Efficacy of a New Hemostatic Dental Sponge in Controlling Bleeding, Pain, and Dry Socket Following Mandibular Posterior Teeth Extraction—A Split-Mouth Randomized Double-Blind Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Dental Gelatin Sponge
2.3. Dates for Trial
2.4. Calculation of Sample Size
2.5. Randomizing and Blinding
2.6. Population
2.7. Inclusion Criteria
2.8. Exclusion Criteria
2.9. The Tooth Extraction Process
2.10. Sponge Insertion
2.11. Blood Absorption Evaluation
2.12. The Amount of Bleeding
2.13. Pain Evaluation
2.14. Occurrence of Dry Socket
2.15. Statistical Analysis of Data
3. Results
3.1. Blood Absorption
3.2. The Amount of Bleeding
3.3. Pain Evaluation
3.4. Occurrence of Dry Socket
4. Discussion
Trial Strength and Limitations
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Obimakinde, O.; Akinpelu, A.; Obimakinde, A. Risk indicators of operative difficulty of impacted mandibular third molar in a Nigerian Tertiary Hospital. Sci. Rep. 2012, 1, 354. [Google Scholar]
- Deliverska, E.G.; Petkova, M. Complications after extraction of impacted third molars-literature review. J. IMAB Annu. Proc. Sci. Pap. 2016, 22, 1202–1211. [Google Scholar] [CrossRef] [Green Version]
- Yamada, S.-I.; Hasegawa, T.; Soutome, S.; Yoshimura, H.; Miyakoshi, M.; Ueda, N.; Okamoto, K.; Hishida, S.; Rokutanda, S.; Nakahara, H.; et al. Prevalence of and risk factors for postoperative hemorrhage after lower third molar extraction on warfarin therapy: A multicenter retrospective study in Japan. Odontology 2020, 108, 462–469. [Google Scholar] [CrossRef]
- Tenglikar, P.; Munnangi, A.; Mangalgi, A.; Uddin, S.F.; Mathpathi, S.; Shah, K. An assessment of factors influencing the difficulty in third molar surgery. Ann. Maxillofac. Surg. 2017, 7, 45. [Google Scholar]
- Sharifi, S.; Zaheri Khosroshahi, A.; Maleki Dizaj, S.; Rezaei, Y. Preparation, physicochemical assessment and the antimicrobial action of hydroxyapatite–gelatin/curcumin nanofibrous composites as a dental biomaterial. Biomimetics 2022, 7, 4. [Google Scholar] [CrossRef]
- Taberner-Vallverdú, M.; Sánchez-Garcés, M.Á.; Gay-Escoda, C. Efficacy of different methods used for dry socket prevention and risk factor analysis: A systematic review. Med. Oral Patol. Oral Cir. Bucal 2017, 22, e750. [Google Scholar] [CrossRef]
- Abdolahinia, E.D.; Barati, G.; Ranjbar-Navazi, Z.; Kadkhoda, J.; Islami, M.; Hashemzadeh, N.; Dizaj, S.M.; Sharifi, S. Application of nanogels as drug delivery systems in multicellular spheroid tumor model. J. Drug Deliv. Sci. Technol. 2022, 68, 103109. [Google Scholar] [CrossRef]
