Comparison of Chest High-Resolution Computed Tomography Findings in Patients with Anti-Melanoma Differentiation-Associated Gene 5 Antibody-Positive and Antibody-Negative Progressive Pulmonary Fibrosis with Polymyositis/Dermatomyositis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Measurement of Laboratory Parameters
2.3. Assessment of Respiratory Symptoms, Arterial Blood Gas Analysis, and Pulmonary Function Testing (PFT)
2.4. Interpretation of Chest HRCT Findings
2.5. Definition of PPF
2.6. Treatments
2.7. Statistical Analysis
3. Results
3.1. Comparison of the Baseline Clinical Characteristics of PPF with Anti-MDA5 Antibody-Positive and Antibody-Negative Cases
3.2. Timing of New Diagnosis with PPF After Treatment Initiation in Patients with PM/DM-ILD
3.3. Comparison of Chest HRCT Findings Regarding PPF Between Anti-MDA5 Antibody Positive and Negative Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PM | polymyositis |
| DM | dermatomyositis |
| IIMs | idiopathic inflammatory myopathies |
| ILD | interstitial lung disease |
| ARS | Aminoacyl-tRNA synthetase |
| MDA5 | melanoma differentiation-associated gene 5 |
| TIF1-γ | transcriptional intermediary factor 1-γ; |
| GCs | glucocorticoids |
| IVCY | intravenous cyclophosphamide |
| PF-ILD | progressive fibrosing ILD |
| HRCT | high-resolution computed tomography |
| PPF | progressive pulmonary fibrosis |
| A/S | acute/subacute |
| DLCO | diffusion capacity of the lung for carbon monoxide |
| AaDO2 | alveolar-arterial oxygen gradient |
| FVC | forced vital capacity |
| GGO | ground-glass opacity |
| CSA | cyclosporin-A |
| TAC | Tacrolimus |
| MPDN | methylprednisolone |
| PE | plasma exchange |
References
- Bohan, A.; Peter, J.B. Polymyositis and dermatomyositis (first of two parts). N. Engl. J. Med. 1975, 292, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Bohan, A.; Peter, J.B. Polymyositis and dermatomyositis (second of two parts). N. Engl. J. Med. 1975, 292, 403–407. [Google Scholar] [CrossRef]
- Johnson, C.; Pinal-Fernandez, I.; Parikh, R.; Paik, J.; Albayda, J.; Mammen, A.L.; Christopher-Stine, L.; Danoff, S. Assessment of mortality in autoimmune myositis with and without associated interstitial lung disease. Lung 2016, 194, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, G.; Gao, D.; Liu, G.; Pan, L.; Ni, L.; Li, Z.; Wang, Q. Factors associated with interstitial lung disease in patients with polymyositis and dermatomyositis: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0155381. [Google Scholar] [CrossRef] [PubMed]
- Gono, T.; Kuwana, M. Inflammatory myopathies: Choosing the right biomarkers to predict ILD in myositis. Nat. Rev. Rheumatol. 2016, 12, 504–506. [Google Scholar] [CrossRef]
