Nonsteroidal Anti-Inflammatory Drug Injections versus Steroid Injections in the Management of Upper and Lower Extremity Orthopedic Conditions: A Systematic Review with Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Systematic Review Registration
2.2. Search Strategy and Selection Criteria
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
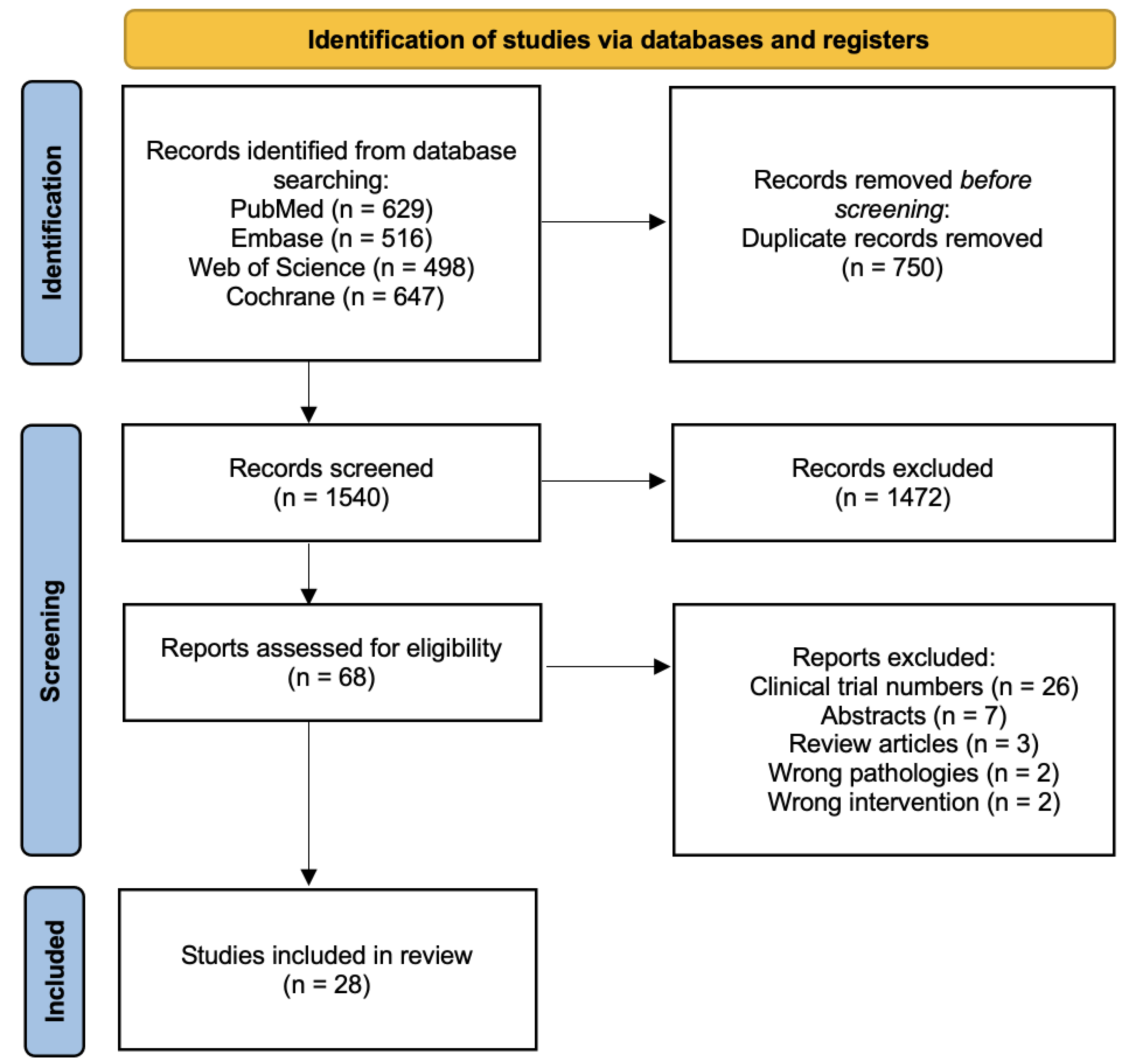
3. Results
3.1. Eligible Studies and Characteristics of Included Studies
| Author | Country | Study Design | Pathology | Population | NSAID | Steroid | Injection Method | Post-injection Protocol | Rehabilitation | Outcome Measures | Follow-up Duration | Adverse Events |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abolhasani 2019 [26] | Iran | RCT | Shoulder impingement syndrome | Ketorolac: 35 patients; 16 M, 19 F Triamcinolone: 35 patients; 17 M, 18 F Betamethasone: 35 patients; 15 M, 20 F Total mean age 37.38 ± 9.45 yrs | Ketorolac: 2 × 1 mL ampoule + 2% lidocaine 4 mL + normal saline 4 mL, total 10 mL | Triamcinolone: 2 × 1 mL ampoule + 2% lidocaine 4 mL + normal saline 4 mL, total 10 mL Betamethasone LA: 2 × 1 mL ampoule + 2% lidocaine 4 mL + normal saline 4 mL, total 10 mL | Posterior approach | NA | NA | VAS; OSS | 2, 4, and 6 wks | NA |
| Ahn 2015 [43] | South Korea | Retrospective comparative study | Adhesive capsulitis | Ketorolac + capsular distension: 64 patients; 11 M, 53 F; mean age 54.63 ± 5.62 yrs; mean duration 7.43 ± 2.32 mo Triamcinolone: 57 patients; 8 M, 49 F; 55.23 ± 4.69 yrs; 7.23 ± 1.94 mo | Ketorolac + capsular distension: 0.5% lidocaine 19 mL + ketorolac 30 mg (1 mL) + capsular distension. | Triamcinolone: 0.5% lidocaine 4 mL + triamcinolone 40 mg (1 mL) | Posterior approach (ultrasound-guided) | Limit shoulder motion for five to ten minutes after injection; second injection offered if less than 50% VAS improvement in two weeks. | Home exercise program | SPADI; VAS; passive ROM | 1, 3, and 6 mo | NSAID: two cases of dizziness and transient muscle weakness Steroid: two cases of steroid-induced synovitis Both groups: no other severe complications |
| Aksakal 2017 [44] | Turkey | RCT | Shoulder impingement syndrome | Lornoxicam: 35 patients; 14 M, 21 F; mean age 53.0 ± 5.5 yrs; mean duration 2–7 wks Betamethasone: 35 patients; 12 M, 23 F; mean age 53.0 ± 5.3 yrs; mean duration 2–8 wks | Lornoxicam: 2 mL (8 mg) | Betamethasone: 1 mL (6.43 mg betamethasone dipropionate, 2.63 mg betamethasone sodium phosphate | Posterior approach (blind) | NA | NA | CMS; UCLA score | 2, 4, and 6 wks | NA |
| Bayat 2018 [45] | Iran | RCT | Knee osteoarthritis (KL grade 2 or 3) | Ketorolac: 19 patients; 1 M, 18 F; mean age 59.9 ± 8.6 yrs; mean duration 3.2 ± 2.6 yrs Triamcinolone: 19 patients; 3 M, 16 F; mean age 61.1 ± 5 yrs; mean duration 4.3 ± 1.9 yrs | Ketorolac: ketorolac 30 mg+ 2% lidocaine 1 mL at baseline | Triamcinolone: triamcinolone 40 mg + 2% lidocaine 2% at baseline | Lateral midpatellar approach in an extended knee using a 22G needle | NA | Exercise therapy—multi-angle isometric exercises along with hamstring stretching three times per day; closed-chain isotonic exercises was started after the first month | WOMAC; Lequesne scale; VAS | 4, and 12 wks | NA |
| Bellamy 2016 [46] | USA | RCT | Knee osteoarthritis (mean KL grade 3) | Ketorolac: 16 patients; 7 M, 9 F; mean age 53 yrs Triamcinolone: 20 patients; 9 M, 11 F; mean age 65 yrs | Ketorolac: 2 mL (toradol 30 mg) in 8 mL of bupivacaine hydrochloride (0.5%) without epinephrine | Triamcinolone acetonide: 2 mL of kenalog 40 mg/mL in 8 mL of bupivacaine hydrochloride (0.5%) without epinephrine | Superolateral approach (ultrasound-guided) | NA | NA | VAS; WOMAC; KS score; Tegner/Lysholm Knee Scoring Scale; Short Form 36; UCLA activity score | 2 wks, 6 wks, 3 mo, and 6 mo | NA |
| Cift 2015 [29] | Turkey | RCT | Shoulder impingement syndrome | Tenoxicam: 20 patients; 10 M, 10 F; mean age 45.3 ± 8.8 yrs Methylprednisolone: 20 patients; 8 M, 12 F; mean age 46.5 ± 11 yrs | Tenoxicam: 20 mg three times by weekly intervals | Methylprednisolone: 40 mg once | Posterior arthroscopy portal (used for the needle entry point; sitting position) | NA | All patients underwent home exercise program including gravity-assisted distraction oscillatory pendulum exercise | VAS; active ROM; DASH | 6 wks and 1 yr | There were no major complications, but patients in the tenoxicam group complained of a moderate burning-like sensation during injection, and two patients had temporary hypotension. |
| Gondal 2021 [30] | Pakistan | RCT | Knee osteoarthritis (KL grade 2 or 3) | Ketorolac: 40 patients; 14 M, 26 F; mean age 55.6 ± 9.2 yrs Triamcinolone: 40 patients; 16 M, 24 F; mean age 57.2 ± 8.30 yrs | Ketorolac: 30 mg + 5 mL lidocaine and 2.5 mL (25 mg) sodium hyaluronate | Triamcinolone: 80 mg + 5 mL lidocaine and 2.5 mL (25 mg) sodium hyaluronate | Ultrasound-guided | NA | NA | VAS; WOMAC | 1 wk, 1 mo, and 3 mo | NA |
| Goyal 2022 [31] | India | RCT | Shoulder impingement syndrome | Ketorolac: 34 patients; 14 M, 20 F; mean age 51.57 ± 13.22 yrs; mean duration 4.86 ± 1.3 mo Methylprednisolone: 33 patients; 10 M, 23 F; mean age 52.7 ± 11.81 yrs; mean duration 5.28 ± 1.1 mo | Ketorolac: 60 mg + 2% lignocaine 5 mL | Methylprednisolone: 40 mg + 2% lignocaine 5 mL | Posterior – lateral approach (palpation-guided) | Injections repeated at four weeks if symptoms persisted. | Rotator cuff strengthening exercises; capsular stretching exercises; shoulder ROM exercises | VAS; SPADI; ROM (flexion, abduction, ER, IR) | 1 mo and 3 mo | NA |
