Complications Associated with Immunosuppressive Agents in Solid Organ Transplant Recipients: A Nationwide Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Study Population
2.2. Ethics Approval and Consent to Participate
2.3. Outcome Definitions
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Incidence Rate of Immunosuppressant-Related Complications by Organ Types
3.3. Onset Timing of Immunosuppressant-Related Complications
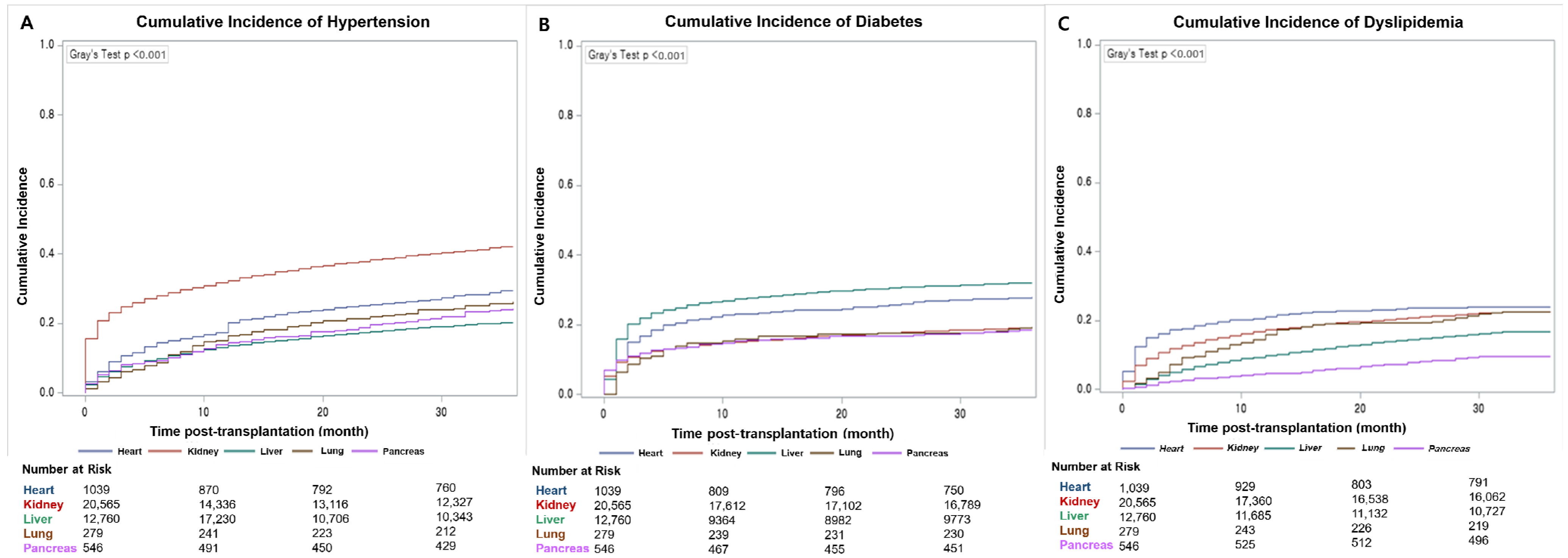
3.4. Cumulative Incidence of Noncommunicable Chronic Complications
3.5. Incidence of Complications by Maintenance Regimen
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Cai, J.; Terasaki, P.I. Induction Immunosuppression Improves Long-Term Graft and Patient Outcome in Organ Transplantation: An Analysis of United Network for Organ Sharing Registry Data. Transplantation 2010, 90, 1511–1515. [Google Scholar] [CrossRef]
- Sayegh, M.H.; Carpenter, C.B. Transplantation 50 Years Later—Progress, Challenges, and Promises. N. Engl. J. Med. 2004, 351, 2761–2766. [Google Scholar] [CrossRef]
- Korea Network for Organ Sharing (KONOS). 2020 Statistical Annual Report on Organ Transplantation and Human Tissue Donation. Seoul: KONOS. June 2020. Available online: https://www.konos.go.kr/board/boardListPage.do?page=sub4_2_1&boardId=30 (accessed on 15 December 2022).
