Immediate Postoperative COVID-19 Infection after Lung Transplantation: A Systematic Review and Case Series
Abstract
:1. Introduction
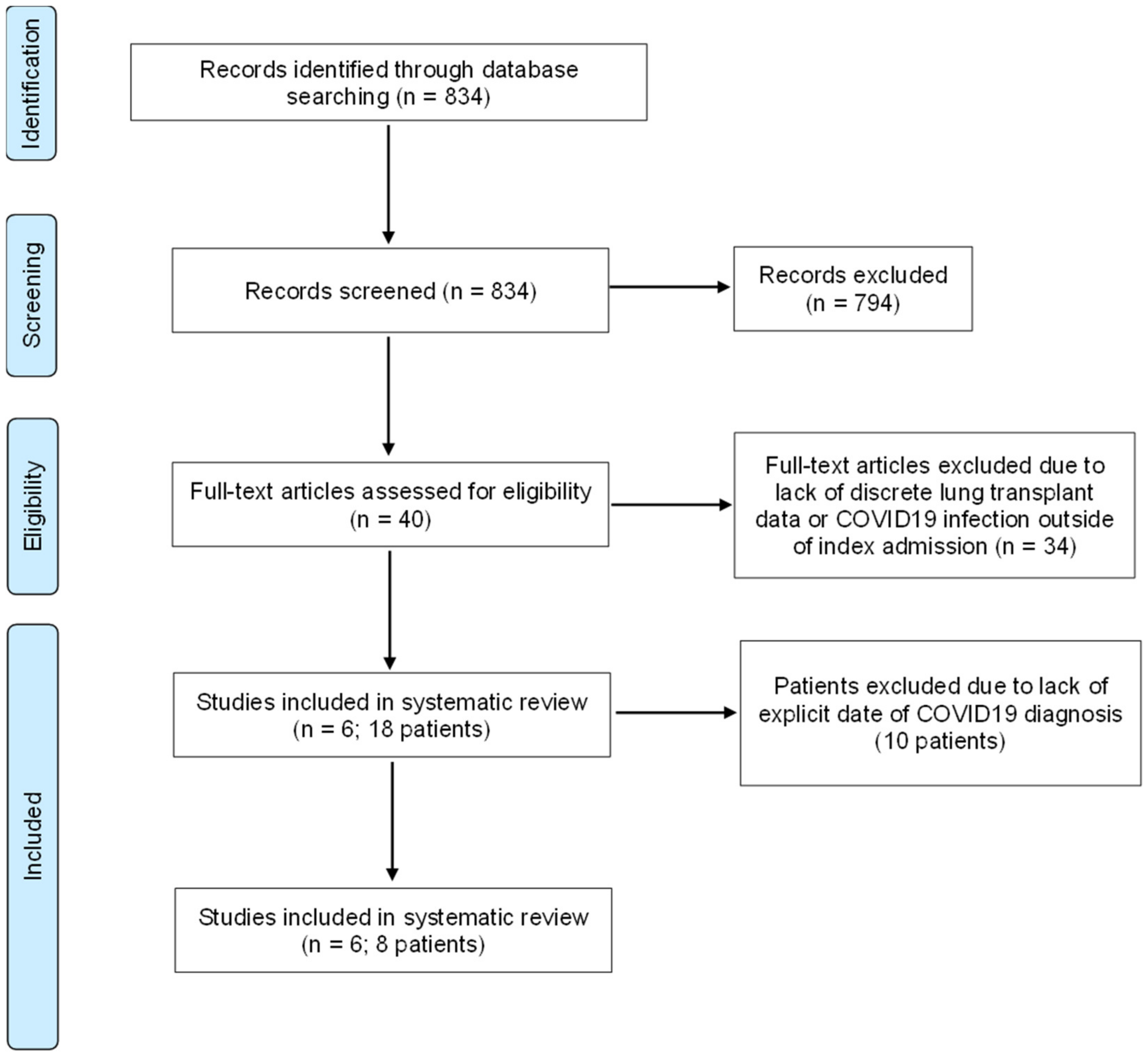
2. Materials and Methods
3. Results
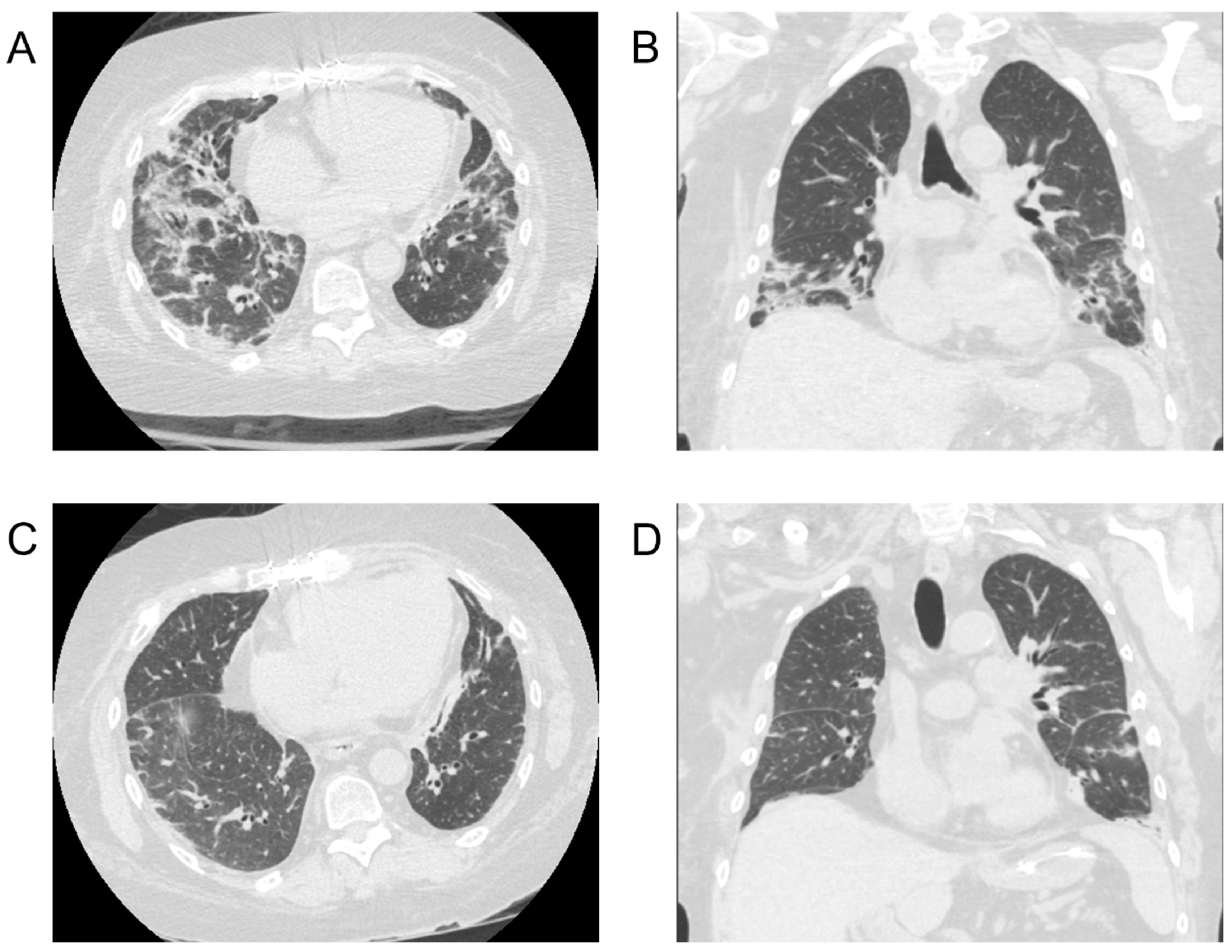
3.1. Case Series
3.2. Systematic Review
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zimmermann, J.; Glueck, O.M.; Fertmann, J.M.; Sienel, W.G.; Yavuz, G.; Damirov, F.; Kovács, J.R.; Tufman, A.; Irlbeck, M.; Kneidinger, N.; et al. COVID-19 in Recent Lung Transplant Recipients: Clinical Outcomes and Management Strategies. Transplant. Proc. 2022, 54, 1504–1516. [Google Scholar] [CrossRef] [PubMed]
- Myers, C.N.; Scott, J.H.; Criner, G.J.; Cordova, F.C.; Mamary, A.J.; Marchetti, N.; Shenoy, K.V.; Galli, J.A.; Mulhall, P.D.; Brown, J.C.; et al. COVID-19 in lung transplant recipients. Transpl. Infect. Dis. 2020, 22, e13364. [Google Scholar] [CrossRef] [PubMed]
- Keller, B.C.; Le, A.; Sobhanie, M.; Colburn, N.; Burcham, P.; Rosenheck, J.; Howsare, M.; Ganapathi, A.M.; Atyia, S.A.; Haden, M.; et al. Early COVID-19 infection after lung transplantation. Am. J. Transplant. 2020, 20, 2923–2927. [Google Scholar] [CrossRef] [PubMed]
- Faccioli, E.; Schiavon, M.; Pezzuto, F.; Dell’Amore, A.; Biondini, D.; Marinello, S.; Persona, P.; Vadori, M.; Loy, M.; Cattelan, A.; et al. A Case of Prolonged Hospital Acquired COVID-19 Pneumonia in a Lung Transplant Recipient: Management and Outcome. J. Heart Lung Transplant. 2022, 41, S375. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Terada, Y.; Nava, R.G.; Puri, V.; Kreisel, D.; Patterson, G.A.; Hachem, R.R.; Takahashi, T. Coronavirus Disease 2019 Positivity Immediately after Lung Transplantation: A Case Report. Transplant Proc. 2022, 54, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Athanazio, R.A.; Costa, A.N.; Carraro, R.M.; Gonzalez, D.; Rached, S.Z.; Samano, M.N.; Teixeira, R.; Campos, S.V. Early COVID-19 infection after lung transplantation in a patient with cystic fibrosis. Clinics 2020, 75, e2274. [Google Scholar] [CrossRef] [PubMed]