- Hassan, O.; Allah, E.; Fouda, A. Evaluation of the role of gelatamp in comparison with gelatine sponge on postoperative complications following odontoctomy of impacted mandibular third molar. Dent. J. 2011, 57, 3659. [Google Scholar]
- Mp, S.K. Local hemostatic agents in the management of bleeding in oral surgery. Asian J. Pharm. Clin. Res. 2016, 9, 35–41. [Google Scholar]
- Nagraj, S.K.; Prashanti, E.; Aggarwal, H.; Lingappa, A.; Muthu, M.S.; Krishanappa, S.K.K.; Hassan, H. Interventions for treating post-extraction bleeding. Cochrane Database Syst. Rev. 2018, 3, CD011930. [Google Scholar]
- Grossi, G.B.; Maiorana, C.; Garramone, R.A.; Borgonovo, A.; Creminelli, L.; Santoro, F. Assessing postoperative discomfort after third molar surgery: A prospective study. J. Oral Maxillofac. Surg. 2007, 65, 901–917. [Google Scholar] [CrossRef]
- Momesso, G.A.C.; Grossi-Oliveira, G.A.; Silva, W.P.P.; Akira, R.; Chiba, F.; Polo, T.O.B.; Neto, T.J.d.L.; Rios, B.R.; Bassi, A.P.F.; Sumida, D.H.; et al. A triple-blind randomized clinical trial of different associations between dexamethasone and non-steroids anti-inflammatories for preemptive action in third molar extractions. Sci. Rep. 2021, 11, 24445. [Google Scholar] [CrossRef]
- Arteagoitia, I.; Diez, A.; Barbier, L.; Santamaría, G.; Santamaría, J. Efficacy of amoxicillin/clavulanic acid in preventing infectious and inflammatory complications following impacted mandibular third molar extraction. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2005, 100, e8–e11. [Google Scholar] [CrossRef]
- Rundgren, M.; Engström, M. A thromboelastometric evaluation of the effects of hypothermia on the coagulation system. Anesth. Analg. 2008, 107, 1465–1468. [Google Scholar] [CrossRef]
- Ozmeric, N.; Mollaoglu, N.; Elgun, S.; Devrim, E. Impact of chlorhexidine mouth rinse use on postextraction infection via nitric oxide pathway. Inflamm. Res. 2010, 59, 437–441. [Google Scholar] [CrossRef]
- Suri, N.; Dutta, A.; Siddiqui, N.; Kaur, K.; Jangra, D. A literature review on dry socket. IP Int. J. Maxillofac. Imaging 2021, 6, 97–100. [Google Scholar] [CrossRef]
- Sadeghi, S.H.; Sharifi, S.; Dizaj, S.M.; Ghavimi, M.A.; Shahi, S.; Ghoreishizadeh, A.; Negahdari, R. Antimicrobial agent containing absorbable gelatin sponge to prevent dry socket: A systematic review. Open Dent. J. 2022, 16, e187421062208111. [Google Scholar] [CrossRef]