- Taylor, A.L.; Watson, C.J.E.; Bradley, J.A. Immunosuppressive agents in solid organ transplantation: Mechanisms of action and therapeutic efficacy. Crit. Rev. Oncol. Hematol. 2005, 56, 23–46. [Google Scholar] [CrossRef]
- Halloran, P.F. Immunosuppressive Drugs for Kidney Transplantation. N. Engl. J. Med. 2004, 351, 2715–2729. [Google Scholar] [CrossRef]
- Rana, A.; Gruessner, A.; Agopian, V.G.; Khalpey, Z.; Riaz, I.B.; Kaplan, B.; Halazun, K.J.; Busuttil, R.W.; Gruessner, R.W.G. Survival Benefit of Solid-Organ Transplant in the United States. JAMA Surg. 2015, 150, 252–259. [Google Scholar] [CrossRef]
- Wojciechowski, D.; Wiseman, A. Long-Term Immunosuppression Management: Opportunities and Uncertainties. Clin. J. Am. Soc. Nephrol. 2021, 16, 1264–1271. [Google Scholar] [CrossRef]
- Raffaele, G. Complications of Post-Transplant Immunosuppression. In Tissue Engineering and Regenerative Medicine; Jose, A.A., Ed.; IntechOpen: London, UK, 2013; Chapter 33. [Google Scholar]
- Katabathina, V.; Menias, C.O.; Pickhardt, P.; Lubner, M.; Prasad, S.R. Complications of Immunosuppressive Therapy in Solid Organ Transplantation. Radiol. Clin. N. Am. 2016, 54, 303–319. [Google Scholar] [CrossRef]
- Moreno, R.; Berenguer, M. Post-liver transplantation medical complications. Ann. Hepatol. 2006, 5, 77–85. [Google Scholar] [CrossRef]
- Fishman, J.A. Infection in Solid-Organ Transplant Recipients. N. Engl. J. Med. 2007, 357, 2601–2614. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Callisen, H.; Libricz, S.; Patel, B. Complications of Solid Organ Transplantation: Cardiovascular, Neurologic, Renal, and Gastrointestinal. Crit. Care Clin. 2019, 35, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Guenette, A.; Husain, S. Infectious Complications Following Solid Organ Transplantation. Crit. Care Clin. 2019, 35, 151–168. [Google Scholar] [CrossRef]
- Neuwirt, H.; Rudnicki, M.; Schratzberger, P.; Pirklbauer, M.; Kronbichler, A.; Mayer, G. Immunosuppression after renal transplantation. Memo—Mag. Eur. Med. Oncol. 2019, 12, 216–221. [Google Scholar] [CrossRef]
- Moini, M.; Schilsky, M.L.; Tichy, E.M. Review on immunosuppression in liver transplantation. World J. Hepatol. 2015, 7, 1355–1368. [Google Scholar] [CrossRef]