- Yoshifuji, H.; Fujii, T.; Kobayashi, S.; Imura, Y.; Fujita, Y.; Kawabata, D.; Usui, T.; Tanaka, M.; Nagai, S.; Umehara, H.; et al. Anti-aminoacyl-tRNA synthetase antibodies in clinical course prediction of interstitial lung disease complicated with idiopathic inflammatory myopathies. Autoimmunity 2006, 39, 233–241. [Google Scholar] [CrossRef]
- Isoda, K.; Kotani, T.; Takeuchi, T.; Kiboshi, T.; Hata, K.; Ishida, T.; Otani, K.; Kamimori, T.; Fujiwara, H.; Shoda, T.; et al. Comparison of long-term prognosis and relapse of dermatomyositis complicated with interstitial pneumonia according to autoantibodies: Anti-aminoacyl tRNA synthetase antibodies versus anti-melanoma differentiation-associated gene 5 antibody. Rheumatol. Int. 2017, 37, 1335–1340. [Google Scholar] [CrossRef]
- Sato, S.; Masui, K.; Nishina, N.; Kawaguchi, Y.; Kawakami, A.; Tamura, M.; Ikeda, K.; Nunokawa, T.; Tanino, Y.; Asakawa, K.; et al. Initial predictors of poor survival in myositis-associated interstitial lung disease: A multicentre cohort of 497 patients. Rheumatology 2018, 57, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.R.; Brown, K.K.; Wells, A.U.; Clerisme-Beaty, E.; Collard, H.R.; Cottin, V.; Devaraj, A.; Inoue, Y.; Le Maulf, F.; Richeldi, L.; et al. Design of the PF-ILD trial: A double-blind, randomised, placebo-controlled phase III trial of nintedanib in patients with progressive fibrosing interstitial lung disease. BMJ Open Respir. Res. 2017, 4, e000212. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.R.; Wells, A.U.; Cottin, V.; Devaraj, A.; Walsh, S.L.F.; Inoue, Y.; Richeldi, L.; Kolb, M.; Tetzlaff, K.; Stowasser, S.; et al. Nintedanib in progressive fibrosing interstitial lung diseases. N. Engl. J. Med. 2019, 381, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: An official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef] [PubMed]
- Planas-Cerezales, L.; Fabbri, L.; Pearmain, L. Add-on therapy for pulmonary fibrosis, a forthcoming era with implications for practice: The BI 101550 and RELIEF trials. Breathe 2023, 19, 230090. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, E.; Cocconcelli, E.; Castelli, G.; Giraudo, C.; Fraia, A.S.; De Zorzi, E.; Gatto, M.; Ienna, L.; Treppo, E.; Malandrino, D.; et al. Interstitial lung disease with and without progressive fibrosing phenotype in patients with idiopathic inflammatory myopathies: Data from a large multicentric cohort. RMD Open 2023, 9, e003121. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Y.; Sun, D.; Yu, S.; Du, X.; Ye, Q. Progressive pulmonary fibrosis in myositis-specific antibody-positive interstitial pneumonia: A retrospective cohort study. Front. Med. 2024, 10, 1325082. [Google Scholar] [CrossRef]
