Robotic First Rib Resection in Thoracic Outlet Syndrome: A Systematic Review of Current Literature
Abstract
1. Introduction
1.1. Diagnosis and Types of TOS
1.2. Neurogenic TOS (nTOS)
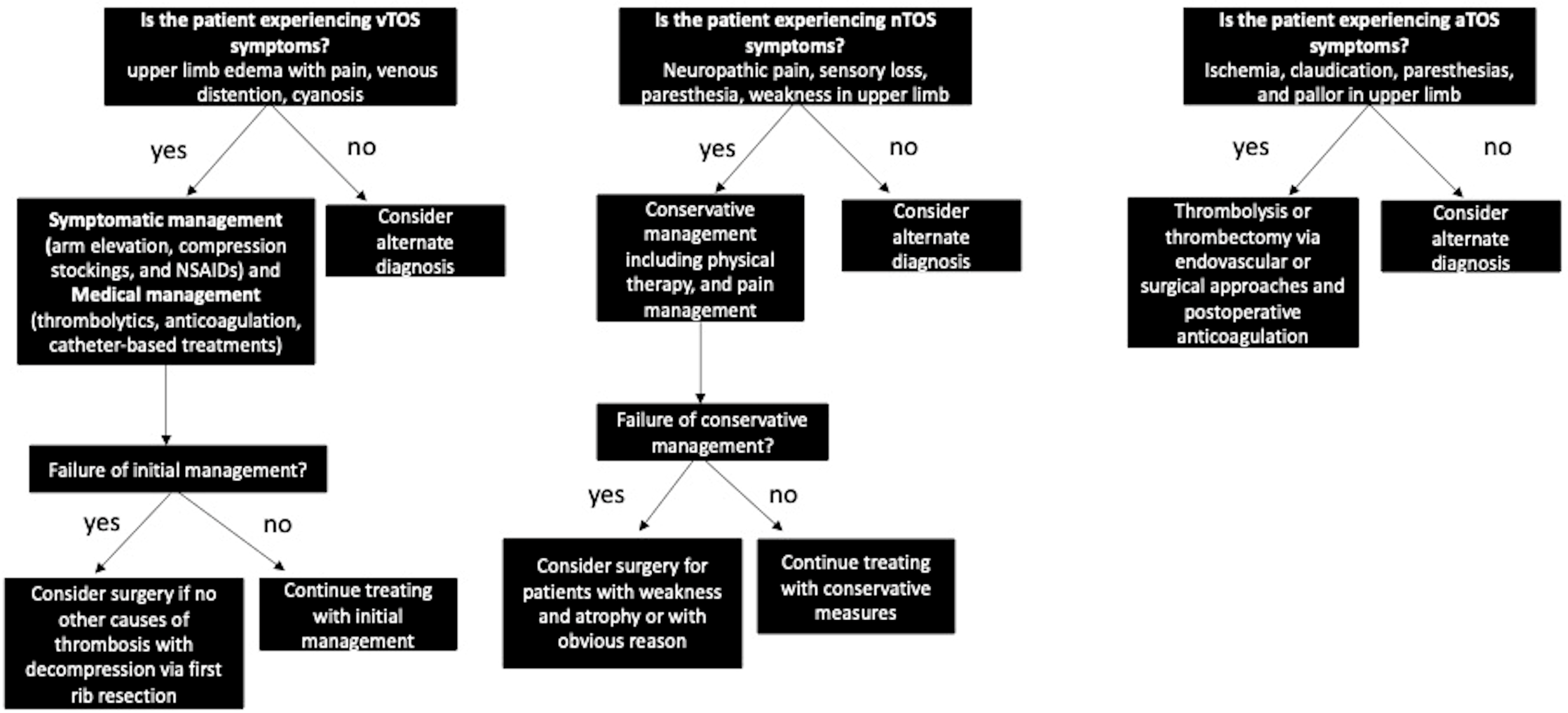
1.3. Venous TOS (vTOS)
1.4. Arterial TOS (aTOS)
1.5. Current Treatment and Management
1.6. Surgery for Treatment of TOS
2. Methods
2.1. Search Strategy
2.2. Study Selection
3. Results
3.1. Prevalence of Robotic-First Rib Resection for Different TOS Subtypes
3.2. Demographics Data: Age and Gender
3.3. Symptom Laterality and Postoperative Pain Scores
3.4. Intraoperative Outcomes after R-FRR
3.5. Operative Outcomes after TOS
4. Discussion
Evidence for R-FRR for the Treatment of TOS
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, M.R.; Prabhakar, A.; Viswanath, O.; Urits, I.; Green, J.B.; Kendrick, J.B.; Brunk, A.J.; Eng, M.R.; Orhurhu, V.; Cornett, E.M.; et al. Thoracic Outlet Syndrome: A Comprehensive Review of Pathophysiology, Diagnosis, and Treatment. Pain Ther. 2019, 8, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Pupovac, S.S.; Lee, P.C.; Zeltsman, D.; Jurado, J.; Hyman, K.; Singh, V. Singh: Robotic-Assisted First Rib Resection: Our Experience and Review of the Literature. Semin. Thorac. Cardiovasc. Surg. 2020, 32, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Nichols, A.W. Diagnosis and management of thoracic outlet syndrome. Curr. Sports Med. Rep. 2009, 8, 240–249. [Google Scholar] [CrossRef]
- Gharagozloo, F.; Meyer, M.; Tempesta, B.J.; Margolis, M.; Strother, E.T.; Tummala, S. Tummala: Robotic en bloc first-rib resection for Paget-Schroetter disease, a form of thoracic outlet syndrome: Technique and initial results. Innovations 2012, 7, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Kimura, H.; Matsumura, N.; Iwamoto, T. Surgical Approaches for Thoracic Outlet Syndrome: A Review of the Literature. J. Hand Surg. Glob. Online 2023, 5, 577–584. [Google Scholar] [CrossRef]
- Ferguson, T.B. The crisis of excellence. J. Thorac. Cardiovasc. Surg. 1982, 84, 161–171. [Google Scholar] [CrossRef]
- Burt, B.M.; Palivela, N.; Cekmecelioglu, D.; Paily, P.; Najafi, B.; Lee, H.-S.; Montero, M. Safety of robotic first rib resection for thoracic outlet syndrome. J. Thorac. Cardiovasc. Surg. 2021, 162, 1297–1305.e1. [Google Scholar] [CrossRef]