| Guner 2013 [32] | Turkey | RCT | Plantar fasciitis | Total: 61 patients (tenoxicam 31, methylprednisolone 30); 14 M, 47 F; mean age 41.4 ± 12.23 yrs | Tenoxicam: 1 mL of tenoxicam (20 mg/2 mL) + 2% lidocaine 1 mL | Methylprednisolone: 1 mL injection of 40 mg of methylprednisolone acetate + 2% lidocaine 1 mL | Medial approach (palpation-guided) | Not allowed to move ten minutes after injection; activity restriction for 4 weeks | Stretching; strengthening exercises | VAS; RM | 6 and 12 mo | NA |
| Jami 2020 [33] | Iran | RCT | Trigger finger | Diclofenac: 42 patients; 10 M, 32 F; mean age 52 ± 9 yrs Methylprednisolone: 42 patients; 11 M, 31 F; mean age 53 ± 7 yrs | Diclofenac: 12.5 mg diclofenac sodium injection | Methylprednisolone: 20 mg methylprednisolone acetate injection | Tendon sheath and 8 mm above the MP | NA | NA | Quinnell grading | 1, 3, and 6 wks; 3, 6, and 12 mo | NA |
| Jurgensmeier 2021 [34] | USA | RCT | Hip and knee osteoarthritis (KL grade 2 or higher) | Total: 120 patients (ketorolac 59, triamcinolone 61); 77 F, 43 M; mean age 65.28 ± 12.6 yrs | Ketorolac: 30 mg + 1% lidocaine 5 mL + 0.5% ropivacaine 5 mL | Triamcinolone: 80 mg + 1% lidocaine 5 mL + 0.5% ropivacaine 5 mL | Ultrasound-guided | NA | NA | HOOS; KOOS; VAS; PROMIS global health score | 1 wk, 1 mo, and 3 mo | Ketorolac: one patient with headache and nausea, which resolved the next day; GI bleeding in a patient who had increased warfarin dose approximately two months after ketorolac injection Triamcinolone: hyperglycemia in two diabetic patients during the first week of post-injection; nausea, hypertension, and flare of temporal arteritis were each recorded once |
| Karimzadeh 2023 [35] | Iran | RCT | Carpal tunnel syndrome (mild to moderate) | Ketorolac: 21 patients; 4 M, 17 F; mean age 50.71 ± 9.92 yrs; mean duration 16.62 ± 0.43 mo Triamcinolone: 22 patients; 6 M, 16 F; mean age 53.05 ± 6.80 yrs; mean duration 16.23 ± 0.57 mo | Ketorolac: 1 mL of 30 mg/mL + 2% lidocaine 0.5 mL | Triamcinolone: 1 mL of 40 mg/mL + 2% lidocaine 0.5 mL | Ultrasound-guided | Relative rest for 24 h; recommended to apply cold compress for ten minutes three times daily; allowed to take acetaminophen 500 mg (without codeine) every 4 to 8 h; not allowed to take other pain-relieving medications such as NSAIDs, dietary supplements, or vitamins for 1 week after the injection. | Allowed to do light to moderate physical activity gradually increase it at their own pace | VAS; BCTQ; electrodiagnostic findings; patient satisfaction; complication | 12 wks | Seven patients from both groups experienced adverse events such as warm sensation, stiffness, and heaviness at the injection site without significant group difference |
| Karthikeyan 2010 [47] | England | RCT | Shoulder impingement syndrome | Tenoxicam: 31 patients; 16 M, 15 F; mean age 58.0 ± 9.8 yrs; mean duration 8 (range 2–12) mo Methylprednisolone: 27 patients; 16 M, 11 F; mean age 60.0 ± 13.0 yrs; mean duration 10 (range 2–12) mo | Tenoxicam: 20 mg + 1% lignocaine 5 mL | Methylprednisolone: 40 mg + 1% lignocaine 5 mL | Anterolateral approach (reduction in pain of at least 50% with Neer’s test ten minutes after injection to confirm accurate placement) | Advised to take simple analgesia if needed but to avoid any preparation containing NSAIDs | Standardized outpatient physiotherapy for all patients | CMS; DASH; OSS | 2, 4, and 6 wks | NA |
| Kim 2021 [36] | South Korea | RCT | Shoulder impingement syndrome | Ketorolac: 30 patients; 19 M, 11 F; mean age 66 ± 6.0 yrs; mean duration 8.8 ± 7.2 mo Triamcinolone: 30 patients; 22 M, 8 F; mean age 68.8 ± 6.0 yrs; mean duration 7.4 ± 5.5 mo | Ketorolac: 1 mL of 30 mg/mL + 2% lidocaine 1 mL | Triamcinolone: 1 mL of 40 mg/mL + 2% lidocaine 1 mL | Palpation-guided | Prescribed with acetaminophen | Home exercise program | VAS; ASES; UCLA score; patient satisfaction | 3, 6, 12 wks | Triamcinolone: one case of uncontrolled diabetes and two cases of facial flushing |
| Kwon 2017 [48] | South Korea | Retrospective comparative study | Shoulder impingement syndrome | Ketorolac: 20 patients; 18 M, 2 F; median age 54.45 (42–68); mean duration 12.20 ± 25.66 wks Triamcinolone: 20 patients; 16 M, 4 F; median age 56.25 (42–69); mean duration 7.25 ± 12.72 wks | Ketorolac: 60 mg + 1% lidocaine 5 mL | Triamcinolone: 40 mg + 1% lidocaine 5 mL | Ultrasound-guided | Advised not to take additional NSAID | Home exercise program | VAS; CMS; ROM (forward flexion, IR, ER) | 1 mo and 3 mo | NA |
| Leow 2018 [37] | Singapore | RCT | Trigger finger | Ketorolac: 59 patients; 23 M, 36 F Triamcinolone: 62 patients; 22 M, 40 F Total mean age of 60 yrs (range 44–87) | Ketorolac: 0.5 mL of 30 mg/mL + 1% lidocaine 1% 0.5 mL (10 mg/mL) | Triamcinolone: 0.5 mL of 10 mg/mL + 1% lidocaine 0.5 mL (10 mg/mL) | Intrasynovial and extrasynovial injection at the level of the A1 pulley | NA | NA | Severity; pain; A1 pulley tenderness and swelling; total active motion and flexion deformity; patient subjective responses; conversion to surgery | 3, 6, 12 and 24 wks | NA |
| Matee 2021 [27] | Pakistan | Quasi-experimental study | Adhesive capsulitis (50%); rotator cuff syndrome (40%); shoulder impingement syndrome (10%) | Ketorolac: 36 patients; 26 M, 10 F; mean age 45 ± 10 yrs Methylprednisolone: 24 patients; 14 M, 10 F; mean age 55 ± 9 yrs | Ketorolac: 1 mL (30 mg) + 2% lignocaine 2 mL | Methylprednisolone: 1 mL (40 mg) + 2% lignocaine 2 mL | Posterior approach glenohumeral injection (blind) | Paracetamol (650 mg) and orphenadrine citrate (50 mg) twice a day, and local application of piroxicam gel 0.5% four times a day | Therapeutic exercises particular to disease | ROM (flexion, extension, abduction, IR, ER) | 4 wks | NA |