- Sontheimer, R.D. Dermatomyositis: An overview of recent progress with emphasis on dermatologic aspects. Dermatol. Clin. 2002, 20, 387–408. [Google Scholar] [CrossRef]
- Gerami, P.; Schope, J.M.; McDonald, L.; Walling, H.W.; Sontheimer, R.D. A systematic review of adult-onset clinically amyopathic dermatomyositis (dermatomyositis siné myositis): A missing link within the spectrum of the idiopathic inflammatory myopathies. J. Am. Acad. Dermatol. 2006, 54, 597–613. [Google Scholar] [CrossRef]
- Hervier, B.; Devilliers, H.; Stanciu, R.; Meyer, A.; Uzunhan, Y.; Masseau, A.; Dubucquoi, S.; Hatron, P.Y.; Musset, L.; Wallaert, B.; et al. Hierarchical cluster and survival analyses of antisynthetase syndrome: Phenotype and outcome are correlated with anti-tRNA synthetase antibody specificity. Autoimmun. Rev. 2012, 12, 210–217. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am. J. Respir. Crit. Care Med. 2002, 165, 277–304. [Google Scholar] [CrossRef]
- Mahler, D.A.; Wells, C.K. Evaluation of clinical methods for rating dyspnea. Chest 1988, 93, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Isoda, K.; Takeuchi, T.; Kotani, T.; Hata, K.; Shoda, T.; Ishida, T.; Yoshida, S.; Kimura, Y.; Makino, S.; Hanafusa, T. Pre-treatment ferritin level and alveolar-arterial oxygen gradient can predict mortality rate due to acute/subacute interstitial pneumonia in dermatomyositis treated by cyclosporine a/glucocorticosteroid combination therapy: A case control study. PLoS ONE 2014, 9, e89610. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Hoshino, K.; Satoh, T.; Fujita, T.; Kawakami, Y.; Fujita, T.; Kuwana, M. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: Association with rapidly progressive interstitial lung disease. Arthritis Rheum. 2009, 60, 2193–2200. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jiang, W.; Jin, Q.; Peng, Q.; Zhang, L.; Lin, S.; Lu, X.; Liu, M.; Wang, Y.; Song, A.; et al. Clinical, radiological and pathological features of anti-MDA5 antibody-associated interstitial lung disease. RMD Open 2023, 9, e003150. [Google Scholar] [CrossRef]
- Fujisawa, T.; Hozumi, H.; Kono, M.; Enomoto, N.; Hashimoto, D.; Nakamura, Y.; Inui, N.; Yokomura, K.; Koshimizu, N.; Toyoshima, M.; et al. Prognostic factors for myositis-associated interstitial lung disease. PLoS ONE 2014, 9, e98824. [Google Scholar] [CrossRef] [PubMed]