- Gharagozloo, F.; Atiquzzaman, N.; Meyer, M.; Tempesta, B.; Werden, S. Robotic first rib resection for thoracic outlet syndrome. J. Thorac. Dis. 2021, 13, 6141–6154. [Google Scholar] [CrossRef]
- Ferrante, M.A.; Ferrante, N.D. The thoracic outlet syndromes: Part 1. Overview of the thoracic outlet syndromes and review of true neurogenic thoracic outlet syndrome. Muscle Nerve 2017, 55, 782–793. [Google Scholar] [CrossRef]
- Ferrante, M.A.; Ferrante, N.D. The thoracic outlet syndromes: Part 2. The arterial, venous, neurovascular, and disputed thoracic outlet syndromes. Muscle Nerve 2017, 56, 663–673. [Google Scholar] [CrossRef]
- Freischlag, J.; Orion, K. Understanding thoracic outlet syndrome. Scientifica 2014, 2014, 248163. [Google Scholar] [CrossRef] [PubMed]
- Franklin, G.M. Work-Related Neurogenic Thoracic Outlet Syndrome: Diagnosis and Treatment. Phys. Med. Rehabil. Clin. N. Am. 2015, 26, 551–561. [Google Scholar] [CrossRef]
- Sanders, R.J.; Hammond, S.L.; Rao, N.M. Diagnosis of thoracic outlet syndrome. J. Vasc. Surg. 2007, 46, 601–604. [Google Scholar] [CrossRef]
- Dengler, N.F.; Pedro, M.T.; Kretschmer, T.; Heinen, C.; Rosahl, S.K.; Antoniadis, G. Neurogenic Thoracic Outlet Syndrome. Dtsch. Arztebl. Int. 2022, 119, 735–742. [Google Scholar] [CrossRef] [PubMed]
- George, E.L.; Arya, S.; Rothenberg, K.A.; Hernandez-Boussard, T.; Ho, V.-T.; Stern, J.R.; Gelabert, H.A.; Lee, J.T. Contemporary Practices and Complications of Surgery for Thoracic Outlet Syndrome in the United States. Ann. Vasc. Surg. 2021, 72, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Grunebach, H.; Arnold, M.W.; Lum, Y.W. Thoracic outlet syndrome. Vasc. Med. 2015, 20, 493–495. [Google Scholar] [CrossRef]
- Habibollahi, P.; Zhang, D.; Kolber, M.K.; Pillai, A.K. Venous thoracic outlet syndrome. Cardiovasc. Diagn. Ther. 2021, 11, 1150–1158. [Google Scholar] [CrossRef]
- Huang, J.; Lauer, J.; Zurkiya, O. Arterial thoracic outlet syndrome. Cardiovasc. Diagn. Ther. 2021, 11, 1118–1124. [Google Scholar] [CrossRef]
- Balderman, J.; Abuirqeba, A.A.; Eichaker, L.; Pate, C.; Earley, J.A.; Bottros, M.M.; Jayarajan, S.N.; Thompson, R.W. Physical therapy management, surgical treatment, and patient-reported outcomes measures in a prospective observational cohort of patients with neurogenic thoracic outlet syndrome. J. Vasc. Surg. 2019, 70, 832–841. [Google Scholar] [CrossRef]