- Dharnidharka, V.R.; Schnitzler, M.A.; Chen, J.; Brennan, D.C.; Axelrod, D.; Segev, D.L.; Schechtman, K.B.; Zheng, J.; Lentine, K.L. Differential risks for adverse outcomes 3 years after kidney transplantation based on initial immunosuppression regimen: A national study. Transpl. Int. 2016, 29, 1226–1236. [Google Scholar] [CrossRef]
- van Delden, C.; Stampf, S.; Hirsch, H.H.; Manuel, O.; Meylan, P.; Cusini, A.; Hirzel, C.; Khanna, N.; Weisser, M.; Garzoni, C.; et al. Burden and Timeline of Infectious Diseases in the First Year After Solid Organ Transplantation in the Swiss Transplant Cohort Study. Clin. Infect. Dis. 2020, 71, e159–e169. [Google Scholar] [CrossRef]
- Kyoung, D.-S.; Kim, H.-S. Understanding and Utilizing Claim Data from the Korean National Health Insurance Service (NHIS) and Health Insurance Review & Assessment (HIRA) Database for Research. J. Lipid. Atheroscler. 2022, 11, 103–110. [Google Scholar] [CrossRef]
- Lim, S.-S.; Lee, W.; Kim, Y.-K.; Kim, J.; Park, J.H.; Park, B.R.; Yoon, J.-H. The cumulative incidence and trends of rare diseases in South Korea: A nationwide study of the administrative data from the National Health Insurance Service database from 2011–2015. Orphanet. J. Rare Dis. 2019, 14, 49. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report 2023; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Aguado, J.M.; Torre-Cisneros, J.; Fortún, J.; Benito, N.; Meije, Y.; Doblas, A.; Muñoz, P. Tuberculosis in solid-organ transplant recipients: Consensus statement of the Group for the Study of Infection in Transplant Recipients (GESITRA). Clin. Infect. Dis. 2009, 48, 1276–1284. [Google Scholar] [CrossRef]
- Hyun, J.; Lee, M.; Jung, I.; Kim, E.; Hahn, S.M.; Kim, Y.R.; Lim, S.; Ihn, K.; Kim, M.Y.; Ahn, J.G.; et al. Changes in tuberculosis risk after transplantation in the setting of decreased community tuberculosis incidence: A national population-based study, 2008–2020. Ann. Clin. Microbiol. Antimicrob. 2024, 23, 1. [Google Scholar] [CrossRef]
- Miller, L.W. Cardiovascular Toxicities of Immunosuppressive Agents. Am. J. Transplant. 2002, 2, 807–818. [Google Scholar] [CrossRef]
- Schwenger, V.; Zeier, M.; Ritz, E. Hypertension after renal transplantation. Ann. Transplant. 2001, 6, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, T.; Hartmann, A. Post-transplant diabetes mellitus in patients with solid organ transplants. Nat. Rev. Endocrinol. 2019, 15, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.H.; Biddle, K.; Augustine, T.; Azmi, S. Post-Transplantation Diabetes Mellitus. Diabetes Ther. 2020, 11, 779–801. [Google Scholar] [CrossRef]