- Nhean, S.; Varela, M.E.; Nguyen, Y.N.; Puri, V.; Kreisel, D.; Patterson, G.A.; Hachem, R.R.; Takahashi, T. COVID-19: A Review of Potential Treatments (Corticosteroids, Remdesivir, Tocilizumab, Bamlanivimab/Etesevimab, and Casirivimab/Imdevimab) and Pharmacological Considerations. J. Pharm. Pract. 2023, 36, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Dougan, M.; Nirula, A.; Azizad, M.; Juarez, A.; Huynh, T.; Udeh, D.; Tseng, A.L. Bamlanivimab plus Etesevimab in Mild or Moderate COVID-19. N. Engl. J. Med. 2021, 385, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, R.L.; Nirula, A.; Chen, P.; Mocherla, B.; Gottlieb, R.L.; Chen, P.; Hebert, C.; Perry, R.; Boscia, J.; Heller, B.; et al. Effect of Bamlanivimab as Monotherapy or in Combination with Etesevimab on Viral Load in Patients with Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Group WHOREAfC-TW; Sterne, J.A.C.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O.; et al. Association Between Administration of Systemic Corticosteroids and Mortality among Critically Ill Patients with COVID-19: A Meta-analysis. JAMA 2020, 324, 1330–1341. [Google Scholar] [PubMed]
- Li, L.Q.; Huang, T.; Wang, Y.Q.; Wang, Z.P.; Liang, Y.; Huang, T.B.; Zhang, H.Y.; Sun, W.; Wang, Y. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J. Med. Virol. 2020, 92, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Verleden, G.M.; Godinas, L.; Lorent, N.; Van Bleyenbergh, P.; Dupont, L.; Delcroix, M.; Yserbyt, J.; Dooms, C.; Vos, R. COVID-19 in lung transplant patients: A case series. Am. J. Transplant. 2020, 20, 3234–3238. [Google Scholar] [CrossRef] [PubMed]
