Long-Term Follow-Up of Custom-Made Porous Hydroxyapatite Cranioplasties: Analysis of Infections in Adult and Pediatric Patients
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. PMCF Database Description
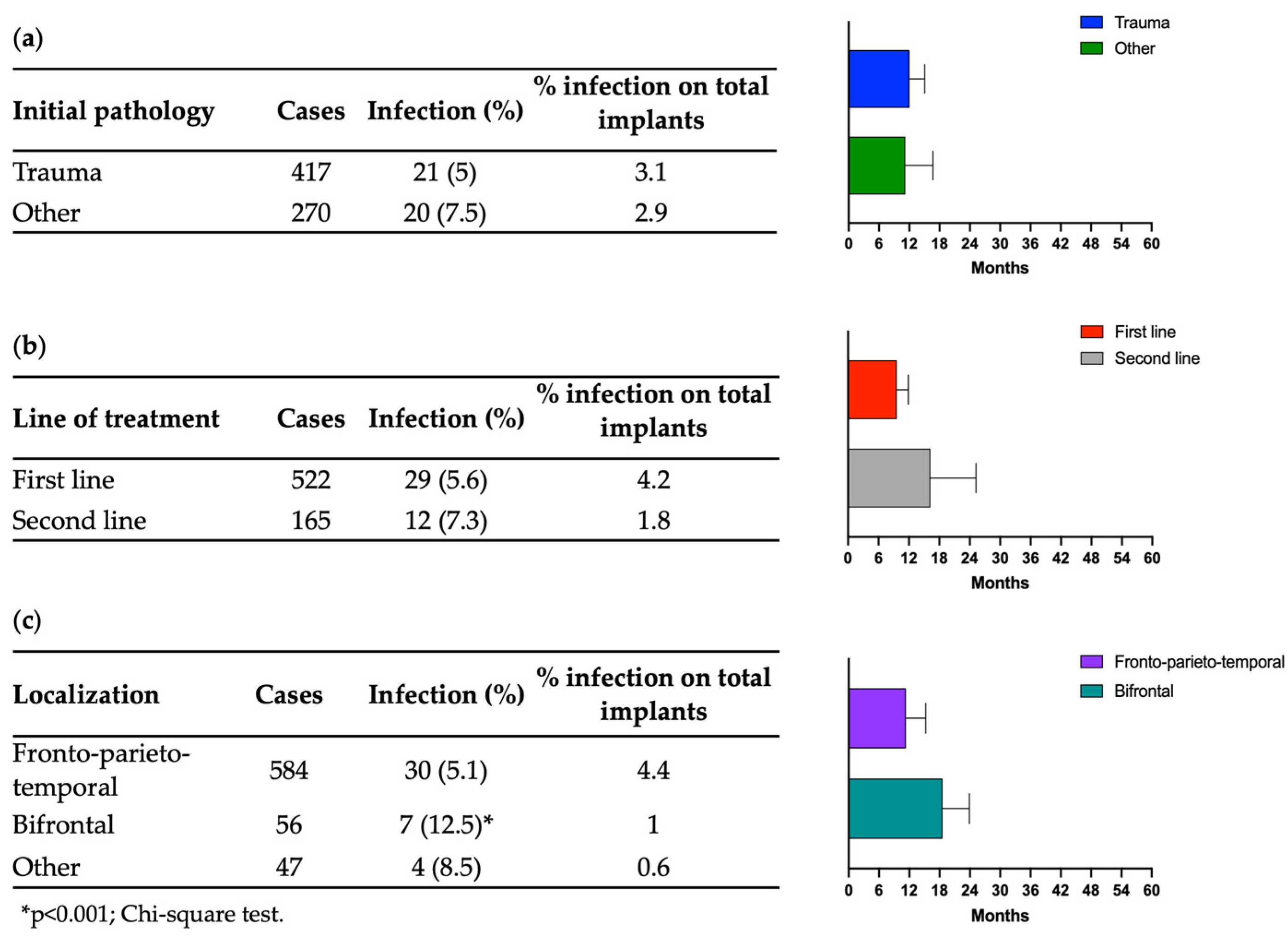
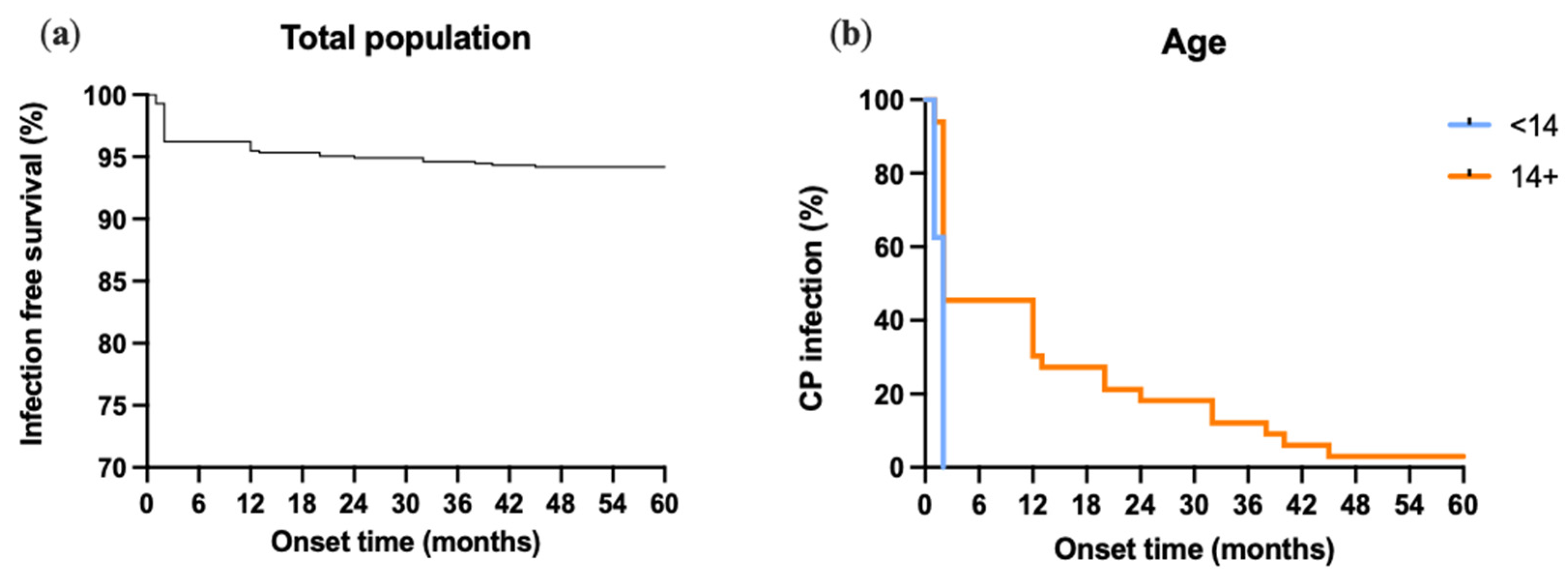
3.2. Infections
4. Discussion
4.1. Practical Recommandations
- PHA cranioplasty needs osteointegration to become strong enough to avoid cranial fractures in the case of a second trauma. Implantation in children and non-collaborating adults should be accompanied with patients keeping protective helmets on for at least for six months.
- In selected cases of post-operative infections, a course of medical therapy can avoid the explantation of the prothesis.
- As for other heterologous materials, bifrontal cranial reconstruction carries an increased risk of post-operative infections and should be avoided whenever possible.