- Lv, C.; Zhang, Y.; Chen, X.; Huang, X.; Xue, M.; Sun, Q.; Wang, T.; Liang, J.; He, S.; Gao, J.; et al. New-onset diabetes after liver transplantation and its impact on complications and patient survival. J. Diabetes 2015, 7, 881–890. [Google Scholar] [CrossRef]
- Agarwal, A.; Prasad, G.V. Post-transplant dyslipidemia: Mechanisms, diagnosis and management. World J. Transplant. 2016, 6, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Eisen, H.J.; Kobashigawa, J.; Starling, R.C.; Pauly, D.F.; Kfoury, A.; Ross, H.; Wang, S.-S.; Cantin, B.; Van Bakel, A.; Ewald, G.; et al. Everolimus versus mycophenolate mofetil in heart transplantation: A randomized, multicenter trial. Am. J. Transplant. 2013, 13, 1203–1216. [Google Scholar] [CrossRef]
- Kasiske, B.L.; de Mattos, A.; Flechner, S.M.; Gallon, L.; Meier-Kriesche, H.-U.; Weir, M.R.; Wilkinson, A. Mammalian target of rapamycin inhibitor dyslipidemia in kidney transplant recipients. Am. J. Transplant. 2008, 8, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Jose, M. The CARI guidelines. Calcineurin inhibitors in renal transplantation: Adverse effects. Nephrology 2007, 12 (Suppl. S1), S66–S74. [Google Scholar] [CrossRef]
- Lee, H.S.; Kang, M.; Kim, B.; Park, Y. Outcomes of kidney transplantation over a 16-year period in Korea: An analysis of the National Health Information Database. PLoS ONE 2021, 16, e0247449. [Google Scholar] [CrossRef]
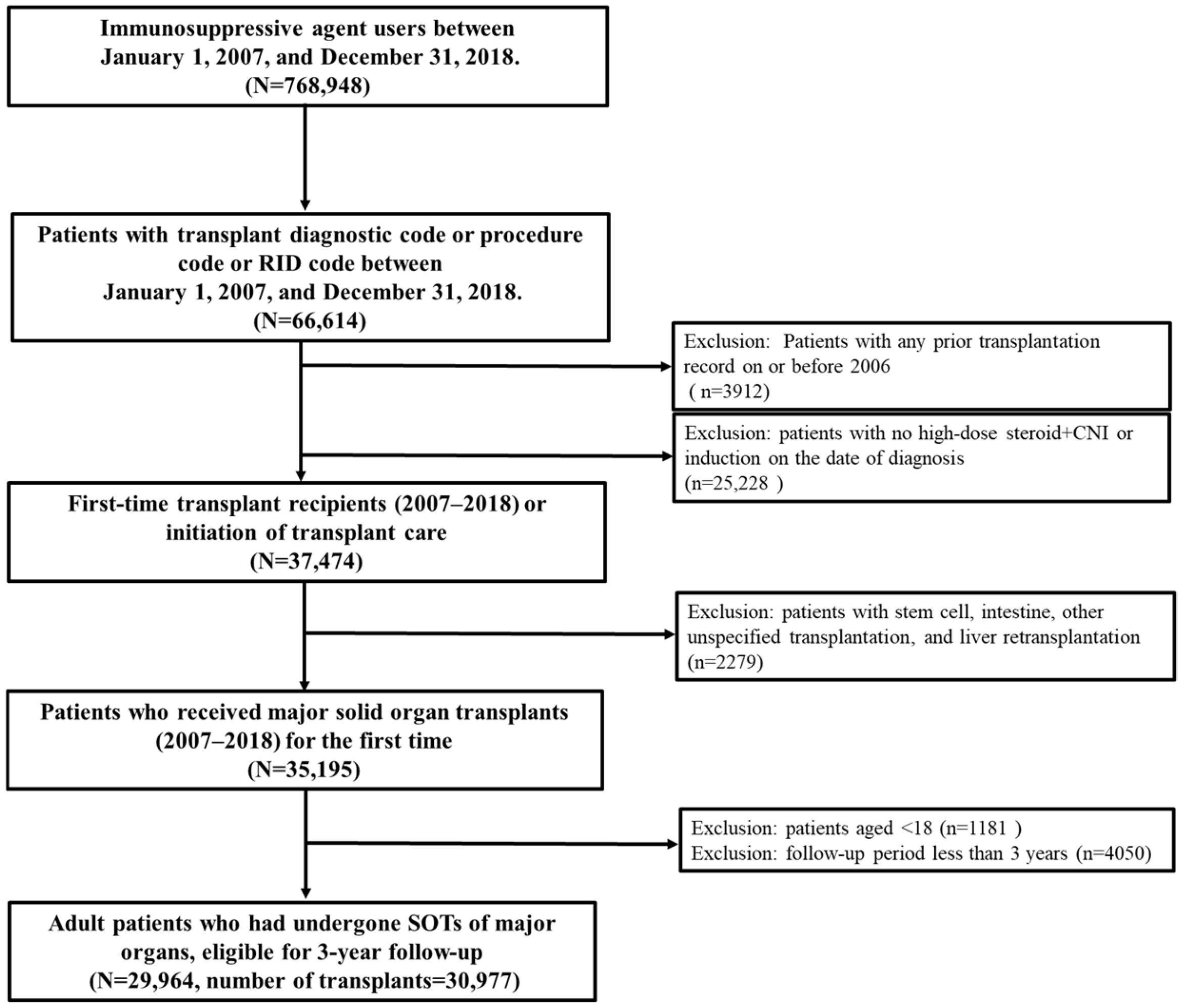

| Variables | Transplant Recipients a (N = 30,977) | With Any Complications (N = 23,915) | Without Complication (N = 7062) | p-Value |
|---|---|---|---|---|
| Age (mean ± SD) | 50.03 ± 11.08 | 50.48 ± 11.0 | 49.54 ± 11.15 | <0.001 |
| 18–34 | 3777 (12.2) | 2716 (11.0) | 1061 (15.1) | <0.001 |
| 35–49 | 10,630 (34.3) | 8002 (33.3) | 2.628 (36.7) | |
| 50–64 | 14,740 (47.6) | 11,655 (49.2) | 3085 (43.6) | |
| ≥65 | 1830 (5.9) | 1542 (6.5) | 288 (4.6) | |
| Male, n (%) | 19,681 (63.5) | 14,154 (64.5) | 5527 (61.2) | <0.001 |
| Comorbid disease | <0.001 | |||
| Hypertension | 23,004 (22.4) | 17,244 (23.1) | 5760 (20.5) | <0.001 |
| Dyslipidemia | 20,332 (19.8) | 14,937 (20.0) | 5395 (19.2) | <0.001 |
| Liver disease | 19,077 (18.5) | 13,043 (17.4) | 6034 (21.5) | <0.001 |