- Kotani, T.; Takeuchi, T.; Yoshimatsu, Y.; Ishida, T.; Yamamoto, N.; Fujiki, Y.; Oda, K.; Isoda, K.; Hata, K.; Kamimori, T.; et al. Initial limited three-level thin-section computed tomography scorings predict the prognosis of acute/subacute interstitial pneumonia in patients with dermatomyositis. Mod. Rheumatol. 2016, 26, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Kunihiro, Y.; Kubo, M.; Kawano, R.; Oishi, K.; Ueda, K.; Gondo, T. HRCT findings of collagen vascular disease-related interstitial pneumonia (CVD-IP): A comparative study among individual underlying diseases. Clin. Radiol. 2018, 73, 833.e1–833.e10. [Google Scholar] [CrossRef]
- Tanizawa, K.; Handa, T.; Nakashima, R.; Kubo, T.; Hosono, Y.; Watanabe, K.; Aihara, K.; Oga, T.; Chin, K.; Nagai, S.; et al. HRCT features of interstitial lung disease in dermatomyositis with anti-CADM-140 antibody. Respir. Med. 2011, 105, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Egashira, R. High-resolution CT findings of myositis-related interstitial lung disease. Medicina 2021, 57, 692. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, W.; Ren, F.; Luo, L.; Huang, D.; Tang, L. Evaluation of prognostic factors in anti-MDA5 antibody-positive patients in Chongqing, China: A retrospective study. Int. J. Gen. Med. 2021, 14, 4775–4781. [Google Scholar] [CrossRef]
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E., Jr.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Waseda, Y.; Johkoh, T.; Egashira, R.; Sumikawa, H.; Saeki, K.; Watanabe, S.; Matsunuma, R.; Takato, H.; Ichikawa, Y.; Hamaguchi, Y.; et al. Antisynthetase syndrome: Pulmonary computed tomography findings of adult patients with antibodies to aminoacyl-tRNA synthetases. Eur. J. Radiol. 2016, 85, 1421–1426. [Google Scholar] [CrossRef]

| Characteristics | Anti-MDA5 Negative (n = 62) | Anti-MDA5 Positive (n = 17) | p-Value |
|---|---|---|---|
| Age, years | 60.7 (51.4–68.5) | 58.4 (53.5–66.8) | 0.61 |
| Females, n (%) | 45 (73) | 12 (71) | 1 |
| CADM/DM/PM/ASSD, n (%) | 15 (24.2)/14 (22.6)/2 (3.2)/31 (50.0) | 9 (52.9)/8 (47.1)/0 (0)/0 (0) | |
| A/S-ILD, n (%) | 32 (51.6) | 17 (100) | 0.0001 |
| Anti-MDA5-Ab, index | 2350 (150–3800) l | ||
| Anti-ARS-Ab (+), DN, n (%) | 50 (80.7), 12 (19.4) | ||
| Jo-1/EJ/PL7/PL12/others | 15/17/10/2/2 | ||
| Anti-Mi-2-Ab (+), n (%) | 2 (3.2) | ||
| Anti-TIF1-γ-Ab (+), n (%) | 1 (1.6) | ||
| Disease duration, weeks | 7.9 (3.9–23.9) | 0.16 (0–1.4) | <0.0001 |
| Follow-up duration, years | 3.3 (0.9–4.6) | 1.0 (0.1–4.1) | 0.04 |
| Modified MRC scale | 1 (0–2) | 1 (1–2) | 0.24 |
| Laboratory findings | |||
| CK, U/L | 107 (69.8–435.8) | 268 (111.5–622.5) | 0.24 |
| ALD, U/L | 6.3 (4.1–16.3) | 8.6 (6.9–10.4) | 0.28 |
| CRP, mg/mL | 0.3 (0.06–0.83) | 0.6 (0.18–2.08) | 0.14 |
| KL-6, U/mL | 1002.5 (569–1432.8) | 789 (374–1180.5) | 0.2 |