| Min 2013 [38] | USA | RCT | Shoulder impingement syndrome | Ketorolac: 17 patients; 13 M, 4 F; mean age 39.6 ± 9.4 yrs Triamcinolone: 15 patients; 12 M, 3 F; mean age 39.1 ± 10.5 yrs | Ketorolac: 60 mg + 1% lidocaine 6 mL with epinephrine | Triamcinolone: 40 mg + 1% lidocaine 6 mL with epinephrine | Posterolateral approach (palpation-guided) | NA | NA | VAS; UCLA score; ROM (abduction and forward flexion) | 4 wks | Triamcinolone: one case of a fainting episode that resolved in five minutes without intervention |
| Park 2015 [49] | South Korea | Retrospective comparative study | Hip osteoarthritis (KL grade 2 or 3) | Ketorolac: 12 M, 36 F; mean age 59.29 ± 8.78 yrs; mean duration 7.10 ± 2.25 mo Triamcinolone: 12 M, 38 F; mean age 58.24 ± 8.52 yrs; mean duration 6.66 ± 2.45 mo | Ketorolac: 30 mg (1 mL) + 0.5% lidocaine 14 mL | Triamcinolone: 40 mg (1 mL) + 0.5% lidocaine 14 mL | Ultrasound-guided | Patients were changed to acetaminophen or tramadol-acetaminophen if they were taking oral NSAIDs | All patients were continued on previous physical therapy | HHS; VAS | 1, 3, and 6 mo | Ketorolac: four local adverse events described as mild, transient sensations of pain and heaviness in the injected joint |
| Shakeel 2012 [39] | Malaysia | RCT | Trigger finger (at least grade 2 by Quinnell) | Diclofenac: 50 patients; 18 M, 32 F; mean age 58 yrs (range 40–75) Triamcinolone: 50 patients; 12 M, 38 F; mean age 57 yrs (range 40–71) | Diclofenac: 0.5 mL of 12.5 mg | Triamcinolone: 0.5 mL of 20 mg | Targeting above the tendon and in the tendon sheath under the A1 pulley (palpation-guided) | NA | NA | Quinnell grading | 3 wks and 3 mo | Diclofenac: two patients (4%) with continuous pain at the injection site; three patients (6%) with nodular swelling at the injection site; two patients (4%) with stiffness of the injected finger; one patient (2%) with recurrence of triggering painTriamcinolone: one patient (2%) with continuous pain at the injection site; nine patients (18%) with recurrence of triggering pain |
| Siddique 2021 [50] | Pakistan | RCT | Shoulder impingement syndrome | Ketorolac: 109 patients; 54 M, 55 F; mean age 39.09 ± 9.9 yrs Methylprednisolone: 109 patients; 63 M, 46 F; mean age 38.08 ± 8.61 yrs | Ketorolac: 60 mg + 2% lidocaine 1 mL | Methylprednisolone: 40 mg mixed + 2% lidocaine 1 mL | NA | NA | NA | VAS; CMS | 4 wks | NA |
| Sindhupakorn 2020 [28] | Thailand | RCT | Soft tissue injuries; bicipital tendinitis (21.7%); de Quervain’s tenosynovitis (31.7%); knee bursitis (4.2%); lateral epicondylitis (15%); myofascial pain syndrome (2.5%); patellar tendinitis (2.5%); plantar fasciitis (22.5%) | Normal saline: 20 patients; 5 M, 15 F; mean age 54.2 ± 7.52 yrsKetorolac 30 mg: 20 patients; 9 M, 11 F; mean age 49.85 ± 9.75 yrs Ketorolac 60 mg: 20 patients; 8 M, 12 F; mean age 50.95 ± 7.54 yrs Triamcinolone 10 mg: 20 patients; 8 M, 12 F; mean age 48.1 ± 8.71 yrs Triamcinolone 20 mg: 20 patients; 5 M, 15 F; mean age 49.8 ± 10.22 yrs Triamcinolone 40 mg: 20 patients; 5 M, 15 F; mean age 52.15 ± 8.65 yrs | Ketorolac 30 mg: 1 mL (30 mg) + 2% xylocaine 2 mL Ketorolac 60 mg: 2 mL (60 mg) + 2% xylocaine 2 mL | Normal saline 0.9%: 1 mL + 2% xylocaine 2 mL Triamcinolone: 1 mL (10 mg) + 2% xylocaine 2 mL Triamcinolone: 2 mL (20 mg) + 2% xylocaine 2 mL Triamcinolone: 1 mL (40 mg) + 2% xylocaine 2 mL | NA | NA | NA | VAS | 0, 10, 30 min; 1, 2, 6 h; 1, 7 days | Control (lidocaine + normal saline) group: one patient dropped out due to pain during injection |
| Suwannaphisit 2022 [40] | Thailand | RCT | De Quervain’s tenosynovitis | Ketorolac: 31 patients; 6 M, 25 F; median age 54.5 yrs [IQR: 10]; mean duration 30 ± 37 days Triamcinolone: 34 patients; 7 M, 27 F; median age 54 yrs [IQR: 14]; mean duration 30 ± 47.1 days | Ketorolac: 1 mL ketorolac (30 mg/mL) + 1% lidocaine 0.5 mL with adrenaline | Triamcinolone: 1 mL triamcinolone acetonide 10 mg/mL + 1% xylocaine 0.5 mL with adrenaline | Injected along the line of the tendon (proximal or distal to radial styloid, at the location of maximal pain) | Allowed to do light activities (avoid lifting weight above 10 kg); wrist motion as tolerated; oral paracetamol 500 mg as needed | NA | VAS; DASH; grip/pinch strength | 6 wks | Steroid: half the patients developed some degree of hypopigmentation |
| Taheri 2017 [41] | Iran | RCT | Shoulder impingement syndrome | Ketorolac: 20 patients; 8 M, 12 F; mean age 49.76 ± 4.83 yrs Methylprednisolone: 20 patients; 9 M, 11 F; mean age 47.53 ± 6.92 yrs | Ketorolac: 60 mg ketorolac mixed + 2% lidocaine 1 mL | Methylprednisolone: 40 mg methylprednisolone mixed + 2% lidocaine 1 mL | Anterolateral approach | Advised to take simple analgesia as needed but to avoid oral NSAIDs | Home exercise program | VAS; CMS | 1 and 3 mo | NA |
| Verma 2022 [51] | India | Retrospective comparative study | Knee osteoarthritis (KL grade 2 or 3) | Ketorolac: 25 patients; 11 M, 14 F; mean age 59.5 ± 10.16 yrs; mean duration 11.59 ± 4.32 mo Triamcinolone: 25 patients; 9 M, 16 F; mean age 58.6 ± 9.2 yrs; mean duration 10.46 ± 3.26 mo | Ketorolac: 10 mg of ketorolac + 0.5% lidocaine 5 mL | Triamcinolone: 40 mg of triamcinolone + 0.5% lidocaine 5 mL | Superolateral approach (three weekly injections with NSAID or steroid and, 2 weeks later, 6 mL (48 mg) of sodium hyaluronate) | Aceclofenac 100 mg BID and oral cefuroxime 250 mg BID for 3 days | NA | VAS; WOMAC; Rubin scale | 1, 2, 5 wks, and 3 mo after first injection | Two patients (unspecified group) with local inflammation and pain |