| Diabetes mellitus | 18,608 (18.1) | 13,252 (17.7) | 5356 (19.0) | <0.001 |
| Heart Failure and cardiomyopathy | 18,618 (18.1) | 13,828 (18.5) | 4790 (17.0) | <0.001 |
| Coronary artery disease | 3251 (3.2) | 2460 (3.3) | 791 (2.8) | <0.001 |
| CCI score (mean ± SD) | 6.11 ± 2.48 | 6.56 ± 2.34 | 6.30 ± 2.31 | <0.001 |
| 0–2 | 752 (2.4) | 466 (2.1) | 286 (3.2) | <0.001 |
| 3–4 | 5216 (16.8) | 3300 (15.0) | 1916 (21.2) | |
| 5–6 | 10,165 (32.8) | 7111 (32.4) | 3054 (33.8) | |
| >6 | 14,844 (47.9) | 11,072 (50.4) | 3772 (41.8) | |
| Organ | <0.001 | |||
| Kidney | 18,401 (59.4) | 13,663 (62.2) | 4738 (52.5) | <0.001 |
| Liver | 10,870 (35.1) | 7053 (32.1) | 3817 (42.3) | <0.001 |
| Heart | 957 (3.1) | 709 (3.2) | 248 (2.7) | 0.0255 |
| Pancreas | 481 (1.6) | 314 (1.4) | 167 (1.8) | 0.0067 |
| Lung | 259 (0.8) | 203 (0.9) | 56 (0.6) | 0.0075 |
| Retransplantation b | 98 (0.3) | 74 (0.3) | 24 (0.3) | 0.2918 |
| Multi-organ transplantation c | 2370 (7.7) | 2010 (8.4) | 360 (5.1) | <0.001 |
| Kidney-liver | 1063 (44.9) | 904 (45.0) | 159 (44.2) | <0.001 |
| Kidney-pancreas | 809 (34.1) | 685 (34.1) | 124 (34.4) | <0.001 |
| Liver-pancreas | 490 (20.7) | 413 (20.5) | 77 (21.4) | <0.001 |
| Heart-lung | 8 (0.3) | 8 (0.4) | 0 (0.0) | 0.1299 |
| Induction regimen | 27,792 (89.7) | 22,132 (92.5) | 6563 (92.9) | <0.001 |
| Basiliximab | 24,683 (88.8) | 19,020 (85.9) | 5962 (90.8) | |
| ATG rabbit | 3109 (11.2) | 3112 (14.1) | 601 (9.2) | |
| Maintenance regimen other than steroids | <0.001 | |||
| Tac + MPA | 17,952 (58.0) | 13,695 (57.3) | 4257 (60.3) | |
| Tacrolimus | 4846 (15.6) | 3559 (14.9) | 1287 (18.2) | |
| CsA + MPA | 2149 (6.9) | 1800 (7.5) | 349 (4.9) | |
| Cyclosporine | 804 (2.6) | 639 (2.7) | 165 (2.3) | |
| Mycophenolate | 397 (1.3) | 301(1.3) | 96(1.4) | |
| Others | 4829 (15.6) | 3825 (16.4) | 908 (12.8) | |
| Duration of steroid therapy | <0.001 | |||
| ≤30 days | 7319 (23.6) | 5009 (20.9) | 2310 (32.7) | |
| 30–90 days | 9431 (30.4) | 7378 (30.9) | 2053 (29.1) | |
| >90 days | 14,227 (46.1) | 11,528 (48.2) | 2699 (38.2) | |
| Complications | Incidence Rate Per 1000 Patient Years (95% CI) | |||||
|---|---|---|---|---|---|---|
| All | Kidney | Liver | Heart | Lung | Pancreas | |
| Overall | 504.1 (499.7–508.6) | 539.0 (533.0–545.1) | 504.9 (497.5–512.4) | 623.0 (592.2–649.5) | 823.9 (766.2–895.8) | 225.9 (211.1–241.4) |