- Abdolahinia, E.D.; Hajisadeghi, S.; Banan, Z.M.; Dadgar, E.; Delaramifar, A.; Izadian, S.; Sharifi, S.; Dizaj, S.M. Potential applications of medicinal herbs and phytochemicals in oral and dental health: Status quo and future perspectives. Oral Dis. 2022. [Google Scholar] [CrossRef]
- Sharifi, S.; Dizaj, S.M.; Ahmadian, E.; Karimpour, A.; Maleki, A.; Memar, M.Y.; Ghavimi, M.A.; Abdolahinia, E.D.; Goh, K.W. A Biodegradable Flexible Micro/Nano-Structured Porous Hemostatic Dental Sponge. Nanomaterials 2022, 12, 3436. [Google Scholar] [CrossRef]
- Singh, M.; Bhate, K.; Kulkarni, D.; Santhosh Kumar, S.; Kathariya, R. The effect of alloplastic bone graft and absorbable gelatin sponge in prevention of periodontal defects on the distal aspect of mandibular second molars, after surgical removal of impacted mandibular third molar: A comparative prospective study. J. Maxillofac. Oral Surg. 2015, 14, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Lu, C. A clinical study of gelatamp colloidal silver gelatin sponge on preventing the complication of teeth extraction. West China J. Stomatol. 2008, 26, 519–521. [Google Scholar]
- Guralnick, W.C.; Berg, L. Gelfoam in oral surgery: A report of two hundred fifty cases. Oral Surg. Oral Med. Oral Pathol. 1948, 1, 632–639. [Google Scholar] [CrossRef]
- Shahi, S.; Dehghani, F.; Abdolahinia, E.D.; Sharifi, S.; Ahmadian, E.; Gajdács, M.; Kavetskyy, T. Effect of gelatinous spongy scaffold containing nano-hydroxyapatite on the induction of odontogenic activity of dental pulp stem cells. J. King Saud Univ. Sci. 2022, 34, 102340. [Google Scholar] [CrossRef]
- Irfan, N.I.; Zubir, A.Z.M.; Suwandi, A.; Haris, M.S.; Jaswir, I.; Lestari, W. Gelatin-based hemostatic agents for medical and dental application at a glance: A narrative literature review. Saudi Dent. J. 2022, 34, 699–707. [Google Scholar] [CrossRef]
- Xi, G.; Liu, W.; Chen, M.; Li, Q.; Hao, X.; Wang, M.; Yang, X.; Feng, Y.; He, H.; Shi, C.; et al. Polysaccharide-based lotus seedpod surface-like porous microsphere with precise and controllable micromorphology for ultrarapid hemostasis. ACS Appl. Mater. Interfaces 2019, 11, 46558–46571. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Musa, A.; Ahmad, M.B.; Hussein, M.Z.; Saiman, M.I.; Sani, H.A. Effect of gelatin-stabilized copper nanoparticles on catalytic reduction of methylene blue. Nanoscale Res. Lett. 2016, 11, 438. [Google Scholar] [CrossRef] [Green Version]