| Xu 2020 [52] | China | Retrospective comparative study | Knee osteoarthritis (KL grade 2 or 3) | Ketorolac: 42 patients; 18 M, 24 F; mean age 59.02 ± 11.25 yrs; mean duration 8.59 ± 4.32 mo Triamcinolone: 42 patients; 16 M, 26 F; mean age 58.16 ± 10.21 yrs; mean duration 8.35 ± 3.86 mo | Ketorolac: 10 mg of ketorolac + 0.5% lidocaine 5 mL | Triamcinolone: 25 mg of triamcinolone + 0.5% lidocaine 5 mL | Superolateral approach (all patients received five weekly injections. NSAID/steroid injection was given during the first 3 weeks; for the last 2 weeks, only sodium hyaluronate (25 mg)) | Imrecoxib 100 mg BID for 3 days; other analgesics not allowed | NA | VAS; WOMAC | 1, 2, and 5 wks after treatment initiation, and 3 mo after the last injection | No major complications in either group NSAID injection: three patients developed mild, focal post-injection pain |
| Yilmaz 2019 [53] | Turkey | RCT | Knee osteoarthritis (KL grade 1 or 2) | Tenoxicam: 30 patients; 11 M, 19 F; mean age 68.07 ± 8.08 yrs; mean duration 51.03 ± 49.29 mo Triamcinolone: 30 patients; 8 M, 22 F; mean age 65.83 ± 10.13 yrs; mean duration 57.67 ± 61.69 mo | Tenoxicam: 2 mL of 20 mg | Triamcinolone: 1 mL of 20 mg | No ultrasound or fluoroscopy guidance | Advised to rest or remain immobilized and avoid any kind of weight loadings for one day; took acetaminophen as needed | NA | VAS; WOMAC | 1, 3, and 6 mo | NA |
| Yu 2018 [42] | China | RCT | Shoulder impingement syndrome | Lornoxicam: 19 patients; 7 M,12 F; mean age 37.2 ± 10.1 yrs (range 27–63) Triclabendazole: 20 patients; 14 M, 8 F; mean age 44.7 ± 9.5 yrs (range 35–68) | Lornoxicam: 8 mg + 2% lidocaine, total 5 mL mixture | Triclabendazole: 40 mg + 2% lidocaine, total 5 mL mixture | Ultrasound-guided | NA | NA | VAS; UCLA score; ROM (abduction) | 60 min, 6 wks | NA |
| Author | Study Design | Pathology | Main Findings |
|---|---|---|---|
| Abolhasani 2019 [26] | RCT | Shoulder impingement syndrome | Ketorolac, triamcinolone, and betamethasone are equally effective in improving VAS and OSS over six weeks. |
| Ahn 2015 [43] | Retrospective comparative study | Adhesive capsulitis | Both groups demonstrated significant improvement in SPADI, VAS, and passive ROM at one, three, and six months. NSAID group showed greater improvement in passive external rotation and abduction at three and six months. |
| Aksakal 2017 [44] | RCT | Shoulder impingement syndrome | Changes in CMS and UCLA scores from baseline were higher in steroid groups at two, four, and six weeks. Steroid injection resulted in significant improvement at all follow-ups compared to previous follow-ups except for UCLA score between weeks four and six, while NSAID injection showed significant improvement from baseline to weeks two but not between weeks two and four and between weeks four and six. |
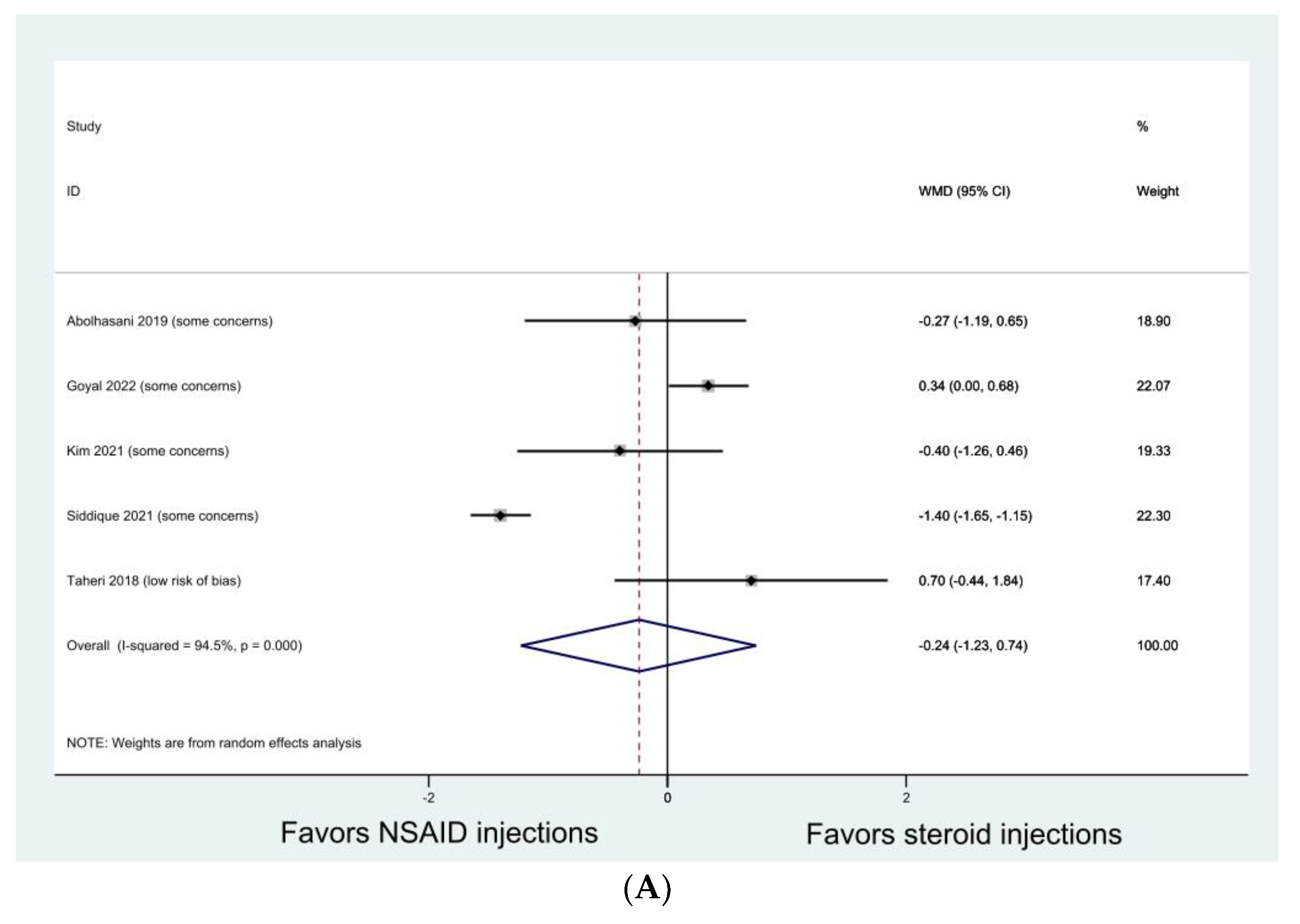
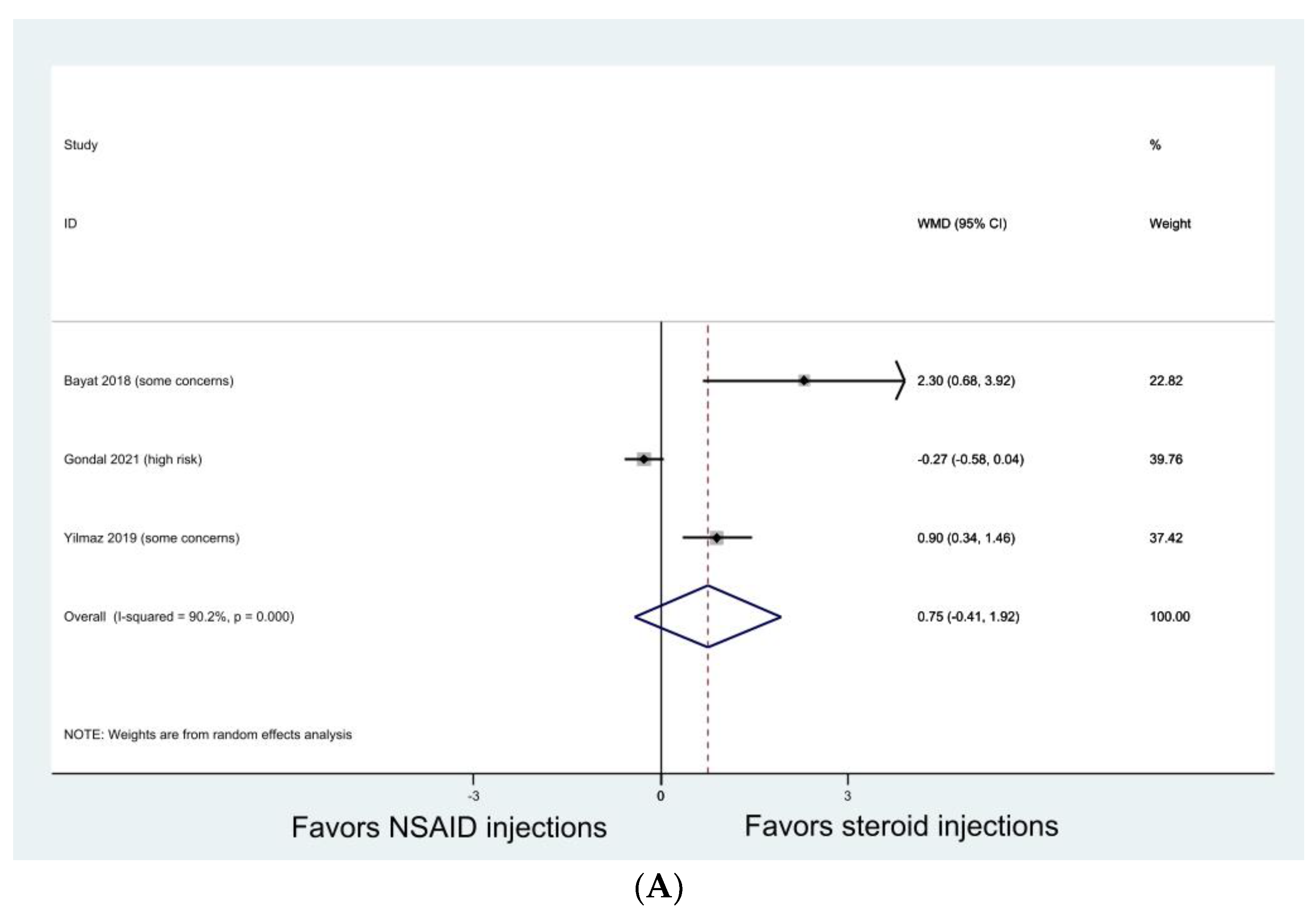
| Bayat 2018 [45] | RCT | Knee osteoarthritis (KL grade 2 or 3) | Pain levels (in all three questionnaires) in triamcinolone group improved more than in ketorolac group after one month. No difference was found between the groups after three months. Intra-group comparisons showed significant improvements in VAS scores and pain subscales of WOMAC and Lequesne after one and three months in both groups; improvements in general WOMAC and Lequesne scores after one and three months in triamcinolone group only. |
| Bellamy 2016 [46] | RCT | Knee osteoarthritis (mean KL grade 3) | Mean VAS for ketorolac and corticosteroid improved significantly from baseline at two weeks and remained improved for 24 weeks without group difference. Corticosteroids appeared to have higher WOMAC scores than ketorolac at the final follow-up. There were no significant differences in KS pain and function, Short Form 36, Tegner/Lysholm, and UCLA activity scores between the two groups throughout the 24 weeks. The cost savings per year using ketorolac instead of triamcinolone would be USD 2259.40, USD 6182.54, and USD 4159.35 for 2013, 2014, and 2015, respectively, with a total saving of USD 12,601.29 over this period. |
| Cift 2015 [29] | RCT | Shoulder impingement syndrome | Both groups demonstrated significant improvement in VAS, DASH, and active ROMs without significant group differences at post-treatment follow-ups. A statistically significant difference in mean change in VAS and DASH favoring NSAID injection. |
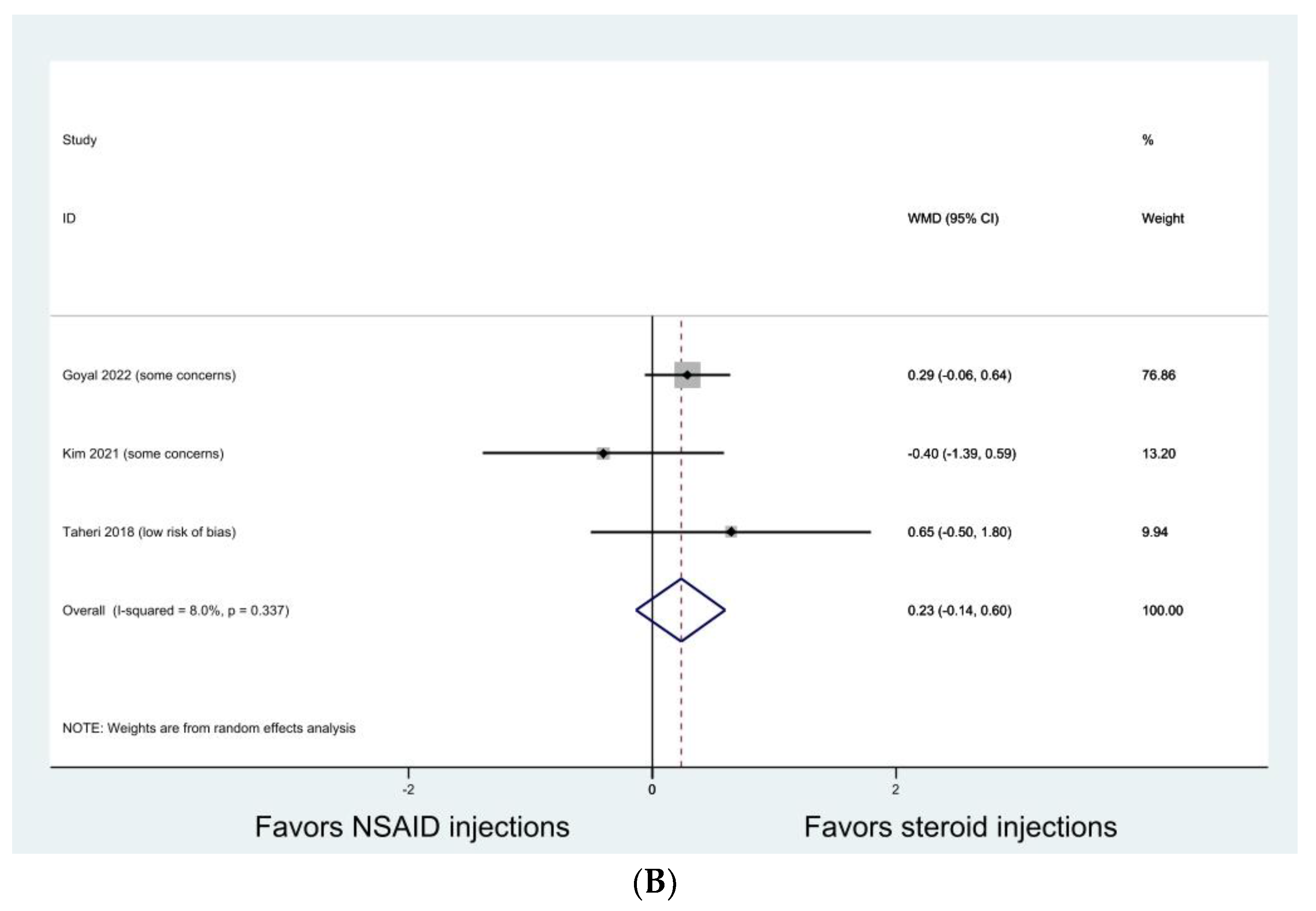
| Gondal 2021 [30] | RCT | Knee osteoarthritis (KL grade 2 or 3) | Both groups showed significant improvement in VAS and WOMAC at one week, one month, and three months without group difference. |
| Goyal 2022 [31] | RCT | Shoulder impingement syndrome | Both groups showed significant improvement in VAS and SPADI at three months without group difference. There was no significant difference in ROM at one or three months between the two groups. Two patients in the NSAID group and one patient in the steroid group required a second injection at four weeks, with one from each group showing improvement, but one patient from the NSAID group underwent arthroscopic subacromial decompression. |
| Guner 2013 [32] | RCT | Plantar fasciitis | Both groups showed significant improvement in terms of VAS at 6 and 12 months compared to pretreatment without group difference. |