| Serious infection | 43.7 (42.5–45.1) | 49.7 (47.8–51.5) | 34.2 (32.3–36.2) | 57.8 (49.5–67.2) | 91.7 (71.6–115.7) | 22.1 (17.6–27.3) |
| Pulmonary infection | 11.0 (1.4–11.7) | 11.0 (10.2–11.9) | 9.5 (8.5–10.6) | 22.4 (17.4–28.6) | 59.4 (43.5–79.2) | 5.2 (3.2–8.0) |
| GI infection | 98.1 (11.9–13.3) | 5.5 (4.9–6.1) | 14.0 (12.8–15.3) | 13.6 (9.7–18.5) | 15.5 (8.0–27.1) | 2.1 (0.9–4.1) |
| Urinary tract infection | 12.6 (11.9–13.3) | 18.8 (17.7–20.2) | 3.8 (3.2–4.5) | 6.5 (3.9–10.1) | 6.5 (2.1–15.1) | 6.0 (3.8–9.0) |
| Skin infection | 5.3 (4.9–5.8) | 6.1 (5.5–6.8) | 3.7 (3.1–4.4) | 9.9 (6.6–14.2) | 5.2 (1.4–13.2) | 4.4 (2.6–7.1) |
| ENT infection | 3.7 (3.4–4.1) | 4.2 (3.7–4.7) | 3.3 (2.7–3.9) | 3.7 (1.9–6.7) | 5.2 (1.4–13.2) | 0.8 (0.2–2.3) |
| Musculoskeletal infection | 1.9 (1.7–2.2) | 2.3 (1.9–2.7) | 1.3 (0.9–1.7) | 2.0 (0.7–4.4) | 0.0 | 3.4 (1.8–5.8) |
| Other infection | 12.1 (11.4–12.8) | 13.5 (12.6–14.5) | 10.0 (9.0–11.1) | 17.3 (12.9–22.8) | 23.2 (13.8–36.7) | 4.7 (2.8–7.4) |
| Opportunistic infection | 113.3 (11.2–115.4) | 105.8 (103.1–108.5) | 126.0 (122.3–129.8) | 152.0 (138.3–166.8) | 278.9 (243–318.7) | 47.8 (41.1–55.2) |
| Tuberculosis | 29.9 (28.8–30.9) | 21.7 (20.5–22.9) | 44.8 (42.6–47.0) | 24.5 (19.2–30.8) | 52.9 (38.0–71.8) | 15.3 (11.7–19.8) |
| Herpes | 73.3 (72.1–75.5) | 72.1 (69.9–74.3) | 76.6 (73.8–79.6) | 106.1 (94.7–118.6) | 189.8 (160.4–223.1) | 27.5 (22.5–33.3) |
| Other opportunistic infection | 9.6 (9.0–10.3) | 12.0 (11.2–13.0) | 4.6 (3.9–5.4) | 21.4 (16.5–27.4) | 36.2 (24.0–52.3) | 4.9 (3.0–7.7) |
| Acute kidney injury | 6.2 (5.7–6.7) | 8.2 (7.4–8.9) | 3.6 (3.0–4.3) | 2.7 (1.2–5.4) | 7.7 (2.8–16.9) | 2.3 (1.1–4.4) |
| Hypertensive emergency | 20.0 (19.1–20.9) | 22.0 (20.8–23.2) | 25.0 (23.4–26.7) | 21.4 (16.5–27.4) | 47.8 (33.6–65.9) | 29.6 (24.4–35.6) |
| Chronic kidney disease | 20.1 (18.6–21.7) | - | 14.7 (13.4–16.0) | 16.0 (11.7–21.3) | 37.5 (25.1–53.8) | 6.5 (4.2–9.6) |
| Hypertension | 118.0 (115.9–120.2) | 151.9 (148.7–155.2) | 73.9 (71.1–76.8) | 104.1 (92.7–116.4) | 94.3 (73.9–118.5) | 34.3 (28.7–40.6) |
| Diabetes mellitus | 82.8 (81.0–84.6) | 68.9 (66.8–71.1) | 116.8 (113.2–120.4) | 99.0 (87.9–111.0) | 69.7 (52.4–91.0) | 26.2 (21.4–31.9) |
| Dyslipidemia | 105.7 (103.1–108.4) | 81.4 (79.0–83.7) | 61.1 (58.5–63.7) | 84.7 (74.5–95.9) | 81.4 (62.5–104.1) | 13.5 (10.1–17.7) |