4.2. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shepetovsky, D.; Mezzini, G.; Magrassi, L. Complications of cranioplasty in relationship to traumatic brain injury: A systematic review and meta-analysis. Neurosurg. Rev. 2021, 44, 3125–3142. [Google Scholar] [CrossRef]
- Malcolm, J.G.; Rindler, R.S.; Chu, J.K.; Grossberg, J.A.; Pradilla, G.; Ahmad, F.U. Complications following cranioplasty and relationship to timing: A systematic review and meta-analysis. J. Clin. Neurosci. 2016, 33, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Paredes, I.; Lagares, A.; San-Juan, R.; Castaño-León, A.M.; Gómez, P.A.; Jimenez-Roldán, L.; Panero, I.; Eiriz, C.; García-Perez, D.; Moreno, L.M.; et al. Reduction in the infection rate of cranioplasty with a tailored antibiotic prophylaxis: A nonrandomized study. Acta Neurochir. 2020, 162, 2857–2866. [Google Scholar] [CrossRef]
- Honeybul, S.; Ho, K.M. Cranioplasty: Morbidity and failure. Br. J. Neurosurg. 2016, 30, 523–528. [Google Scholar] [CrossRef]
- Im, S.H.; Jang, D.K.; Han, Y.M.; Kim, J.T.; Chung, D.S.; Park, Y.S. Long-term incidence and predicting factors of cranioplasty infection after decompressive craniectomy. J. Korean Neurosurg. Soc. 2012, 52, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Rosseto, R.S.; Giannetti, A.V.; de Souza Filho, L.D.; Faleiro, R.M. Risk Factors for Graft Infection after Cranioplasty in Patients with Large Hemicranial Bony Defects. World Neurosurg. 2015, 84, 431–437. [Google Scholar] [CrossRef]
- Kim, J.S.; Park, I.S.; Kim, S.K.; Park, H.; Kang, D.H.; Lee, C.H.; Hwang, S.H.; Jung, J.M.; Han, J.W. Analysis of the Risk Factors Affecting the Surgical Site Infection after Cranioplasty Following Decompressive Craniectomy. Korean J. Neurotrauma 2015, 11, 100–105. [Google Scholar] [CrossRef]
- Lee, C.H.; Chung, Y.S.; Lee, S.H.; Yang, H.J.; Son, Y.J. Analysis of the factors influencing bone graft infection after cranioplasty. J. Trauma Acute Care Surg. 2012, 73, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Alkhaibary, A.; Alharbi, A.; Abbas, M.; Algarni, A.; Abdullah, J.M.; Almadani, W.H.; Khairy, I.; Alkhani, A.; Aloraidi, A.; Khairy, S. Predictors of Surgical Site Infection in Autologous Cranioplasty: A Retrospective Analysis of Subcutaneously Preserved Bone Flaps in Abdominal Pockets. World Neurosurg. 2020, 133, e627–e632. [Google Scholar] [CrossRef]
- Mustroph, C.M.; Malcolm, J.G.; Rindler, R.S.; Chu, J.K.; Grossberg, J.A.; Pradilla, G.; Ahmad, F.U. Cranioplasty Infection and Resorption Are Associated with the Presence of a Ventriculoperitoneal Shunt: A Systematic Review and Meta-Analysis. World Neurosurg. 2017, 103, 686–693. [Google Scholar] [CrossRef]
- Zanotti, B.; Zingaretti, N.; Verlicchi, A.; Robiony, M.; Alfieri, A.; Parodi, P.C. Cranioplasty: Review of Materials. J. Craniofac. Surg. 2016, 27, 2061–2072. [Google Scholar] [CrossRef]
- Malcolm, J.G.; Mahmooth, Z.; Rindler, R.S.; Allen, J.W.; Grossberg, J.A.; Pradilla, G.; Ahmad, F.U. Autologous Cranioplasty is Associated with Increased Reoperation Rate: A Systematic Review and Meta-Analysis. World Neurosurg. 2018, 116, 60–68. [Google Scholar] [CrossRef]
- Do, T.H.; Lu, J.; Palzer, E.F.; Cramer, S.W.; Huling, J.D.; Johnson, R.A.; Zhu, P.; Jean, J.N.; Howard, M.A.; Sabal, L.T.; et al. Rates of operative intervention for infection after synthetic or autologous cranioplasty: A National Readmissions Database analysis. J. Neurosurg. 2022, 138, 514–521. [Google Scholar] [CrossRef]
- Pfnür, A.; Tosin, D.; Petkov, M.; Sharon, O.; Mayer, B.; Wirtz, C.R.; Knoll, A.; Pala, A. Exploring complications following cranioplasty after decompressive hemicraniectomy: A retrospective bicenter assessment of autologous, PMMA and CAD implants. Neurosurg. Rev. 2024, 47, 72. [Google Scholar] [CrossRef]