| Jami 2020 [33] | RCT | Trigger finger | Both treatments were effective in improving symptoms, but the rate of improvement was better in the steroid group. |
| Jurgensmeier 2021 [34] | RCT | Hip and knee osteoarthritis (KL grade 2 or higher) | Both groups showed significant improvement in HOOS, KOOS, and VAS without group differences. |
| Karimzadeh 2023 [35] | RCT | Carpal tunnel syndrome (mild to moderate) | Both injections relieved pain, increased function, and improved electrodiagnostic findings in patients with mild to moderate carpal tunnel syndrome. Triamcinolone was more effective than ketorolac in terms of analgesic effect and improvement in symptom severity and function. |
| Karthikeyan 2010 [47] | RCT | Shoulder impingement syndrome | Both groups showed significant improvement in CMS at six weeks, but the improvement was higher in the steroid group. Patients in the steroid group demonstrated significantly higher improvement in the DASH and the OSS than those in the NSAID group at two, four, and six weeks. |
| Kim 2021 [36] | RCT | Shoulder impingement syndrome | Both groups showed significant improvements in VAS, ASES, and UCLA scores without group differences during all follow-up periods. |
| Kwon 2017 [48] | Retrospective comparative study | Shoulder impingement syndrome | Pain improvement was significantly greater in the steroid group at one month, but there was no significant difference in pain improvement at three months. Significant improvement in CMS without group difference. External rotation was significantly greater in the NSAID group at three months. |
| Leow 2018 [37] | RCT | Trigger finger | Steroid injection provided better short-term outcomes in terms of severity, resolution of triggering, and reduction in pain, swelling, and flexion deformity at 12 weeks. However, the longer-term outcomes of NSAID and steroids at 24 weeks were similar. |
| Matee 2021 [27] | Quasi-experimental study | Adhesive capsulitis (50%); rotator cuff syndrome (40%); shoulder impingement syndrome (10%) | Both injections resulted in significant improvement in ROM at four weeks without group difference. |
| Min 2013 [38] | RCT | Shoulder impingement syndrome | Both injections resulted in significant improvement of ROM and pain at four weeks, but abduction and mean improvement in the UCLA score were significantly higher in the NSAID group. |
| Park 2015 [49] | Retrospective comparative study | Hip osteoarthritis (KL grade 2 or 3) | Significant improvement in HHS and VAS at one, three, and six months compared to baseline without group difference. |
| Shakeel 2012 [39] | RCT | Trigger finger (at least grade 2 by Quinnell) | There was a significant initial improvement in Quinnell grading for the corticosteroid group compared to the NSAID group from zero to three weeks, but from three weeks to three months, the grades were ranked again, and the NSAID group did significantly better. There was no significant difference in Quinnell grading improvement between the two groups at three months. |
| Siddique 2021 [50] | RCT | Shoulder impingement syndrome | NSAID injections resulted in significantly lower pain scores, while steroid injections resulted in significantly higher CMS at four weeks. |
| Sindhupakorn 2020 [28] | RCT | Soft tissue injuries; bicipital tendinitis (21.7%); de Quervain’s tenosynovitis (31.7%); knee bursitis (4.2%); lateral epicondylitis (15%); myofascial pain syndrome (2.5%); patellar tendinitis (2.5%); plantar fasciitis (22.5%) | Both ketorolac and triamcinolone showed comparable efficacy in reducing pain intensity. Specifically, 60 mg of ketorolac demonstrated similar or even better pain reduction at several time points compared to various doses of triamcinolone. Moreover, 20 mg of triamcinolone facilitated the quickest return to normal activity post injection, with an average time of 12.35 min, while 60 mg of ketorolac had the highest success rate in returning to normal activity within 30 min. |
| Suwannaphisit 2022 [40] | RCT | De Quervain’s tenosynovitis | The steroid group showed higher improvement in VAS and DASH scores at six weeks. Both groups showed significant improvement in grip strength at six weeks. Furthermore, 25/31 in the ketorolac group received additional injections with triamcinolone. |
| Taheri 2017 [41] | RCT | Shoulder impingement syndrome | Both groups showed significant improvement in VAS and CMS at one and three months without group difference. |
| Verma 2022 [51] | Retrospective comparative study | Knee osteoarthritis (KL grade 2 or 3) | Both groups showed significant improvement in VAS and WOMAC at three months after the first injection without group difference. |
| Xu 2020 [52] | Retrospective comparative study | Knee osteoarthritis (KL grade 2 or 3) | Both groups showed significant improvement in VAS and WOMAC at three months without group difference. |
| Yilmaz 2019 [53] | RCT | Knee osteoarthritis (KL grade 1 or 2) | Both NSAID and steroid injection alone provided improvement in VAS and WOMAC at one month but not at three and six months. Combined NSAID and steroid injections resulted in improvement in VAS and WOMAC at one, three, and six months. |
| Yu 2018 [42] | RCT | Shoulder impingement syndrome | Pain improvement was seen in both interventions in both 60 min and six weeks post intervention. UCLA scores improvement was seen in both interventions in both 60 min and six weeks post intervention, but no difference was found between groups. Improvement of abduction was seen in both interventions in both 60 min and six weeks post-intervention, and no difference was found between groups at 60 min after, but NSAID group showed better recovery in shoulder abduction at six weeks after intervention. |
