.047 |
| % Paid professional activity | 86.15 (56) | 73.68 (14) | 0.291 |
| Monthly net income family %Low income < €1500 %€1500–5000 % >€5000 | 4.62 (3) 86.15 (56) 9.23 (6) | 11.11 (2) 83.33 (15) 5.56 (1) | 0.534 |
| % Living without partner | 16.92 (11) | 21.05 (4) | 0.736 |
| % Currently smoking | 6.15 (4) | 10.53 (2) | 0.614 |
| % Multiparity | 58.46 (38) | 47.37 (9) | 0.439 |
| % History of GDM in previous pregnancy | 17.02 (8) | 41.67 (5) | 0.113 |
| % History of PCOS | 3.23 (2) | 5.88 (1) | 0.522 |
| % History of miscarriage | 44.62 (29) | 36.84 (7) | 0.607 |
| Pre-pregnancy BMI (kg/m2) | 27.2 ± 4.67 | 28.8 ± 8.19 | 0.673 |
| % First degree family history of T2DM | 29.51 (18) | 47.37 (9) | 0.173 |
| % Insulin use in pregnancy | 35.38 (23) | 42.11 (8) | 0.600 |
| Gestational weight gain | |||
| Total Weight gain (first visit till delivery) (Kg) | 8.5 ± 6.31 | 5.9 ± 6.38 | 0.103 |
| % Excessive weight gain | 16.95 (10) | 11.11 (2) | 0.721 |
| % Inadequate weight gain | 47.46 (28) | 72.22 (13) | 0.104 |
| Baseline (6–16 weeks postpartum) | |||
| % Breastfeeding | 22.58 (14) | 47.37 (9) | 0.046 |
| Timing OGTT (months) | 3.0 ± 0.57 | 2.7 ± 0.61 | 0.067 |
| FPG (mmol/L) 30 min glucose OGTT (mmol/L) 1-h glucose OGTT (mmol/L) 2-h glucose OGTT (mmol/L) | 5.3 ± 0.57 8.9 ± 1.37 9.4 ± 1.92 8.1 ± 1.32 | 5.4 ± 0.63 9.2 ± 1.39 9.9 ± 1.70 8.1 ± 1.36 | 0.222 0.374 0.136 0.769 |
| % Metabolic syndrome | 22.58 (14) | 47.37 (9) | 0.046 |
| BMI (kg/m2) | 27.6 ± 4.65 | 29.0 ± 8.34 | 0.612 |
| % Overweight (BMI 25.0–29.9) % Obese (BMI ≥ 30) | 36.92 (24) 32.31 (21) | 5.26 (1) 47.37 (9) | 0.019 |
| Mean systolic blood pressure (mmHg) | 118.5 ± 12.43 | 121.5 ± 11.78 | 0.146 |
| Mean diastolic blood pressure (mmHg) | 74.5 ± 10.69 | 77.4 ± 9.13 | 0.284 |
| % Hypertension (systolic BP ≥ 140 and/or diastolic BP ≥ 90 mmHg) | 7.69 (5) | 21.05 (4) | 0.199 |
| % Waist circumference >80 cm | 87.10 (54) | 73.68 (14) | 0.172 |
| % Waist circumference > 88 cm | 61.29 (38) | 68.42 (13) | 0.787 |
| % PPWR > 0 kg % PPWR > 5 kg | 56.92 (37) 13.85 (9) | 47.37 (9) 15.79 (3) | 0.601 1.000 |
| HbA1c (%) | 5.4 ± 0.36 | 5.5 ± 0.47 | 0.610 |
| HbA1c (mmol/mol) | 35.3 ± 3.93 | 36.1 ± 5.14 | 0.610 |
| Fasting total cholesterol (mmol/L) Fasting HDL-cholesterol (mmol/L) Fasting LDL-cholesterol (mmol/L) Triglycerides (mmol/L) | 4.8 ± 0.86 1.5 ± 0.38 2.9 ± 0.84 1.2 ± 0.74 | 5.1 ± 0.97 1.4 ± 0.36 3.2 ± 0.84 1.2 ± 0.54 | 0.178 0.268 0.135 0.333 |
| Matsuda insulin sensitivity | 3.8 ± 2.12 | 3.5 ± 2.34 | 0.354 |
| HOMA-IR | 2.5 ± 1.35 | 3.3 ± 2.86 | 0.413 |
| HOMA-B | 123.5 ± 72.72 | 130.3 ± 75.13 | 0.858 |
| ISSI-2 | 1.4 ± 0.32 | 1.3 ± 0.36 | 0.613 |
| Insulinogenic index/HOMA-IR | 0.2 ± 0.10 | 0.2 ± 0.14 | 0.775 |