- Maenhoudt, W.; Hallaert, G.; Kalala, J.P.; Baert, E.; Dewaele, F.; Bauters, W.; Van Roost, D. Hydroxyapatite cranioplasty: A retrospective evaluation of osteointegration in 17 cases. Acta Neurochir. 2018, 160, 2117–2124. [Google Scholar] [CrossRef]
- Stefini, R.; Zanotti, B.; Nataloni, A.; Martinetti, R.; Scafuto, M.; Colasurdo, M.; Tampieri, A. The Efficacy of Custom-Made Porous Hydroxyapatite Prostheses for Cranioplasty: Evaluation of Postmarketing Data on 2697 Patients. J. Appl. Biomater. Funct. Mater. 2015, 13, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Millward, C.P.; Doherty, J.A.; Mustafa, M.A.; Humphries, T.J.; Islim, A.I.; Richardson, G.E.; Clynch, A.L.; Gillespie, C.S.; Keshwara, S.M.; Kolamunnage-Dona, R.; et al. Cranioplasty with hydroxyapatite or acrylic is associated with a reduced risk of all-cause and infection-associated explantation. Br. J. Neurosurg. 2022, 36, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Lindner, D.; Schlothofer-Schumann, K.; Kern, B.C.; Marx, O.; Müns, A.; Meixensberger, J. Cranioplasty using custom-made hydroxyapatite versus titanium: A randomized clinical trial. J. Neurosurg. 2017, 126, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.M.P.; Amorim, R.L.O.; Brasil, S.; Gattás, G.S.; de Andrade, A.F.; Junior, F.M.P.; Bor-Seng-Shu, E.; Iaccarino, C.; Teixeira, M.J.; Paiva, W.S. Improvement in neurological outcome and brain hemodynamics after late cranioplasty. Acta Neurochir. 2021, 163, 2931–2939. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, F.; Giordano, M.; Caccavella, V.M.; Ioannoni, E.; Gelormini, C.; Caricato, A.; Olivi, A.; Montano, N. A systematic review and meta-analysis of factors involved in bone flap resorption after decompressive craniectomy. Neurosurg. Rev. 2022, 45, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Servadei, F.; Iaccarino, C. The therapeutic cranioplasty still needs an ideal material and surgical timing. World Neurosurg. 2015, 83, 133–135. [Google Scholar] [CrossRef]
- Rae, A.I.; O’Neill, B.E.; Godil, J.; Fecker, A.L.; Ross, D. Low-Cost Wound Healing Protocol Reduces Infection and Reoperation Rates after Cranioplasty: A Retrospective Cohort Study. Neurosurgery 2023, 93, 1220–1227. [Google Scholar] [CrossRef]
- Sauvigny, T.; Giese, H.; Höhne, J.; Schebesch, K.M.; Henker, C.; Strauss, A.; Beseoglu, K.; Spreckelsen, N.V.; Hampl, J.A.; Walter, J.; et al. A multicenter cohort study of early complications after cranioplasty: Results of the German Cranial Reconstruction Registry. J. Neurosurg. 2021, 137, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Riordan, M.A.; Simpson, V.M.; Hall, W.A. Analysis of Factors Contributing to Infections After Cranioplasty: A Single-Institution Retrospective Chart Review. World Neurosurg. 2016, 87, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Giese, H.; Antritter, J.; Unterberg, A.; Beynon, C. Long-Term Results of Neurological Outcome, Quality of Life, and Cosmetic Outcome After Cranioplastic Surgery: A Single Center Study of 202 Patients. Front. Neurol. 2021, 12, 702339. [Google Scholar] [CrossRef] [PubMed]
- Fricia, M.; Nicolosi, F.; Ganau, M.; Cebula, H.; Todeschi, J.; Santin, M.D.N.; Nannavecchia, B.; Morselli, C.; Chibbaro, S. Cranioplasty with Porous Hydroxyapatite Custom-Made Bone Flap: Results from a Multicenter Study Enrolling 149 Patients Over 15 Years. World Neurosurg. 2019, 121, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Frassanito, P.; Massimi, L.; Tamburrini, G.; Bianchi, F.; Nataloni, A.; Canella, B.; Caldarelli, M. Customed hydroxyapatite for cranial repair in a specific pediatric age group (7 tp 13 years old): A multicenter post marketing surveillance study. Child’s Nerv. Syst. 2018, 34, 2283–2289. [Google Scholar] [CrossRef] [PubMed]