3.2. Study Quality and Risk of Bias Assessment
3.3. Shoulder Impingement Syndrome
3.4. Knee Osteoarthritis
3.5. Hip Osteoarthritis
3.6. Trigger Finger
3.7. Adhesive Capsulitis
3.8. Plantar fasciitis
3.9. Carpal Tunnel Syndrome
3.10. De Quervain’s Tenosynovitis
3.11. Other Soft Tissue Injuries
4. Discussion
4.1. Clinical Implications and Future Research Direction
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, A.; Mak, D.; Davies, A.M.; James, S.L.; Botchu, R. Musculoskeletal Corticosteroid Administration: Current Concepts. Can. Assoc. Radiol. J. 2019, 70, 29–36. [Google Scholar] [CrossRef]
- Hepper, C.T.; Halvorson, J.J.; Duncan, S.T.; Gregory, A.J.; Dunn, W.R.; Spindler, K.P. The efficacy and duration of intra-articular corticosteroid injection for knee osteoarthritis: A systematic review of level I studies. J. Am. Acad. Orthop. Surg. 2009, 17, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Akgün, K.; Birtane, M.; Akarirmak, U. Is local subacromial corticosteroid injection beneficial in subacromial impingement syndrome? Clin. Rheumatol. 2004, 23, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, P.; Liu, S.; Li, H.; Jiang, J.; Chen, S.; Chen, J. Intra-articular Steroid Injection for Frozen Shoulder: A Systematic Review and Meta-analysis of Randomized Controlled Trials With Trial Sequential Analysis. Am. J. Sports Med. 2017, 45, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, J.R.; Lee, K.S.; Blankenbaker, D.G.; del Rio, A.M.; Keene, J.S. Ultrasound-guided corticosteroid injections for treatment of greater trochanteric pain syndrome: Greater trochanter bursa versus subgluteus medius bursa. AJR Am. J. Roentgenol. 2013, 201, W313–W317. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.H. Steroid versus placebo injection for trigger finger. J. Hand Surg. Am. 1996, 21, 530. [Google Scholar] [CrossRef] [PubMed]
- Kerezoudis, P.; Rinaldo, L.; Alvi, M.A.; Hunt, C.L.; Qu, W.; Maus, T.P.; Bydon, M. The Effect of Epidural Steroid Injections on Bone Mineral Density and Vertebral Fracture Risk: A Systematic Review and Critical Appraisal of Current Literature. Pain Med. 2018, 19, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, M.; Gross, A.E. Achilles tendon rupture following steroid injection. Report of three cases. J. Bone Joint Surg. Am. 1983, 65, 1345–1347. [Google Scholar] [CrossRef]
- Mahler, F.; Fritschy, D. Partial and complete ruptures of the Achilles tendon and local corticosteroid injections. Br. J. Sports Med. 1992, 26, 7–14. [Google Scholar] [CrossRef]
- Thompson, A.R.; Ensrud, E.R. Rapid Onset of Femoral Head Osteonecrosis After a Single Intra-articular Hip Joint Injection of Corticosteroid. Am. J. Phys. Med. Rehabil. 2020, 99, e54–e55. [Google Scholar] [CrossRef]
- Al-Omari, A.A.; Aleshawi, A.J.; Marei, O.A.; Younes, H.M.B.; Alawneh, K.Z.; Alquran, E.; Mohaidat, Z.M. Avascular necrosis of the femoral head after single steroid intra-articular injection. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 193–197. [Google Scholar] [CrossRef]
- Yamamoto, T.; Schneider, R.; Iwamoto, Y.; Bullough, P.G. Rapid destruction of the femoral head after a single intraarticular injection of corticosteroid into the hip joint. J. Rheumatol. 2006, 33, 1701–1704. [Google Scholar]
- Choudhry, M.N.; Malik, R.A.; Charalambous, C.P. Blood Glucose Levels Following Intra-Articular Steroid Injections in Patients with Diabetes: A Systematic Review. JBJS Rev. 2016, 4, e5. [Google Scholar] [CrossRef] [PubMed]
- Gaujoux-Viala, C.; Dougados, M.; Gossec, L. Efficacy and safety of steroid injections for shoulder and elbow tendonitis: A meta-analysis of randomised controlled trials. Ann. Rheum. Dis. 2009, 68, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Jevsevar, D.S.; Shores, P.B.; Mullen, K.; Schulte, D.M.; Brown, G.A.; Cummins, D.S. Mixed Treatment Comparisons for Nonsurgical Treatment of Knee Osteoarthritis: A Network Meta-analysis. J. Am. Acad. Orthop. Surg. 2018, 26, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Machado, G.C.; Abdel-Shaheed, C.; Underwood, M.; Day, R.O. Non-steroidal anti-inflammatory drugs (NSAIDs) for musculoskeletal pain. BMJ 2021, 372, n104. [Google Scholar] [CrossRef] [PubMed]
- Osani, M.C.; Vaysbrot, E.E.; Zhou, M.; McAlindon, T.E.; Bannuru, R.R. Duration of Symptom Relief and Early Trajectory of Adverse Events for Oral Nonsteroidal Antiinflammatory Drugs in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Arthritis Care Res. 2020, 72, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Selig, D.J.; Kress, A.T.; Horton, I.M.; Livezey, J.R.; Sadik, E.J.; DeLuca, J.P. Pharmacokinetics, safety and efficacy of intra-articular non-steroidal anti-inflammatory drug injections for the treatment of osteoarthritis: A narrative review. J. Clin. Pharm. Ther. 2022, 47, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Sardana, V.; Burzynski, J.; Hasan, K.; Zalzal, P. Are non-steroidal anti-inflammatory drug injections an alternative to steroid injections for musculoskeletal pain?: A systematic review. J. Orthop. 2018, 15, 812–816. [Google Scholar] [CrossRef]
- Ziradkar, R.; Best, T.M.; Quintero, D.; Paultre, K. Nonsteroidal Anti-inflammatory and Corticosteroid Injections for Shoulder Impingement Syndrome: A Systematic Review and Meta-analysis. Sports Health 2023, 15, 579–591. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 13 April 2023).