- Colmenero, J.; Rodriguez-Peralvarez, M.; Salcedo, M.; Arias-Milla, A.; Muñoz-Serrano, A.; Graus, J.; Nuño, J.; Gastaca, M.; Bustamante-Schneider, J.; Cachero, A.; et al. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J. Hepatol. 2021, 74, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Nie, J.; Wang, H.; Zhao, Q.; Xiong, Y.; Deng, L.; Song, S.; Ma, Z.; Mo, P.; Zhang, Y. Characteristics of Peripheral Lymphocyte Subset Alteration in COVID-19 Pneumonia. J. Infect. Dis. 2020, 221, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Waisberg, D.R.; Abdala, E.; Nacif, L.S.; Ducatti, L.; Haddad, L.B.; Martino, R.B.; Pinheiro, R.S.; Arantes, R.M.; Galvao, F.H.; Gouveia, L.N.; et al. Coronavirus Disease 2019 in the Early Postoperative Period of Liver Transplantation: Is the Outcome Really So Positive? Liver Transpl. 2021, 27, 1357–1359. [Google Scholar] [CrossRef] [PubMed]
- Babidge, W.J.; Tivey, D.R.; Kovoor, J.G.; Weidenbach, K.; Collinson, T.G.; Hewett, P.J.; Hugh, T.J.; Padbury, R.T.A.; Hill, N.M.; Maddern, G.J. Surgery triage during the COVID-19 pandemic. ANZ J. Surg. 2020, 90, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Doglietto, F.; Vezzoli, M.; Gheza, F.; Lussardi, G.L.; Domenicucci, M.; Vecchiarelli, L.; Zanin, L.; Saraceno, G.; Signorini, L.; Panciani, P.P.; et al. Factors Associated with Surgical Mortality and Complications Among Patients with and Without Coronavirus Disease 2019 (COVID-19) in Italy. JAMA Surg. 2020, 155, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Wong, S.Y.; Chai, L.Y.A.; Lee, S.C.; Lee, M.X.; Muthiah, M.D.; Tay, S.H.; Teo, C.B.; Tan, B.K.J.; Chan, Y.H.; et al. Efficacy of COVID-19 vaccines in immunocompromised patients: Systematic review and meta-analysis. BMJ 2022, 376, e068632. [Google Scholar] [CrossRef] [PubMed]