| FFQ Total fruit (g) Total vegetables (g) Total meat (g) Total fish (g) Total discretionary foods (g) Daily protein intake (g) Daily fat intake (g) Daily carbohydrate intake (g) Daily fiber intake (g) Daily water intake (ml) Dietary Health Index (%) | 130.3 ± 108.03 191.9 ± 100.64 113.5 ± 59.25 22.2 ± 19.54 319.4 ± 274.63 65.8 ± 21.27 56.8 ± 17.52 191.0 ± 61.01 18.3 ± 6.57 2122.32 ± 552.78 75.6 ± 12.07 | 129.8 ± 99.17 191.4 ± 97.7 111.5 ± 58.02 16.1 ± 9.11 210.6 ± 188.93 63.4 ± 18.40 53.4 ± 19.21 172.6 ± 50.51 17.2 ± 5.36 2005.2 ± 533.72 78.0 ± 10.80 | 1.000 0.995 0.921 0.391 0.198 0.996 0.276 0.345 0.584 0.307 0.436 |
| IPAQ/METs category at time of OGTT % Low % Moderate % High | 13.11 (8) 44.26 (27) 42.62 (26) | 18.75 (3) 31.25 (5) 50.00 (8) | 0.596 |
| SF36 Physical functioning Role physical Role emotional Vitality Mental health Social functioning Pain General health | 86.1 ± 20.23 77.3 ± 26.11 87.3 ± 17.19 61.4 ± 16.03 78.9 ± 10.19 89.2 ± 17.38 80.2 ± 21.99 72.5 ± 16.45 | 83.5 ± 21.42 67.3 ± 35.53 62.8 ± 32.96 50.4 ± 22.15 63.5 ± 18.52 67.7 ± 29.34 75.4 ± 26.28 57.7 ± 20.43 | 0.196 0.380 0.004 0.067 0.001 0.003 0.579 0.004 |
| STAI-6 | 11.2 ± 2.31 | 14.9 ± 4.34 | <0.001 |
| One year post-randomization | |||
| % Breastfeeding | 81.54 (53) | 73.68 (14) | 0.520 |
| Timing OGTT (months) | 15.1 ± 0.84 | 15.1 ± 0.82 | 0.834 |
| FPG (mmol/L) 30 min glucose OGTT (mmol/L) 1-h glucose OGTT (mmol/L) 2-h glucose OGTT (mmol/L) | 5.4 ± 0.77 8.6 ± 1.61 8.6 ± 2.50 7.3 ± 2.26 | 5.7 ± 0.69 9.4 ± 1.55 9.6 ± 1.53 7.8 ± 1.37 | 0.033 0.063 0.021 0.088 |
| % IFG % IGT % IFG + IGT | 29.41 (10) 47.06 (16) 23.53 (8) | 28.57 (4) 28.57 (4) 42.86 (6) | 0.411 |
| % T2DM | 6.15 (4) | 5.26 (1) | 1.000 |
| % Metabolic syndrome | 25.00 (16) | 47.37 (9) | 0.087 |
| BMI (kg/m2) | 27.1 ± 4.98 | 28.8 ± 8.96 | 0.688 |
| % Overweight (BMI 25.0–29.9) % Obese (BMI ≥ 30) | 35.38 (23) 27.69 (18) | 0.00 (0) 47.37 (9) | 0.003 |
| Mean systolic blood pressure (mmHg) | 117.0 ± 12.86 | 123.9 ± 11.83 | 0.054 |
| Mean diastolic blood pressure (mmHg) | 75.2 ± 9.23 | 80.5 ± 8.63 | 0.031 |
| % Hypertension (systolic BP ≥ 140 and/or diastolic BP ≥ 90 mmHg) | 9.23 (6) | 26.32 (5) | 0.114 |
| % Waist circumference >80 cm | 75.00 (48) | 63.16 (12) | 0.383 |
| % Waist circumference > 88 cm | 50.00 (32) | 52.63 (10) | 1.000 |
| % PPWR > 0 kg % PPWR > 5 kg | 52.31 (34) 16.92 (11) | 36.84 (7) 5.26 (1) | 0.300 0.282 |
| HbA1c (%) | 5.3 ± 0.44 | 5.4 ± 0.38 | 0.181 |
| HbA1c (mmol/mol) | 34.8 ± 4.83 | 35.9 ± 4.13 | 0.181 |
| Fasting total cholesterol (mmol/L) Fasting HDL-cholesterol (mmol/L) Fasting LDL-cholesterol (mmol/L) Triglycerides (mmol/L) | 4.9 ± 0.87 1.5 ± 0.43 2.9 ± 0.75 1.2 ± 0.87 | 5.3 ± 1.38 1.4 ± 0.35 3.2 ± 1.20 1.6 ± 0.71 | 0.317 0.231 0.351 0.005 |
| Matsuda insulin sensitivity | 4.0 ± 2.36 | 3.0 ± 2.04 | 0.069 |
| HOMA-IR | 2.7 ± 1.99 | 4.5 ± 4.94 | 0.196 |
| HOMA-B | 118.6 ± 53.40 | 138.8 ± 85.79 | 0.484 |
| ISSI-2 | 1.7 ± 0.72 | 1.4 ± 0.47 | 0.089 |
| Insulinogenic index/HOMA-IR | 0.2 ± 0.23 | 0.2 ± 0.08 | 0.253 |