| Sp-D, ng/mL | 167 (106–277) a | 43.3 (27.5–70.4) b | <0.0001 |
| Ferritin, ng/mL | 176.5 (78.8–350.8) c | 1054 (536.5–1817.5) d | <0.0001 |
| AaDO2 | 19.0 (9.0–26.5) e | 35.1 (22.7–68.9) d | 0.001 |
| PFT findings | |||
| Predicted FVC, % | 76.8 (64.8–88) f | 76 (67.1–90.6) g | 0.8 |
| Predicted DLCO, % | 46.0 (33.5–58.5) h | 40.8 (36.8–48.4) i | 0.57 |
| Treatment | |||
| GCs, mg/day | 47.5 (33.8–55) | 55 (50–65) | 0.01 |
| CSA, mg/day | 250 (200–275) j | 287.5 (243.8–306.3) b | 0.89 |
| TAC, mg/day | 6 (3–8) f | 1 (0–6) k | 0.03 |
| MPDN pulse, n (%) | 8 (13.1) | 11 (64.7) | <0.0001 |
| IVCY, n (%) | 30 (48.4) | 17 (100) | <0.0001 |
| IVIG, n (%) | 3 (5.3) | 8 (47.1) | 0.0002 |
| PE, n (%) | 4 (6.6) | 12 (70.6) | <0.0001 |
| MMF, n (%) | 5 (8.1) | 5 (29.4) | 0.03 |
| RTX, n (%) | 1 (1.6) | 4 (23.5) | 0.01 |
| TOF, n (%) | 1 (1.6) | 9 (52.9) | <0.0001 |
| Nintedanib, n (%) | 6 (9.7) | 1 (5.9) | 1 |
| Outcomes | |||
| Dead due to ILD, n (%) | 4 (6.6) | 4 (25) | 0.053 |
| Characteristics | Non PPF (n = 65) | PPF (n = 14) | p-Value |
|---|---|---|---|
| Age, years | 60.3 (52.2–68.3) | 61.9 (51.1–67.8) | 0.96 |
| Females, n (%) | 46 (71) | 11 (79) | 0.75 |
| CADM/DM/PM/ASSD, n (%) | 17 (26.2)/20 (30.8)/1 (1.5)/27 (41.5) | 7 (50.0)/2 (14.3)/1 (7.1)/4 (28.6) | |
| A/S-ILD, n (%) | 38 (58.5) | 11 (78.6) | 0.23 |
| Anti-MDA5-Ab (+), Anti-ARS-Ab (+), DN, n (%) | 12 (18.5), 44 (67.7), 9 (13.9) | 5 (35.7), 6 (42.9), 3 (21.4) | |
| Anti-MDA5-Ab, index | 1450 (150–3250) m | 3675 (862.5-5287.5) n | 0.21 |
| Anti-Mi-2-Ab (+), n (%) | 2 (3.2) | 0 (0) | 1 |
| Anti-TIF1-γ-Ab (+), n (%) | 1 (1.6) | 0 (0) | 1 |
| Disease duration, weeks | 7.0 (1.6–17.0) | 3.8 (0.5–21.5) | 0.77 |
| Follow-up duration, years | 2.9 (0.9–4.6) | 1.2 (0.1–3.5) | 0.06 |
| Modified MRC scale | 1 (0–2) | 1.5 (1–3) | 0.19 |
| Laboratory findings | |||
| CK, U/L | 123 (70.5–435.5) | 268 (89.8–717.5) | 0.12 |
| ALD, U/L | 7.1 (4.6–14.7) | 7.6 (4.7–13.0) | 0.63 |
| CRP, mg/mL | 0.25 (0.09–1.70) | 0.6 (0.27–1.54) | 0.18 |
| KL-6, U/mL | 967 (512.5–1272.5) | 1162 (734–1866.6) | 0.15 |
| Sp-D, ng/mL | 147.5 (68.9–267) a | 116.5 (44.4–293) b | 0.61 |
| Ferritin, ng/mL | 204.7 (96.3–609.8) c | 382 (134.6–1340) d | 0.14 |
| AaDO2 | 19.8 (10.1–29.3) e | 31.4 (19.4–79.1) f | 0.03 |
| PFT findings | |||
| Predicted FVC, % | 78.3 (66.1–89.2) g | 63.1 (59.8–69.5) b | 0.01 |
| Predicted DLCO, % | 47.4 (36.3–60.9) h | 35.7 (25.4–47.3) i | 0.03 |
| Treatment | |||
| GCs, mg/day | 50 (35–55) | 57.5 (33.8–66.3) | 0.19 |
| CSA, mg/day | 250 (218.8–300) j | 250 (250–300) k | 0.89 |
| TAC, mg/day | 5.5 (2.8–7.3) l | 6 (3–7) b | 0.81 |
| MPDN pulse, n (%) | 13 (20.3) | 6 (42.9) | 0.09 |