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Biggerstaff, B.J.; Tweedie, R.L. Incorporating variability in estimates of heterogeneity in the random effects model in meta-analysis. Stat. Med. 1997, 16, 753–768. [Google Scholar] [CrossRef]
- Abolhasani, H.; Falsafi, M.; Khafri, S.; Frydoni, M.B. Comparison of efficacy of ketorolac, triamcinolone and betamethasone injections in the treatment of shoulder impingement syndrome. J. Evol. Med. Dent. Sci. 2019, 8, 2050+. [Google Scholar] [CrossRef]
- Matee, S.; Anwar, W.; Wahid, S.; Ayaz, S.B.; Shahid, R.; Sheikh, N.A. Comparison of intraarticular methylprednisolone and ketorolac injections in improving range of motion for different shoulder joint disorders. Pak. Armed Forces Med. J. 2021, 71, 818–822. [Google Scholar] [CrossRef]
- Sindhupakorn, B.; Jomkoh, D.; Namkuntee, T. Randomized, double-blind, Placebo-Controlled Study to Compare the Efficacy of Combination of Lidocaine with ketorolac or triamcinolone versus Lidocaine Alone for Soft Tissue Injuries. J. Orthop. 2020, 20, 135–143. [Google Scholar] [CrossRef]
- Çift, H.; Özkan, F.; Tolu, S.; Şeker, A.; Mahiroğulları, M. Comparison of subacromial tenoxicam and steroid injections in the treatment of impingement syndrome. Jt. Dis. Relat. Surg. 2015, 26, 16–20. [Google Scholar] [CrossRef]
- Gondal, S.S.; Zareen, A.; Anwar, M.H.; Zaheer, J.; Salim, M. Comparison of analgesic and functional outcomes of Intra-articular Ketorolac versus Triamcinolone Acetone Injection in Patients of Knee Osteoarthritis. J. Rawalpindi Med. Coll. 2021, 25, 512. [Google Scholar] [CrossRef]
- Goyal, T.; Paul, S.; Sethy, S.S.; Choudhury, A.K. Outcomes of ketorolac versus depomedrol infiltrations for subacromial impingement syndrome: A randomized controlled trial. Musculoskelet. Surg. 2022, 106, 29–34. [Google Scholar] [CrossRef]
- Guner, S.; Onder, H.; Guner, S.I.; Ceylan, M.F.; Gökalp, M.A.; Keskin, S. Effectiveness of local tenoxicam versus corticosteroid injection for plantar fasciitis treatment. Orthopedics 2013, 36, e1322–e1326. [Google Scholar] [CrossRef]
- Mohammadi Jami, S.; Khafri, S.; Bahrami Feraydoni, M. Comparison of Methylprednisolone Injection Versus Diclofenac Injection in Treatment of Trigger Finger. J. Babol Univ. Med. Sci. 2020, 22, 275–282. [Google Scholar]
- Jurgensmeier, K.; Jurgensmeier, D.; Kunz, D.E.; Fuerst, P.G.; Warth, L.C.; Daines, S.B. Intra-articular Injections of the Hip and Knee With Triamcinolone vs Ketorolac: A Randomized Controlled Trial. J. Arthroplasty 2021, 36, 416–422. [Google Scholar] [CrossRef]
- Karimzadeh, A.; Esmaily, H.; Raeissadat, S.A.; Esmaelzade, M.; Aghamiri, S.H.; Bolandnazar, N.S. Comparison of the Effect of Intracarpal Injection of Ketorolac With Triamcinolone in Carpal Tunnel Syndrome: A Randomized Controlled Trial. Ann. Pharmacother. 2023, 58, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.B.; Lee, W.S.; Won, J.S. The effects of a single-dose subacromial injection of a nonsteroidal anti-inflammatory drug in geriatric patients with subacromial impingement syndrome: A randomized double-blind study. Clin. Shoulder Elb. 2021, 24, 4–8. [Google Scholar] [CrossRef]
- Leow, M.Q.H.; Hay, A.S.R.; Ng, S.L.; Choudhury, M.M.; Li, H.; McGrouther, D.A.; Tay, S.C. A randomized controlled trial comparing ketorolac and triamcinolone injections in adults with trigger digits. J. Hand Surg. Eur. Vol. 2018, 43, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Min, K.S.; St Pierre, P.; Ryan, P.M.; Marchant, B.G.; Wilson, C.J.; Arrington, E.D. A double-blind randomized controlled trial comparing the effects of subacromial injection with corticosteroid versus NSAID in patients with shoulder impingement syndrome. J. Shoulder Elbow Surg. 2013, 22, 595–601. [Google Scholar] [CrossRef]
- Shakeel, H.; Ahmad, T.S. Steroid injection versus NSAID injection for trigger finger: A comparative study of early outcomes. J. Hand Surg. Am. 2012, 37, 1319–1323. [Google Scholar] [CrossRef]
- Suwannaphisit, S.; Suwanno, P.; Fongsri, W.; Chuaychoosakoon, C. Comparison of the effect of ketorolac versus triamcinolone acetonide injections for the treatment of de Quervain’s tenosynovitis: A double-blind randomized controlled trial. BMC Musculoskelet. Disord. 2022, 23, 831. [Google Scholar] [CrossRef]
- Taheri, P.; Dehghan, F.; Mousavi, S.; Solouki, R. Comparison of Subacromial Ketorolac Injection versus Corticosteroid Injection in the Treatment of Shoulder Impingement Syndrome. J. Res. Pharm. Pract. 2017, 6, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Zhang, D.; Yang, J.; Liu, M.; Tan, L. Clinical efficacy of ultrasound-guided subacromial drug injection in the treatment of subacromial impingement syndrome. Zhonghua Wai Ke Za Zhi 2018, 56, 781–785. [Google Scholar]
- Ahn, J.K.; Kim, J.; Lee, S.J.; Park, Y.; Bae, B.; Lee, W. Effects of Ultrasound-guided intra-articular ketorolac injection with capsular distension. J. Back Musculoskelet. Rehabil. 2015, 28, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Aksakal, M.; Ermutlu, C.; Özkaya, G.; Özkan, Y. Lornoxicam injection is inferior to betamethasone in the treatment of subacromial impingement syndrome: A prospective randomized study of functional outcomes. Orthopade 2017, 46, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Bayat, M.; Raeissadat, S.A.; Mirzakhani, K.; Adili, M. A Comparison between the Efficacy of Intra-articular Injections of Ketorolac and Triamcinolone in Patients with Knee Osteoarthritis. J. Isfahan Med. Sch. 2018, 36, 411–418. [Google Scholar]
- Bellamy, J.L.; Goff, B.J.; Sayeed, S.A. Economic Impact of Ketorolac vs Corticosteroid Intra-Articular Knee Injections for Osteoarthritis: A Randomized, Double-Blind, Prospective Study. J. Arthroplasty 2016, 31, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, S.; Kwong, H.T.; Upadhyay, P.K.; Parsons, N.; Drew, S.J.; Griffin, D. A double-blind randomised controlled study comparing subacromial injection of tenoxicam or methylprednisolone in patients with subacromial impingement. J. Bone Joint Surg. Br. 2010, 92, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Lee, Y.H.; Kim, H.M.; Kim, J.M.; Jung, H.S.; Yi, S.R. Early Clinical Outcomes after Subacromial Injection of Ketorolac in Patients with Shoulder Impingement Syndrome: A Comparison with Steroid Injection. J. Korean Orthop. Assoc. 2017, 52, 170–177. [Google Scholar] [CrossRef]
- Park, K.D.; Kim, T.K.; Bae, B.W.; Ahn, J.; Lee, W.Y.; Park, Y. Ultrasound guided intra-articular ketorolac versus corticosteroid injection in osteoarthritis of the hip: A retrospective comparative study. Skeletal Radiol. 2015, 44, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Siddique, T.; Harl, H.A.; Gillani, S.F.U.H.S.; Asif, S.; Hanif, M.M. Comparison of subacromial ketorolac injection vs subacromial steroid injection in the treatment of shoulder impingement syndrome. Pak. J. Med. Health Sci. 2021, 15, 1028–1030. [Google Scholar]
- Verma, V.; Kunwar, A.; Yadav, A.; Verma, S. Outcome of intra-articular corticosteroid vs. intra-articular ketorolac in symptomatic knee osteo-arthritis: A retrospective study. Int. Surg. J. 2022, 9, 639–643. [Google Scholar] [CrossRef]
- Xu, J.; Qu, Y.; Li, H.; Zhu, A.; Jiang, T.; Chong, Z.; Wang, B.; Shen, P.; Xie, Z. Effect of Intra-articular Ketorolac Versus Corticosteroid Injection for Knee Osteoarthritis: A Retrospective Comparative Study. Orthop. J. Sports Med. 2020, 8, 2325967120911126. [Google Scholar] [CrossRef]