- Piri, P.; Esmaeili, A.; Mahdipour, A.; Asayesh, H. The effect of using Gelatamp on pain and gingival bleeding after tooth extraction: A randomize clinical trial. Qom. Univ. Med. Sci. J. 2018, 12, 10–18. [Google Scholar] [CrossRef]
- Thuruthel, M.J.; Kumar, L.S.; Kurien, N.M.; Tharakan, M. Efficacy of gelatamp in controlling the postoperative sequelae following mandibular posterior teeth extraction-A split-mouth study. J. Oral Biol. Craniofacial Res. 2023, 13, 96–103. [Google Scholar] [CrossRef]
- Scott, J.; Huskisson, E. Graphic representation of pain. Pain 1976, 2, 175–184. [Google Scholar] [CrossRef]
- Amaratunga, N.D.S.; Senaratne, C. A clinical study of dry socket in Sri Lanka. Br. J. Oral Maxillofac. Surg. 1988, 26, 410–418. [Google Scholar] [CrossRef]
- Guralnick, W.C. Absorbable gelatin sponge and thrombin in oral surgery. Am. J. Orthod. Oral Surg. 1946, 32, 792–794. [Google Scholar] [CrossRef]
- Liu, W.; Yang, C.; Gao, R.; Zhang, C.; Ou-Yang, W.; Feng, Z.; Zhang, C.; Pan, X.; Huang, P.; Kong, D.; et al. Polymer Composite Sponges with Inherent Antibacterial, Hemostatic, Inflammation-Modulating and Proregenerative Performances for Methicillin-Resistant Staphylococcus aureus-Infected Wound Healing. Adv. Healthc. Mater. 2021, 10, 2101247. [Google Scholar] [CrossRef]
- Zhang, Y.; Guan, J.; Wu, J.; Ding, S.; Yang, J.; Zhang, J.; Dong, A.; Deng, L. N-alkylated chitosan/graphene oxide porous sponge for rapid and effective hemostasis in emergency situations. Carbohydr. Polym. 2019, 219, 405–413. [Google Scholar] [CrossRef]
- Mahmoodzadeh, A.; Moghaddas, J.; Jarolmasjed, S.; Kalan, A.E.; Edalati, M.; Salehi, R. Biodegradable cellulose-based superabsorbent as potent hemostatic agent. Chem. Eng. J. 2021, 418, 129252. [Google Scholar] [CrossRef]
- Kim, J.-C.; Choi, S.-S.; Wang, S.-J.; Kim, S.-G. Minor complications after mandibular third molar surgery: Type, incidence, and possible prevention. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 102, e4–e11. [Google Scholar] [CrossRef]
- Tropp, J.; Rivnay, J. Design of biodegradable and biocompatible conjugated polymers for bioelectronics. J. Mater. Chem. C 2021, 9, 13543–13556. [Google Scholar] [CrossRef]