- Carlon, T.A.; Sudheendra, D. Interventional Therapy for Upper Extremity Deep Vein Thrombosis. Semin. Interv. Radiol. 2017, 34, 54–60. [Google Scholar] [CrossRef][Green Version]
- Novak, C.B.; Collins, E.D.; Mackinnon, S.E. Outcome following conservative management of thoracic outlet syndrome. J. Hand Surg. Am. 1995, 20, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Peek, J.; Vos, C.G.; Ünlü, M.; Schreve, M.A.; Van de Mortel, R.H.W.; De Vries, J.-P.P.M. Long-Term Functional Outcome of Surgical Treatment for Thoracic Outlet Syndrome. Diagnostics 2018, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Min, B.-J.; Jo, W.-M.; Shin, J.S. Video-assisted thoracoscopic surgery for intrathoracic first rib resection in thoracic outlet syndrome. J. Thorac. Dis. 2017, 9, 2022–2028. [Google Scholar] [CrossRef]
- Nuutinen, H.; Riekkinen, T.; Aittola, V.; Mäkinen, K.; Kärkkäinen, J.M. Thoracoscopic Versus Transaxillary Approach to First Rib Resection in Thoracic Outlet Syndrome. Ann. Thorac. Surg. 2018, 105, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, A.; Lutz, J.; Dorn, P.; Minervini, F.; Kestenholz, P.; Gelpke, H.; Schmid, R.A.; Kocher, G.J. Robotic-Assisted Thoracoscopic Resection of the First Rib for Vascular Thoracic Outlet Syndrome: The New Gold Standard of Treatment? J. Clin. Med. 2021, 10, 3952. [Google Scholar] [CrossRef]
- Palivela, N.; Lee, H.-S.; Jang, H.-J.; Paily, P.; Montero, M.; Najafi, B.; Burt, B.M. Improvement of Disability in Neurogenic Thoracic Outlet Syndrome by Robotic First Rib Resection. Ann. Thorac. Surg. 2022, 114, 919–925. [Google Scholar] [CrossRef]
- Hoexum, F.; Jongkind, V.; Coveliers, H.M.; Yeung, K.K.; Wisselink, W. Robot-assisted transthoracic first rib resection for venous thoracic outlet syndrome. Vascular 2022, 30, 217–224. [Google Scholar] [CrossRef]
- Azenha, L.F.; Kocher, G.J.; Kestenholz, P.B.; Gioutsos, K.; Minervini, F. Thoracic outlet syndrome: A retrospective analysis of robotic assisted first rib resections. J. Robot. Surg. 2023, 17, 891–896. [Google Scholar] [CrossRef]

| TOS Classification | Predominant Affected Structure | Key Symptoms | Diagnostic Methods |
|---|---|---|---|
| Neurogenic TOS (nTOS) | Brachial plexus | - Neuropathic pain—Sensory loss—Paresthesia of ulnar nerve distribution—Weakness of upper extremity—Neck pain—Occipital headaches—Diminished pulse—Raynaud’s phenomenon—Gilliatt-Sumner hand (severe cases) | - Clinical diagnosis—Physical exam maneuvers (scalene tenderness, neck rotation, head tilt, upper limb tension test, Adson test, EAST)—Imaging (Duplex US, chest and neck radiographs, MRI, CT, EMG)—Neuromuscular blockade of scalene muscles |