| IVCY, n (%) | 36 (55.4) | 11 (78.6) | 0.14 |
| IVIG, n (%) | 7 (11.3) | 4 (33.3) | 0.07 |
| PE, n (%) | 11 (17.2) | 5 (35.7) | 0.15 |
| MMF, n (%) | 8 (12.3) | 2 (14.3) | 1 |
| RTX, n (%) | 4 (6.2) | 1 (7.1) | 1 |
| TOF, n (%) | 7 (10.9) | 3 (21.4) | 0.37 |
| Nintedanib, n (%) | 5 (7.7) | 2 (14.3) | 0.6 |
| Outcomes | |||
| Dead due to ILD, n (%) | 5 (7.8) | 3 (23.1) | 0.13 |
| Characteristics | Anti-MDA5 Negative (n = 9) | Anti-MDA5 Positive (n = 5) | p-Value |
|---|---|---|---|
| Age, years | 58 (47.3–68.7) | 65.9 (60.9–67.7) | 0.42 |
| Females, n (%) | 8 (89) | 3 (60) | 0.51 |
| CADM/DM/PM/ASSD, n (%) | 3 (33.3)/1 (11.1)/1 (11.1)/4 (44.4) | 4 (80.0)/1 (20.0)/0 (0)/0 (0) | |
| A/S-ILD, n (%) | 6 (66.7) | 5 (100) | 0.26 |
| Anti-MDA5-Ab, index | 3675 (862.5-5287.5) c | ||
| Anti-ARS-Ab positive, DN, n (%) | 7 (70), 3 (30) | ||
| Anti-Mi-2-Ab (+), n (%) | 0 (0) | ||
| Anti-TIF1-γ-Ab (+), n (%) | 0 (0) | ||
| Disease duration, weeks | 19.6 (3.9–29.4) | 0.29 (0–1.4) | 0.003 |
| Follow-up duration, years | 3.3 (1.0–4.6) | 0.1 (0.1–0.7) | 0.011 |
| Modified MRC scale | 1 (0.5–2.5) | 2 (1–3.5) | 0.41 |
| Laboratory findings | |||
| CK, U/L | 300 (107.5–1089) | 236 (74–693.5) | 0.69 |
| ALD, U/L | 9 (3.8–30.4) | 6.8 (5.75–8.85) | 0.59 |
| CRP, mg/mL | 0.5 (0.08–0.96) | 1.49 (0.89–2.56) | 0.07 |
| KL-6, U/mL | 1376 (934.5–1876.1) | 771 (533.5–1700) | 0.23 |
| Sp-D, ng/mL | 246 (174–489.5) a | 48.3 (24.2–71.2) | 0.012 |
| Ferritin, ng/mL | 225.2 (96.275–36.5) b | 1085 (638.5–2152.5) | 0.016 |
| AaDO2 | 21.6 (16.6–31.4) | 85.3 (36.9–103.5) | 0.008 |
| PFT findings | |||
| Predicted FVC, % | 61.9 (59.5–65.3) b | 78.6 (62.3–94.9) c | 0.36 |
| Predicted DLCO, % | 32.4 (23.2–44.9) d | 43.2 (36.7–49.6) c | 0.31 |
| Treatment | |||
| GCs (n = 14), mg/kg | 50 (30–60) | 60 (60–75) | 0.044 |
| CSA (n = 7), mg/day | 250 (100–287.5) e | 300 (250–300) f | 0.34 |
| TAC (n = 10), mg/day | 6 (4–6.3) g | 3.5 (0–10.8) e | 1 |
| MPDN pulse, n (%) | 1 (11.1) | 5 (100) | 0.003 |
| IVCY, n (%) | 6 (66.7) | 5 (100) | 0.26 |
| IVIG, n (%) | 1 (14.3) | 3 (60) | 0.22 |
| PE, n (%) | 1 (11.1) | 4 (80) | 0.023 |
| MMF, n (%) | 1 (11.1) | 1 (20) | 1 |
| RTX, n (%) | 0 (0) | 1 (20) | 0.36 |
| TOF, n (%) | 1 (11.1) | 2 (40) | 0.51 |
| Nintedanib, n (%) | 1 (11.1) | 1 (20) | 1 |
| Outcomes | |||
| Dead due to ILD, n (%) | 1 (11.1) | 2 (40) | 0.51 |
| >6 mo | 6–12 mo | 12–24 mo | 24–36 mo | >60 mo | |
|---|---|---|---|---|---|
| Anti-MDA5-Ab negative (n = 9), n (%) | 2 (22.2) | 1 (11.1) | 1 (11.1) | 3 (33.3) | 2 (22.2) |
| Anti-MDA5-Ab positive (n = 5), n (%) | 5 (100) |
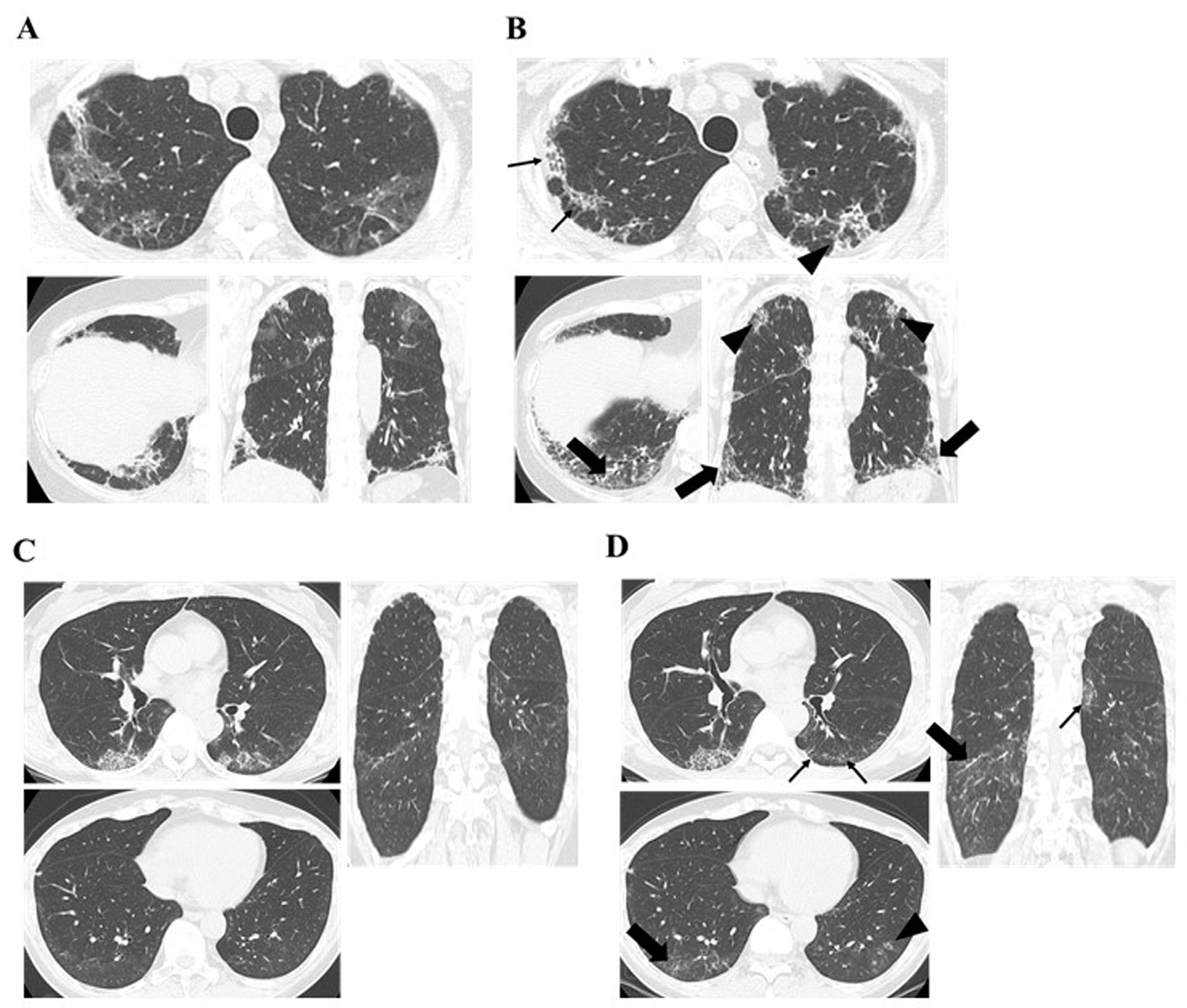