- Ehab, K.; Abouldahab, O.; Hassan, A.; Fawzy El-Sayed, K.M. Alvogyl and absorbable gelatin sponge as palatal wound dressings following epithelialized free gingival graft harvest: A randomized clinical trial. Clin. Oral Investig. 2020, 24, 1517–1525. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Guan, Q.L.; Li, Y.X.; Guo, J.L.; Jiang, L.; Jia, M.Y.; Deng, Y. Use of “gelatamp” colloidal silver gelatin sponge to prevent dry socket after extracting mandibular impacted teeth. Shanghai J. Stomatol. 2013, 22, 108–110. [Google Scholar]
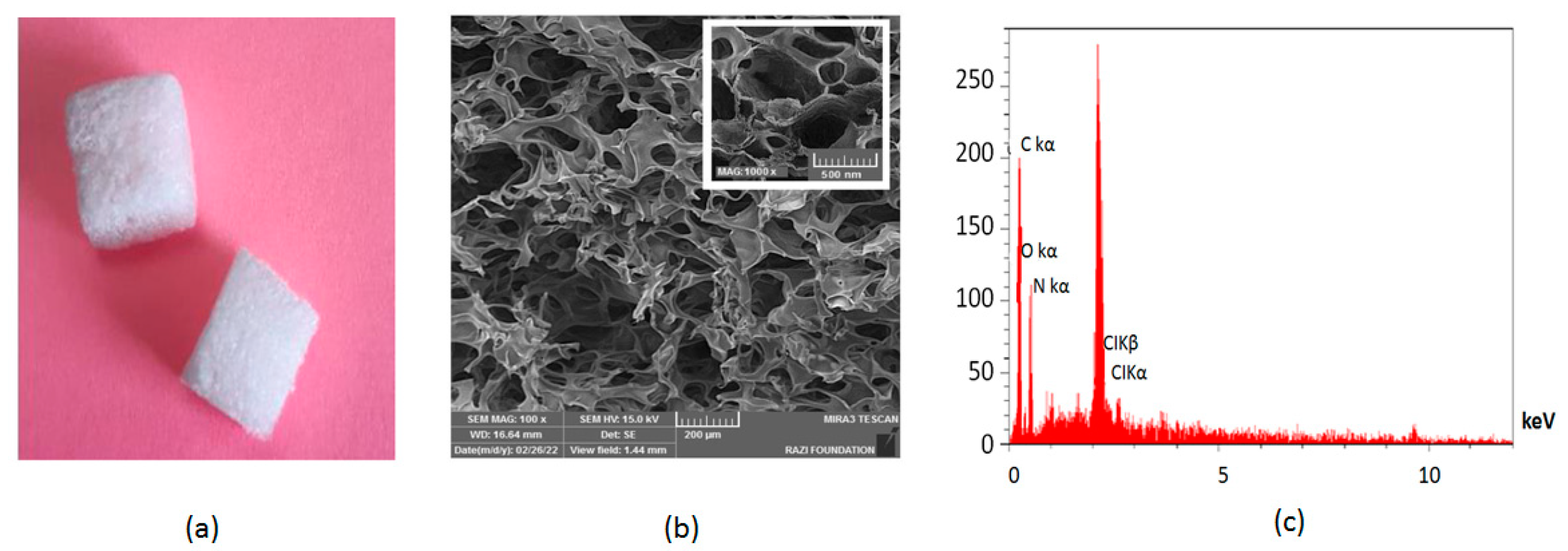
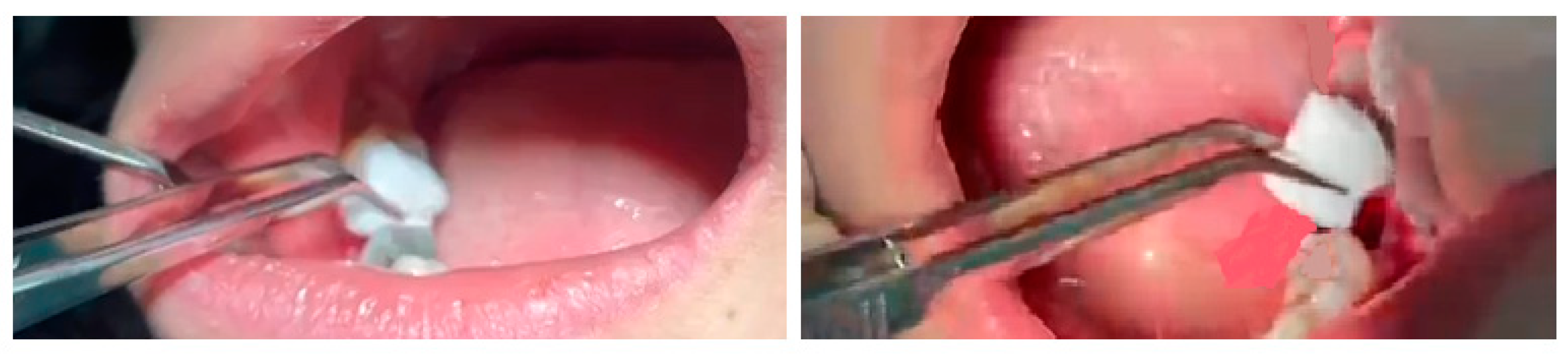

| Variable | Category | Mean ± SD/n(%) |
|---|---|---|
| Age | - | 31.3 ± 4.02 |
| Gender | Male | 12 |
| Female | 14 |
| Number of Gauzes | Control Group | Test Group | p-Value | |
|---|---|---|---|---|
| 2 | Number | 6 | 7 | 0.845 |
| Percent | 22.2% | 25.9% | ||
| 3 | Number | 18 | 16 | |
| Percent | 66.7% | 59.3% | ||
| 4 | Number | 3 | 4 | |
| Percent | 11.1% | 14.8% | ||
| Total | Number | 27 | 27 | |
| Percent | 100.0% | 100.0% |
| Time (h) after Tooth Extraction | Control Group (Mean) | Test Group (Mean) |
|---|---|---|
| within 1 | 1.11 ± 0.23 | 1.90 ± 0.45 |
| within 1–4 | 0.23 ± 0.42 | 0.37 ± 0.31 |
| After 4 | 0.03 ± 0.21 | 0.21 ± 0.40 |
| Time (h) | Control Group (Mean) | Test Group (Mean) |
|---|---|---|
| 12 | 4.0741 ± 0.71 | 3.0056 ± 0.80 |
| 24 | 4.4259 ± 0.45 | 2.0815 ± 0.66 |
| 48 | 2.4630 ± 0.78 | 1.0667 ± 0.78 |
| 72 | 1.9630 ± 0.65 | 1.0041 ± 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoudi, A.; Ghavimi, M.A.; Maleki Dizaj, S.; Sharifi, S.; Sajjadi, S.S.; Jamei Khosroshahi, A.R. Efficacy of a New Hemostatic Dental Sponge in Controlling Bleeding, Pain, and Dry Socket Following Mandibular Posterior Teeth Extraction—A Split-Mouth Randomized Double-Blind Clinical Trial. J. Clin. Med. 2023, 12, 4578. https://doi.org/10.3390/jcm12144578
Mahmoudi A, Ghavimi MA, Maleki Dizaj S, Sharifi S, Sajjadi SS, Jamei Khosroshahi AR. Efficacy of a New Hemostatic Dental Sponge in Controlling Bleeding, Pain, and Dry Socket Following Mandibular Posterior Teeth Extraction—A Split-Mouth Randomized Double-Blind Clinical Trial. Journal of Clinical Medicine. 2023; 12(14):4578. https://doi.org/10.3390/jcm12144578
Chicago/Turabian StyleMahmoudi, Armin, Mohammad Ali Ghavimi, Solmaz Maleki Dizaj, Simin Sharifi, Seyyede Shabnam Sajjadi, and Amir Reza Jamei Khosroshahi. 2023. "Efficacy of a New Hemostatic Dental Sponge in Controlling Bleeding, Pain, and Dry Socket Following Mandibular Posterior Teeth Extraction—A Split-Mouth Randomized Double-Blind Clinical Trial" Journal of Clinical Medicine 12, no. 14: 4578. https://doi.org/10.3390/jcm12144578