| Venous TOS (vTOS) | Subclavian vein | - Upper limb edema—Pain—Venous distension—Cyanosis—Risk of thrombosis | - Imaging (Duplex US, chest radiograph, CT, MRI venography, catheter-directed venography) |
| Arterial TOS (aTOS) | Subclavian artery | - Ischemia—Paresthesia—Claudication—Pallor in digits and hand—Absent radial pulse | - Imaging (neck radiographs, CT, MRI/MRA, Duplex US, catheter arteriography) |
| PMID: | Number of Patients | R-FRR Cases | nTOS | aTOS | vTOS | Non-Specific TOS | Females | Males | Age (Years) | R Handed | Left Sided | Bilateral | Preoperative VAS Score | Preoperative DASH Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 34795965 | 162 | 162 | 79 | 0 | 83 | 0 | 84 | 78 | 24 ± 8.5 (vTOS) 34 ± 9.5 (nTOS) | N/A | N/A | N/A | N/A | 60.3 ± 2.1 (nTOS) |
| 34592270 | 34 | 38 | 4 | 3 | 20 | 11 | 21 | 13 | 32.5 | N/A | 9 | 4 | N/A | N/A |
| 33046231 | 66 | 72 | 50 | 0 | 22 | 0 | 47 | 19 | 36 | 59 | 28 | 6 | N/A | N/A |
| 33832359 | 15 | 15 | 0 | 0 | 15 | 0 | 7 | 8 | 32.9 (mean) | N/A | 6 | 0 | N/A | N/A |
| 35350142 | 11 | 11 | 1 | 2 | 8 | 0 | N/A | N/A | 28 | N/A | 3 | 0 | N/A | N/A |
| 29696329 | 8 | 8 | 4 | 0 | 3 | 0 | 4 | 4 | N/A | N/A | 2 | 0 | N/A | N/A |
| 36327061 | 42 | 47 | 5 | 7 | 22 | 13 | 24 | 18 | 47 (mean) | N/A | 16 | 0 | N/A | N/A |
| 32446920 | 17 | 17 | 8 | 0 | 9 | 0 | 9 | 8 | 45 ± 11 (mean) | N/A | N/A | N/A | N/A | N/A |
| 30085044 | 83 | 83 | 0 | 0 | 83 | 0 | 34 | 49 | 24 ± 8.5 Y (mean) | N/A | N/A | N/A | N/A | N/A |
| 34501401 | 23 | 24 | 0 | 10 | 13 | 0 | 16 | 7 | N/A | N/A | 6 | 0 | N/A | N/A |
| 22576034 | 5 | 5 | 0 | 0 | 5 | 0 | 1 | 4 | 34.6 ± 10 | N/A | N/A | N/A | N/A | N/A |
| 34419432 | 37 | 40 | 37 | 0 | 0 | 0 | 32 | 5 | 36 | 34 | 8 | 3 | 6.0 ± 2.5 | 64.2 ± 17.1 |
| PMID: | Blood Loss | Operative Time (minutes) | Complications | LOS | Postoperative Complications | VAS Score | DASH Score |
|---|---|---|---|---|---|---|---|
| 34795965 | N/A | 87.6 ± 10.8 (nTOS), 127.6 ± 20.8 min (vTOS) | none | 3 days (nTOS), 4 days (vTOS) | none (nTOS) | N/A | 5 ± 2.3 (immediate post-op nTOS), 3.5 ± 1.1 (nTOS 6 months), N/A vTOS |
| 34592270 | N/A | 133 ± 44.7 min | none | 2 ± 2.1 days | none | N/A | N/A |