| Chest HRCT Findings of PPF | Anti-MDA5-Ab Negative (n = 9) | Anti-MDA5-Ab Positive (n = 5) | p-Value | |
|---|---|---|---|---|
| Increased extent or severity of traction bronchiectasis and bronchiolectasis | n (%) | 8 (88.9) | 5 (100) | 1 |
| months | 13.9 (7.2–19.5) | 3.3 (0.3–5.2) | 0.016 | |
| New ground-glass opacity with traction bronchiectasis | n (%) | 7 (77.8) | 5 (100) | 0.51 |
| months | 14.6 (6.0–32.3) | 3.3 (0.3–5.2) | 0.023 | |
| New fine reticulation | n (%) | 6 (66.7) | 3 (60) | 1 |
| months | 15.8 (4.9–42.1) | 4.8 (3.3–5.7) | 0.16 | |
| Increased extent or increased coarseness of reticular abnormality | n (%) | 6 (66.7) | 2 (40) | 0.58 |
| months | 14.2 (9.5–33.6) | 5.2 (4.8–5.7) | 0.067 | |
| New or increased honeycombing | n (%) | 3 (33.3) | 0 (0) | 0.26 |
| months | 40 (33.9–71.6) | |||
| Increased lobar volume loss. | n (%) | 1 (11.1) | 2 (40) | 0.51 |
| months | 6 | 3.1 (0.4–5.7) | 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, N.; Kotani, T.; Koyama, M.; Matsuda, S.; Sakamoto, A.; Shou, Y.; Oe, K.; Takeuchi, T.; Osuga, K. Comparison of Chest High-Resolution Computed Tomography Findings in Patients with Anti-Melanoma Differentiation-Associated Gene 5 Antibody-Positive and Antibody-Negative Progressive Pulmonary Fibrosis with Polymyositis/Dermatomyositis. J. Clin. Med. 2025, 14, 1601. https://doi.org/10.3390/jcm14051601
Sato N, Kotani T, Koyama M, Matsuda S, Sakamoto A, Shou Y, Oe K, Takeuchi T, Osuga K. Comparison of Chest High-Resolution Computed Tomography Findings in Patients with Anti-Melanoma Differentiation-Associated Gene 5 Antibody-Positive and Antibody-Negative Progressive Pulmonary Fibrosis with Polymyositis/Dermatomyositis. Journal of Clinical Medicine. 2025; 14(5):1601. https://doi.org/10.3390/jcm14051601
Chicago/Turabian StyleSato, Noboro, Takuya Kotani, Mitsuhiro Koyama, Shogo Matsuda, Aya Sakamoto, Yoshihiro Shou, Katsumasa Oe, Tohru Takeuchi, and Keigo Osuga. 2025. "Comparison of Chest High-Resolution Computed Tomography Findings in Patients with Anti-Melanoma Differentiation-Associated Gene 5 Antibody-Positive and Antibody-Negative Progressive Pulmonary Fibrosis with Polymyositis/Dermatomyositis" Journal of Clinical Medicine 14, no. 5: 1601. https://doi.org/10.3390/jcm14051601
APA StyleSato, N., Kotani, T., Koyama, M., Matsuda, S., Sakamoto, A., Shou, Y., Oe, K., Takeuchi, T., & Osuga, K. (2025). Comparison of Chest High-Resolution Computed Tomography Findings in Patients with Anti-Melanoma Differentiation-Associated Gene 5 Antibody-Positive and Antibody-Negative Progressive Pulmonary Fibrosis with Polymyositis/Dermatomyositis. Journal of Clinical Medicine, 14(5), 1601. https://doi.org/10.3390/jcm14051601