- Leitao, I.C.; Calil, P.T.; Galliez, R.M.; Moreira, F.R.R.; Mariani, D.; Castineiras, A.C.P.; da Silva, G.P.D.; Maia, R.A.; Correa, I.A.; Monteiro, F.L.L.; et al. Prolonged SARS-CoV-2 Positivity in Immunocompetent Patients: Virus Isolation, Genomic Integrity, and Transmission Risk. Microbiol. Spectr. 2021, 9, e0085521. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Number of Patients | Age (Range) | Sex (nF, nM) | Transplant Indication | Time to Diagnosis (Days) | Comorbidities | Presenting Symptoms | COVID-19 Diagnostic Methods | Imaging Modality | Imaging Findings | LOS, Days | Survived Infection, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yokoyama et al. (2022) [5] | 1 | 71 | (0, 1) | HP | 1 | None | Cough, Dyspnea, Fatigue | Nasopharyngeal Swab, Bronchoalveolar Lavage, Saliva | CXR | None | 14 | 1/1 (100) |
| Faccioli et al. (2022) [4] | 1 | 52 | (1, 0) | IPF | 12 | NR | Cough | Nasopharyngeal Swab | CT | Consolidation | NR | 1/1 (100) |
| Zimmermann et al. (2022) [1] | 6 | 60 (38–69) | (4, 2) | IPF (3), IPAF (1), HP (1), Sarcoidosis (1) | NR (4), 18, 22 | HF (3), PHTN (3), HTN (2), PVD (1), CHD (1), Afib (1) | Fatigue (6), Dyspnea (5), Cough (5), Diarrhea (4), Fever (3), Asthenia (3), Nausea (2), Expectoration (2), Headache (2), Myalgia (1), Abdominal Pain (1) | Nasopharyngeal and Oropharyngeal Swab | CT | GGO (6), Consolidation (4), Intersitial Abnormalities (4), Pleural Effusion (3) | 50 (25–122) | 4/4 (100), 1/2 * (50) |
| Myers et al. (2020) [2] | 8 | 60.8 (43–75) | (1, 7) | IPF (5), COPD (2), CF (1) | NR (6), 7, 14 | Prior Smoker (7), Afib (3), CKD (3), DM2 (3), HL (3), DVT (2), HTN (2), CHF (1), CAD (1), OSA (1) | Cough (6), Dyspnea (6), Fever (4), Nausea (3), Fatigue (3) | NR | CT | GGO, Consolidation | 8.6 (2–16) | 6/6 (100), 0/2 * (0) |
| Athanazio et al. (2020) [6] | 1 | 37 | (0, 1) | CF | 13 | None | Dyspnea | Nasopharyngeal Swab | CT | GGO, Consolidation, Pleural Effusion | 39 | 1/1 (100) |