| 33046231 | 20 mL | 140 | 47.8 h | 5 (phrenic n injury, brachial plexus palsy, sensory, any) | 4.7 | N/A | |
| 33832359 | 79.5 mL | 147.9 min (mean) | 3 (conversion to transaxillary) | 3.5 days (mean) | pneumonia, pleural effusion, hemothorax | short term: general 7.1, work 5.8, sport/recreation 13.1; long-term: general 6, work 5.4, sport/recreation 12.5 | |
| 35350142 | N/A | 180 min (median) | none | 2 days (median) | 1 (pneumothorax requiring chest tube) | 0.6 (mean) | N/A |
| 29696329 | not significant | 108 min (median) | none | 2 days | none | N/A | N/A |
| 36327061 | N/A | 122 ± 40 min | none | 3 ± 1 days | 2 (pneumothorax requiring chest tube, recurrent pleural effusion) | 2 (SD = 1) | N/A |
| 32446920 | N/A | 113.2 ± 55.3 min (mean) | N/A | 1.8 ± 1.9 days (mean) | N/A | N/A | |
| 30085044 | N/A | 127 min ± 20.8 min | none | 4 days (median) | none | N/A | N/A |
| 34501401 | N/A | 117 | N/A | 2 days | 1 (postop hematoma) | 3 | |
| 22576034 | N/A | 195 ± 24.6 min | none | 3 days (median) | NONE | N/A | N/A |
| 34419432 | N/A | N/A | N/A | N/A | N/A | 1st postop = 2.8 ± 2.1, 2nd post-op = 1.4 ± 1.5 | 1st post op = 35.0 ± 21.3, 2nd post-op = 30.2 ± 19.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes, M.; Alaparthi, S.; Roedl, J.B.; Moreta, M.C.; Evans, N.R.; Grenda, T.; Okusanya, O.T. Robotic First Rib Resection in Thoracic Outlet Syndrome: A Systematic Review of Current Literature. J. Clin. Med. 2023, 12, 6689. https://doi.org/10.3390/jcm12206689
Reyes M, Alaparthi S, Roedl JB, Moreta MC, Evans NR, Grenda T, Okusanya OT. Robotic First Rib Resection in Thoracic Outlet Syndrome: A Systematic Review of Current Literature. Journal of Clinical Medicine. 2023; 12(20):6689. https://doi.org/10.3390/jcm12206689
Chicago/Turabian StyleReyes, Maikerly, Sneha Alaparthi, Johannes B. Roedl, Marisa C. Moreta, Nathaniel R. Evans, Tyler Grenda, and Olugbenga T. Okusanya. 2023. "Robotic First Rib Resection in Thoracic Outlet Syndrome: A Systematic Review of Current Literature" Journal of Clinical Medicine 12, no. 20: 6689. https://doi.org/10.3390/jcm12206689
APA StyleReyes, M., Alaparthi, S., Roedl, J. B., Moreta, M. C., Evans, N. R., Grenda, T., & Okusanya, O. T. (2023). Robotic First Rib Resection in Thoracic Outlet Syndrome: A Systematic Review of Current Literature. Journal of Clinical Medicine, 12(20), 6689. https://doi.org/10.3390/jcm12206689