| Osteoporosis | 6.1 (5.5–6.7) | 9.6 (8.8–10.5) | 10.7 (9.6–11.8) | 9.5 (6.3–13.8) | 60.7 (44.6–80.7) | 3.1 (1.6–5.4) |
| Complications | Complication Episodes (N) | Incidence Rate Per 1000 Patient Year (95% CI) | |||
|---|---|---|---|---|---|
| 0–1 Month | 2–6 Month | 7 Month–1 Year | 2–3 Year | ||
| Overall | 41,332 | 662.8 (631.4–695.0) | 606.9 (593.4–620.6) | 394.1 (384.3–404.0) | 263.2 (259.7–267.9) |
| Serious infection | 4368 | 76.8 (66.5–88.4) | 124.5 (118.5–130.8) | 82.4 (77.9–87.0) | 58.1 (56.2–60.0) |
| GI infection | 863 | 21.2 (15.9–27.6) | 18.0 (15.7–20.5) | 9.2 (7.7–10.8) | 7.3 (6.6–8.0) |
| ENT infection | 372 | 0.8 (0.1–2.8) | 3.7 (2.7–4.9) | 4.7 (3.7–5.9) | 4.1 (3.6–4.6) |
| Pulmonary infection | 1099 | 4.7 (2.4–8.2) | 18.3 (16.0–20.8) | 17.4 (15.4–19.6) | 14.0 (13.1–15.0) |
| Urinary tract infection | 1258 | 10.6 (7.0–15.4) | 28.0 (25.2–31.1) | 20.5 (18.3–22.9) | 17.0 (15.9–18.0) |
| Musculoskeletal infection | 193 | 0.0 | 1.4 (0.8–2.2) | 2.0 (1.4–2.9) | 1.7 (1.4–2.1) |
| Skin infection | 529 | 5.5 (3.0–9.2) | 10.0 (8.3–11.9) | 6.1 (4.9–7.4) | 4.2 (3.7–4.8) |
| Other infection | 1209 | 23.9 (18.3–30.7) | 32.3 (29.2–35.5) | 13.4 (11.6–15.3) | 5.9 (5.3–6.6) |
| Opportunistic infection | 11,313 | 74.5 (64.3–85.9) | 178.8 (171.5–186.3) | 182.8 (176.2–189.6) | 113.9 (111.2–116.6) |
| Tuberculosis | 2981 | 18.8 (13.9–25.0) | 48.2 (44.5–52.2) | 74.8 (70.6–79.3) | 31.6 (30.2–33.0) |
| Herpes | 7371 | 27.8 (21.7–35.1) | 85.5 (80.5–90.7) | 96.0 (91.2–100.9) | 78.1 (75.9–80.4) |
| Other opportunistic infection | 961 | 27.8 (21.7–35.1) | 45.0 (41.5–48.9) | 12.0 (10.3–13.8) | 4.2 (3.7–4.7) |
| Acute kidney injury | 618 | 24.3 (18.6–31.2) | 16.2 (14.0–18.5) | 7.3 (6.1–8.8) | 4.0 (3.5–4.5) |
| Hypertensive emergency | 1998 | 261.9 (242.4–282.5) | 44.4 (40.8–48.3) | 20.4 (18.2–22.7) | 16.0 (15.0–17.1) |
| Chronic kidney disease | 621 | 52.8 (28.9–88.6) | 124.1 (105.8–144.6) | 64.5 (52.7–78.1) | 53.4 (47.9–59.4) |
| Hypertension | 11,788 | 142.5 (137.7–147.4) | 27.3 (26.3–28.2) | 11.5 (10.9–12.1) | 6.7 (6.5–6.9) |
| Diabetes mellitus | 2334 | 101.7 (96.6–106.9) | 54.2 (52.5–55.9) | 10.6 (10.0–11.3) | 5.1 (4.9–5.4) |
| Dyslipidemia | 7159 | 43.6 (40.3–47.1) | 38.1 (36.8–39.6) | 14.8 (14.0–15.6) | 6.7 (6.4–7.0) |
| Osteoporosis | 1013 | 2.6 (1.9–3.5) | 1.6 (1.3–1.9) | 1.1 (0.9–1.3) | 0.9 (0.8–1.0) |