| Keller et al. (2020) [3] | 1 | 69 | (1, 0) | IPF | 8 | GERD, HL, Psoriasis | NR | Nasopharyngeal Swab | CXR, CT | GGO, Consolidation | NR | 1/1 (100) |
| Changes in Immunosuppression | n (%) |
|---|---|
| Decrease or Hold Antimetabolite | 6 (75) |
| Decrease or Hold CNI | 2 (25) |
| Decrease or Hold Steroids | 2 (25) |
| Antibiotic/Antiviral Treatment of Choice | |
| Monoclonal Antibody | 3 (38) |
| Hydroxychloroquine | 1 (13) |
| Remdesivir | 5 (63) |
| Oseltamivir | 0 (0) |
| Zosyn | 1 (13) |
| Azithromycin | 1 (13) |
| Meropenem | 1 (13) |
| None | 1 (13) |
| O2 Support | |
| Mechanical Ventilation | 4 (50) |
| Mechanical Ventilation + Prone Positioning | 1 (13) |
| ECMO | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donohue, J.K.; Hyzny, E.J.; Clifford, S.; Chan, E.G.; Coster, J.N.; Furukawa, M.; Sanchez, P.G. Immediate Postoperative COVID-19 Infection after Lung Transplantation: A Systematic Review and Case Series. J. Clin. Med. 2023, 12, 7028. https://doi.org/10.3390/jcm12227028
Donohue JK, Hyzny EJ, Clifford S, Chan EG, Coster JN, Furukawa M, Sanchez PG. Immediate Postoperative COVID-19 Infection after Lung Transplantation: A Systematic Review and Case Series. Journal of Clinical Medicine. 2023; 12(22):7028. https://doi.org/10.3390/jcm12227028
Chicago/Turabian StyleDonohue, Jack K., Eric J. Hyzny, Sarah Clifford, Ernest G. Chan, Jenalee Nicole Coster, Masashi Furukawa, and Pablo G. Sanchez. 2023. "Immediate Postoperative COVID-19 Infection after Lung Transplantation: A Systematic Review and Case Series" Journal of Clinical Medicine 12, no. 22: 7028. https://doi.org/10.3390/jcm12227028
APA StyleDonohue, J. K., Hyzny, E. J., Clifford, S., Chan, E. G., Coster, J. N., Furukawa, M., & Sanchez, P. G. (2023). Immediate Postoperative COVID-19 Infection after Lung Transplantation: A Systematic Review and Case Series. Journal of Clinical Medicine, 12(22), 7028. https://doi.org/10.3390/jcm12227028