| FFQ Total fruit (g) Total vegetables (g) Total meat (g) Total fish (g) Total discretionary foods (g) Daily protein intake (g) Daily fat intake (g) Daily carbohydrate intake (g) Daily fiber intake (g) Daily water intake (ml) Dietary Health Index (%) | 137.0 ± 95.16 210.6 ± 98.91 103.9 ± 53.78 19.5 ± 20.16 313.4 ± 268.15 58.4 ± 17.76 50.0 ± 15.98 169.2 ± 56.44 16.9 ± 5.48 2044.4 ± 596.70 76.2 ± 10.97 | 106.6 ± 63.33 178.9 ± 113.16 99.4 ± 65.13 16.7 ± 10.92 223.5 ± 214.68 56.5 ± 16.27 46.8 ± 23.14 151.3 ± 49.85 14.9 ± 4.83 1674.1 ± 611.01 73.6 ± 10.30 | 0.269 0.195 0.477 0.831 0.231 0.521 0.155 0.231 0.105 0.025 0.199 |
| IPAQ/METs category at time of OGTT % Low % Moderate % High | 14.29 (8) 39.29 (22) 46.43 (26) | 11.76 (2) 29.41 (5) 58.82 (10) | 0.800 |
| SF36 Physical functioning Role physical Role emotional Vitality Mental Health Social functioning Pain General health | 90.4 ± 13.41 73.8 ± 17.72 85.4 ± 17.87 83.3 ± 12.26 73.4 ± 12.16 62.1 ± 17.95 75.7 ± 15.80 55.2 ± 14.22 | 80.5 ± 25.76 62.8 ± 23.98 57.0 ± 24.10 57.6 ± 16.87 50.8 ± 12.94 36.8 ± 23.38 58.4 ± 21.39 42.6 ± 10.19 | 0.083 0.027 <0.001 <0.001 <0.001 <0.001 0.002 <0.001 |
| STAI-6 | 11.6 ± 2.66 | 16.4 ± 4.00 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanlaer, Y.; Minschart, C.; Van den Keybus, K.; Myngheer, N.; Maes, T.; De Block, C.; Bochanen, N.; Van Pottelbergh, I.; Abrams, P.; Vinck, W.; et al. Mental Health and Metabolic Outcomes in Early Postpartum in Women with Prediabetes After Gestational Diabetes: A Secondary Analysis of the MELINDA Trial. J. Clin. Med. 2025, 14, 3592. https://doi.org/10.3390/jcm14103592
Vanlaer Y, Minschart C, Van den Keybus K, Myngheer N, Maes T, De Block C, Bochanen N, Van Pottelbergh I, Abrams P, Vinck W, et al. Mental Health and Metabolic Outcomes in Early Postpartum in Women with Prediabetes After Gestational Diabetes: A Secondary Analysis of the MELINDA Trial. Journal of Clinical Medicine. 2025; 14(10):3592. https://doi.org/10.3390/jcm14103592
Chicago/Turabian StyleVanlaer, Yana, Caro Minschart, Karolijn Van den Keybus, Nele Myngheer, Toon Maes, Christophe De Block, Niels Bochanen, Inge Van Pottelbergh, Pascale Abrams, Wouter Vinck, and et al. 2025. "Mental Health and Metabolic Outcomes in Early Postpartum in Women with Prediabetes After Gestational Diabetes: A Secondary Analysis of the MELINDA Trial" Journal of Clinical Medicine 14, no. 10: 3592. https://doi.org/10.3390/jcm14103592
APA StyleVanlaer, Y., Minschart, C., Van den Keybus, K., Myngheer, N., Maes, T., De Block, C., Bochanen, N., Van Pottelbergh, I., Abrams, P., Vinck, W., Leuridan, L., Driessens, S., Billen, J., Matthys, C., Bogaerts, A., Laenen, A., Mathieu, C., & Benhalima, K. (2025). Mental Health and Metabolic Outcomes in Early Postpartum in Women with Prediabetes After Gestational Diabetes: A Secondary Analysis of the MELINDA Trial. Journal of Clinical Medicine, 14(10), 3592. https://doi.org/10.3390/jcm14103592