- Yilmaz, E. The evaluation of the effectiveness of intra-articular steroid, tenoxicam, and combined steroid-tenoxicam injections in the treatment of patients with knee osteoarthritis. Clin. Rheumatol. 2019, 38, 3243–3252. [Google Scholar] [CrossRef]
- Lo, C.K.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef]
- Beitzel, K.; McCarthy, M.B.; Cote, M.P.; Apostolakos, J.; Russell, R.P.; Bradley, J.; ElAttrache, N.S.; Romeo, A.A.; Arciero, R.A.; Mazzocca, A.D. The effect of ketorolac tromethamine, methylprednisolone, and platelet-rich plasma on human chondrocyte and tenocyte viability. Arthroscopy 2013, 29, 1164–1174. [Google Scholar] [CrossRef]
- Shapiro, P.S.; Rohde, R.S.; Froimson, M.I.; Lash, R.H.; Postak, P.; Greenwald, A.S. The effect of local corticosteroid or ketorolac exposure on histologic and biomechanical properties of rabbit tendon and cartilage. Hand 2007, 2, 165–172. [Google Scholar] [CrossRef]
- Zeng, C.; Lane, N.E.; Hunter, D.J.; Wei, J.; Choi, H.K.; McAlindon, T.E.; Li, H.; Lu, N.; Lei, G.; Zhang, Y. Intra-articular corticosteroids and the risk of knee osteoarthritis progression: Results from the Osteoarthritis Initiative. Osteoarthr. Cartil. 2019, 27, 855–862. [Google Scholar] [CrossRef]
- Coombes, B.K.; Bisset, L.; Vicenzino, B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: A systematic review of randomised controlled trials. Lancet 2010, 376, 1751–1767. [Google Scholar] [CrossRef] [PubMed]
- Ekici, A.G.; Akyol, O.; Ekici, M.; Sitilci, T.; Topacoglu, H.; Ozyuvaci, E. Intra-articular injection of dexketoprofen in rat knee joint: Histopathologic assessment of cartilage & synovium. Indian J. Med. Res. 2014, 140, 227–230. [Google Scholar] [PubMed]
- Abrams, G.D.; Chang, W.; Dragoo, J.L. In Vitro Chondrotoxicity of Nonsteroidal Anti-inflammatory Drugs and Opioid Medications. Am. J. Sports Med. 2017, 45, 3345–3350. [Google Scholar] [CrossRef] [PubMed]
- Dogan, N.; Erdem, A.F.; Gundogdu, C.; Kursad, H.; Kizilkaya, M. The effects of ketorolac and morphine on articular cartilage and synovium in the rabbit knee joint. Can. J. Physiol. Pharmacol. 2004, 82, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Gencosmanoglu, B.E.; Eryavuz, M.; Dervisoglu, S. Effects of some nonsteroidal anti-inflammatory drugs on articular cartilage of rats in an experimental model of osteoarthritis. Res. Exp. Med. 2001, 200, 215–226. [Google Scholar]
- Riggin, C.N.; Tucker, J.J.; Soslowsky, L.J.; Kuntz, A.F. Intra-articular tibiofemoral injection of a nonsteroidal anti-inflammatory drug has no detrimental effects on joint mechanics in a rat model. J. Orthop. Res. 2014, 32, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Saricaoglu, F.; Dal, D.; Atilla, P.; Iskit, A.B.; Tarhan, O.; Aşan, E.; Aypar, U.A. Effect of intraarticular injection of lornoxicam on the articular cartilage & synovium in rat. Indian J. Med. Res. 2008, 127, 362–365. [Google Scholar] [PubMed]
- Kalbhen, D.A. The influence of NSAIDs on morphology of articular cartilage. Scand. J. Rheumatol. Suppl. 1988, 77, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.D.; Stride, M.; Scott, A. Tendons—Time to revisit inflammation. Br. J. Sports Med. 2014, 48, 1553–1557. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.L.; Silbernagel, K.G.; Thorborg, K.; Kirwan, P.D.; Galatz, L.M.; Abrams, G.D.; Murrell, G.A.C.; McInnes, I.B.; Rodeo, S.A. Tendinopathy. Nat. Rev. Dis. Prim. 2021, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- O’Dowd, A. Update on the Use of Platelet-Rich Plasma Injections in the Management of Musculoskeletal Injuries: A Systematic Review of Studies From 2014 to 2021. Orthop. J. Sports Med. 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Moya, D.; Ramón, S.; Schaden, W.; Wang, C.J.; Guiloff, L.; Cheng, J.H. The Role of Extracorporeal Shockwave Treatment in Musculoskeletal Disorders. J. Bone Joint Surg. Am. 2018, 100, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.A.; Lackner, J.B.; Steilen-Matias, D.; Harris, D.K. A Systematic Review of Dextrose Prolotherapy for Chronic Musculoskeletal Pain. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2016, 9, 139–159. [Google Scholar] [CrossRef]
- Townsend, C.; Von Rickenbach, K.J.; Bailowitz, Z.; Gellhorn, A.C. Post-Procedure Protocols Following Platelet-Rich Plasma Injections for Tendinopathy: A Systematic Review. PMR 2020, 12, 904–915. [Google Scholar] [CrossRef]
- Rhim, H.C.; Shin, J.; Kang, J.; Dyrek, P.; Crockett, Z.; Galido, P.; Wade, C.; Hollander, K.; Borg-Stein, J.; Sampson, S.; et al. Use of extracorporeal shockwave therapies for athletes and physically active individuals: A systematic review. Br. J. Sports Med. 2024, 58, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Joo, Y.B.; Song, J.H. Preoperative intra-articular steroid injections within 3 months increase the risk of periprosthetic joint infection in total knee arthroplasty: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2023, 18, 148. [Google Scholar] [CrossRef] [PubMed]
- Puzzitiello, R.N.; Patel, B.H.; Nwachukwu, B.U.; Allen, A.A.; Forsythe, B.; Salzler, M.J. Adverse Impact of Corticosteroid Injection on Rotator Cuff Tendon Health and Repair: A Systematic Review. Arthroscopy 2020, 36, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Magra, M.; Maffulli, N. Nonsteroidal antiinflammatory drugs in tendinopathy: Friend or foe. Clin. J. Sport. Med. 2006, 16, 1–3. [Google Scholar] [CrossRef]
- Paavola, M.; Kannus, P.; Järvinen, T.A.; Järvinen, T.L.; Józsa, L.; Järvinen, M. Treatment of tendon disorders. Is there a role for corticosteroid injection? Foot Ankle Clin. 2002, 7, 501–513. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhim, H.C.; Ruiz, J.; Taseh, A.; Afunugo, W.; Crockett, Z.; Schon, J.; Pan, X.; Shin, J.; Schowalter, S.; Jang, K.-M.; et al. Nonsteroidal Anti-Inflammatory Drug Injections versus Steroid Injections in the Management of Upper and Lower Extremity Orthopedic Conditions: A Systematic Review with Meta-Analysis. J. Clin. Med. 2024, 13, 1132. https://doi.org/10.3390/jcm13041132
Rhim HC, Ruiz J, Taseh A, Afunugo W, Crockett Z, Schon J, Pan X, Shin J, Schowalter S, Jang K-M, et al. Nonsteroidal Anti-Inflammatory Drug Injections versus Steroid Injections in the Management of Upper and Lower Extremity Orthopedic Conditions: A Systematic Review with Meta-Analysis. Journal of Clinical Medicine. 2024; 13(4):1132. https://doi.org/10.3390/jcm13041132
Chicago/Turabian StyleRhim, Hye Chang, Joseph Ruiz, Atta Taseh, Wilma Afunugo, Zack Crockett, Jason Schon, Xiaoyu Pan, Jaehyung Shin, Sean Schowalter, Ki-Mo Jang, and et al. 2024. "Nonsteroidal Anti-Inflammatory Drug Injections versus Steroid Injections in the Management of Upper and Lower Extremity Orthopedic Conditions: A Systematic Review with Meta-Analysis" Journal of Clinical Medicine 13, no. 4: 1132. https://doi.org/10.3390/jcm13041132
APA StyleRhim, H. C., Ruiz, J., Taseh, A., Afunugo, W., Crockett, Z., Schon, J., Pan, X., Shin, J., Schowalter, S., Jang, K.-M., & Robinson, D. M. (2024). Nonsteroidal Anti-Inflammatory Drug Injections versus Steroid Injections in the Management of Upper and Lower Extremity Orthopedic Conditions: A Systematic Review with Meta-Analysis. Journal of Clinical Medicine, 13(4), 1132. https://doi.org/10.3390/jcm13041132