- Amelot, A.; Nataloni, A.; François, P.; Cook, A.R.; Lejeune, J.P.; Baroncini, M.; Hénaux, P.L.; Toussaint, P.; Peltier, J.; Buffenoir, K.; et al. Security and reliability of CUSTOMBONE cranioplasties: A prospective multicentric study. Neurochirurgie 2021, 67, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Yeap, M.C.; Tu, P.H.; Liu, Z.H.; Hsieh, P.C.; Liu, Y.T.; Lee, C.Y.; Lai, H.Y.; Chen, C.T.; Huang, Y.C.; Wei, K.C.; et al. Long-Term Complications of Cranioplasty Using Stored Autologous Bone Graft, Three-Dimensional Polymethyl Methacrylate, or Titanium Mesh after Decompressive Craniectomy: A Single-Center Experience after 596 Procedures. World Neurosurg. 2019, 128, e841–e850. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.; Kim, J.; Kim, J.H.; Chong, K.; Yoon, W.K.; Kwon, T.H.; Kim, J.H. Analysis of Complications after Cranioplasty with a Customized Three-Dimensional Titanium Mesh Plate. World Neurosurg. 2019, 123, e39–e44. [Google Scholar] [CrossRef]
- Schön, S.N.; Skalicky, N.; Sharma, N.; Zumofen, D.W.; Thieringer, F.M. 3D-Printer-Assisted Patient-Specific Polymethyl Methacrylate Cranioplasty: A Case Series of 16 Consecutive Patients. World Neurosurg. 2021, 148, e356–e362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yuan, Y.; Li, X.; Sun, T.; Zhou, Y.; Yu, H.; Guan, J. A Large Multicenter Retrospective Research on Embedded Cranioplasty and Covered Cranioplasty. World Neurosurg. 2018, 112, e645–e651. [Google Scholar] [CrossRef] [PubMed]
- Rosinski, C.L.; Patel, S.; Geever, B.; Chiu, R.G.; Chaker, A.N.; Zakrzewski, J.; Rosenberg, D.M.; Parola, R.; Shah, K.; Behbahani, M.; et al. A Retrospective Comparative Analysis of Titanium Mesh and Custom Implants for Cranioplasty. Neurosurgery 2020, 86, E15–E22. [Google Scholar] [CrossRef] [PubMed]
- Di Rienzo, A.; Colasanti, R.; Dobran, M.; Formica, F.; Della Costanza, M.; Carrassi, E.; Aiudi, D.; Iacoangeli, M. Management of infected hydroxyapatite cranioplasty: Is salvage feasible? Brain Spine 2022, 2, 100907. [Google Scholar] [CrossRef]
- Zaed, I.; Faedo, F.; Chibbaro, S.; Cannizzaro, D.; Tomei, M.; Servadei, F.; Cardia, A. Prevalence of Postoperative Complications of Autologous and Heterologous Cranioplasty in the Pediatric Population: A Systematic Review of the Literature. Pediatr. Neurosurg. 2022, 57, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Rossini, Z.; Franzini, A.; Zaed, I.; Zingaretti, N.; Nicolosi, F.; Zanotti, B. Custom-Made Porous Hydroxyapatite Cranioplasty in Patients with Tumor Versus Traumatic Brain Injury: A Single-Center Case Series. World Neurosurg. 2020, 138, e922–e929. [Google Scholar] [CrossRef]
- Gooch, M.R.; Gin, G.E.; Kenning, T.J.; German, J.W. Complications of cranioplasty following decompressive craniectomy: Analysis of 62 cases. Neurosurg. Focus 2009, 26, E9. [Google Scholar] [CrossRef]
- Mukherjee, S.; Thakur, B.; Haq, I.; Hettige, S.; Martin, A.J. Complications of titanium cranioplasty--a retrospective analysis of 174 patients. Acta Neurochir. 2014, 156, 989–998, discussion 998. [Google Scholar] [CrossRef]