| Organ | Regimen | Exposure | Total | Renal a | Infection b | Osteoporosis | Diabetes | Hypertension | Dyslipidemia |
|---|---|---|---|---|---|---|---|---|---|
| (Patient-Years) | Per 1000 Patient Years | ||||||||
| Kidney | Tac + MPA | 47,041 | 411.0 | - | 127.9 | 51.1 | 52.2 | 105.1 | 55.8 |
| CsA + MPA | 7634 | 443.2 | - | 147.2 | 50.6 | 50.4 | 104 | 66.1 | |
| Tac | 4879 | 484.3 | - | 146.5 | 51.9 | 71.3 | 121.7 | 65.4 | |
| Liver | Tac + MPA | 20,875 | 406.4 | 16.0 | 134.1 | 54.7 | 94.3 | 61.4 | 45.9 |
| Tac | 8143 | 398.3 | 17.4 | 138.2 | 55.9 | 92.2 | 58 | 36.6 | |
| CsA + MPA | 7634 | 467.4 | 19.1 | 147.2 | 59.9 | 93.7 | 87.6 | 59.9 | |
| Heart | Tac + MPA | 2290 | 468.5 | 12.2 | 196.9 | 49.8 | 83.4 | 78.2 | 48.0 |
| CsA + MPA | 295 | 508.1 | 20.3 | 220.2 | 44.0 | 57.6 | 108.4 | 57.6 | |
| Tac + MPA + mTORi | 181 | 698.6 | 16.6 | 237.4 | 60.7 | 110.4 | 160.1 | 110.4 | |
| Pancreas | Tac + MPA | 1392 | 757.1 | 236 | 137.9 | 57.5 | 34.4 | 58.9 | 23.0 |
| Tac | 80 | 275.0 | 50.0 | 62.5 | 62.5 | 0.0 | 87.5 | 12.5 | |
| Lung | Tac + MPA | 356 | 1293.6 | 42.2 | 236.2 | 115.3 | 73.1 | 64.7 | 61.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, A.Y.; Jeong, J.; Heo, K.-N.; Park, S.; Ah, Y.-M.; Han, J.M.; Lee, J.-Y.; Min, S.I. Complications Associated with Immunosuppressive Agents in Solid Organ Transplant Recipients: A Nationwide Analysis. J. Clin. Med. 2025, 14, 3602. https://doi.org/10.3390/jcm14103602
Lee AY, Jeong J, Heo K-N, Park S, Ah Y-M, Han JM, Lee J-Y, Min SI. Complications Associated with Immunosuppressive Agents in Solid Organ Transplant Recipients: A Nationwide Analysis. Journal of Clinical Medicine. 2025; 14(10):3602. https://doi.org/10.3390/jcm14103602
Chicago/Turabian StyleLee, Ah Young, Jonghyun Jeong, Kyu-Nam Heo, Soyoung Park, Young-Mi Ah, Ji Min Han, Ju-Yeun Lee, and Sang Il Min. 2025. "Complications Associated with Immunosuppressive Agents in Solid Organ Transplant Recipients: A Nationwide Analysis" Journal of Clinical Medicine 14, no. 10: 3602. https://doi.org/10.3390/jcm14103602
APA StyleLee, A. Y., Jeong, J., Heo, K.-N., Park, S., Ah, Y.-M., Han, J. M., Lee, J.-Y., & Min, S. I. (2025). Complications Associated with Immunosuppressive Agents in Solid Organ Transplant Recipients: A Nationwide Analysis. Journal of Clinical Medicine, 14(10), 3602. https://doi.org/10.3390/jcm14103602