- Marchac, D.; Greensmith, A. Long-term experience with methylmethacrylate cranioplasty in craniofacial surgery. J. Plast. Reconstr. Aesthetic Surg. 2008, 61, 744–752, discussion 753. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.J.; Rosenfeld, J.V.; Murray, L.; Arabi, Y.M.; Davies, A.R.; D’Urso, P.; Kossmann, T.; Ponsford, J.; Seppelt, I.; Reilly, P.; et al. Decompressive craniectomy in diffuse traumatic brain injury. N. Engl. J. Med. 2011, 364, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, P.J.; Kolias, A.G.; Timofeev, I.S.; Corteen, E.A.; Czosnyka, M.; Timothy, J.; Anderson, I.; Bulters, D.O.; Belli, A.; Eynon, C.A.; et al. Trial of Decompressive Craniectomy for Traumatic Intracranial Hypertension. N. Engl. J. Med. 2016, 375, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, P.J.; Adams, H.; Mohan, M.; Devi, B.I.; Uff, C.; Hasan, S.; Mee, H.; Wilson, M.H.; Gupta, D.K.; Bulters, D.; et al. Decompressive Craniectomy versus Craniotomy for Acute Subdural Hematoma. N. Engl. J. Med. 2023, 388, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
| Number | % | |
|---|---|---|
| Number of patients | 687 | 100 |
| Gender | ||
| Male | 461 | 67.1 |
| Female | 226 | 32.9 |
| Age | ||
| Mean age | 37.2 | |
| Pediatric (2–13 years) | 80 | 11.6 |
| Male | 50 | 62.5 |
| Female | 30 | 37.5 |
| Adult (14+ years) | 607 | 88.4 |
| Male | 411 | 67.7 |
| Female | 196 | 32.3 |
| Initial pathology | ||
| Trauma | 417 | 60.7 |
| Vascular disease | 118 | 17.2 |
| Tumor | 118 | 17.2 |
| Malformation | 20 | 2.9 |
| Other | 14 | 2 |
| Line of treatment | ||
| First line | 522 | 76 |
| Second line | 165 | 24 |
| Localization | ||
| Fronto-parieto-temporal | 584 | 85 |
| Bifrontal | 56 | 8.2 |
| Other | 47 | 6.8 |
| Complications (n = 80) | Number | % |
|---|---|---|
| Global infection | 41 | 6 |
| Pediatric (2–13 years) | 8 * | 10 |
| Adult (14+ years) | 33 * | 5.4 |
| Global fracture | 17 | 2.5 |
| Pediatric (2–13 years) | 12 * | 15 |
| Adult (14+ years) | 5 * | 0.8 |
| Global displacement | 7 | 1 |
| Pediatric (2–13 years) | 3 | 3.8 |
| Adult (14+ years) | 4 | 0.7 |
| Other | 15 | 2.2 |
| Pediatric (2–13 years) | - | - |
| Adult (14+ years) | 15 | 2.5 |
| Early Infections (n = 26; 3.8%) | Late Infections (n = 15; 2.2%) | |||
|---|---|---|---|---|
| Cases (%) | Explant (%) | Cases (%) | Explant (%) | |
| Initial pathology | ||||
| Trauma | 12 (1.8) | 8 (1.2) | 9 (1.3) | 7 (1) |
| Vascular disease | 9 (1.3) | 7 (1) | 2 (0.3) | 1 (0.2) |
| Tumor | 3 (0.4) | 3 (0.4) | 2 (0.3) | 1 (0.2) |
| Malformation | 2 (0.3) | 2 (0.3) | - | - |
| Other | - | - | 2 (0.3) | 1 (0.2) |
| Line of treatment | ||||
| First-line | 18 (2.6) | 13 (1.9) | 11 (1.6) | 7 (1) |
| Second-line | 8 (1.2) | 7 (1) | 4 (0.6) | 3 (0.4) |
| Localization | ||||
| Fronto-parieto-temporal | 20 (2.9) | 15 (2.2) | 10 (1.5) | 8 (1.2) |
| Bifrontal | 2 (0.3) | 1 (0.2) | 5 (0.7) | 2 (0.3) |
| Other | 4 (0.6) | 4 (0.6) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannella, F.C.; Faedo, F.; Fumagalli, M.; Norata, G.D.; Zaed, I.; Servadei, F. Long-Term Follow-Up of Custom-Made Porous Hydroxyapatite Cranioplasties: Analysis of Infections in Adult and Pediatric Patients. J. Clin. Med. 2024, 13, 1133. https://doi.org/10.3390/jcm13041133
Mannella FC, Faedo F, Fumagalli M, Norata GD, Zaed I, Servadei F. Long-Term Follow-Up of Custom-Made Porous Hydroxyapatite Cranioplasties: Analysis of Infections in Adult and Pediatric Patients. Journal of Clinical Medicine. 2024; 13(4):1133. https://doi.org/10.3390/jcm13041133
Chicago/Turabian StyleMannella, Francesca Carolina, Francesca Faedo, Marta Fumagalli, Giuseppe Danilo Norata, Ismail Zaed, and Franco Servadei. 2024. "Long-Term Follow-Up of Custom-Made Porous Hydroxyapatite Cranioplasties: Analysis of Infections in Adult and Pediatric Patients" Journal of Clinical Medicine 13, no. 4: 1133. https://doi.org/10.3390/jcm13041133
APA StyleMannella, F. C., Faedo, F., Fumagalli, M., Norata, G. D., Zaed, I., & Servadei, F. (2024). Long-Term Follow-Up of Custom-Made Porous Hydroxyapatite Cranioplasties: Analysis of Infections in Adult and Pediatric Patients. Journal of Clinical Medicine, 13(4), 1133. https://doi.org/10.3390/jcm13041133









