Relationship between Muscle Mass, Bone Density and Vascular Calcifications in Elderly People with SARS-CoV-2 Pneumonia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Laboratory Assessment
2.3. HRCT
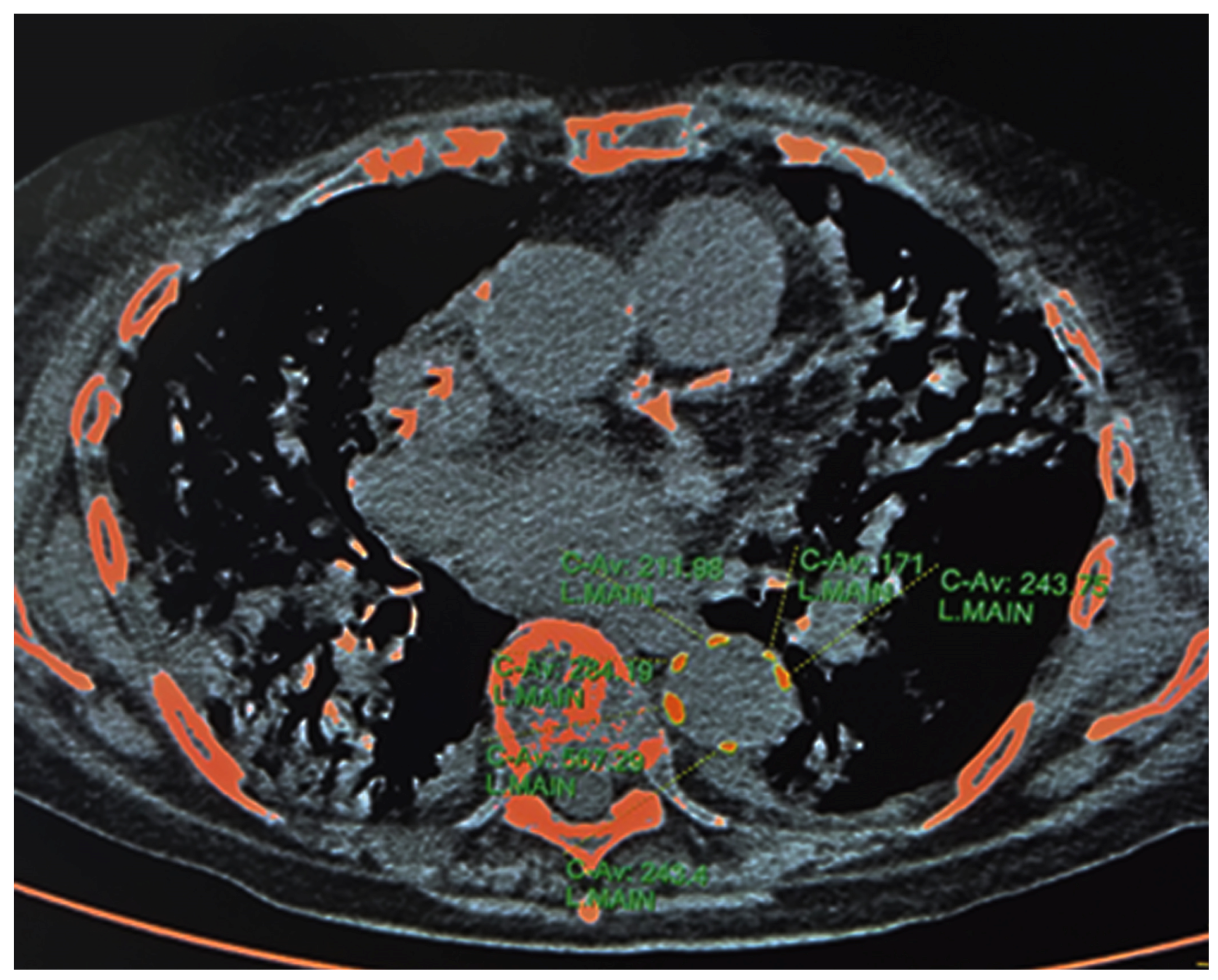
2.3.1. HRCT—Calcium Content of Descending Thoracic Aorta
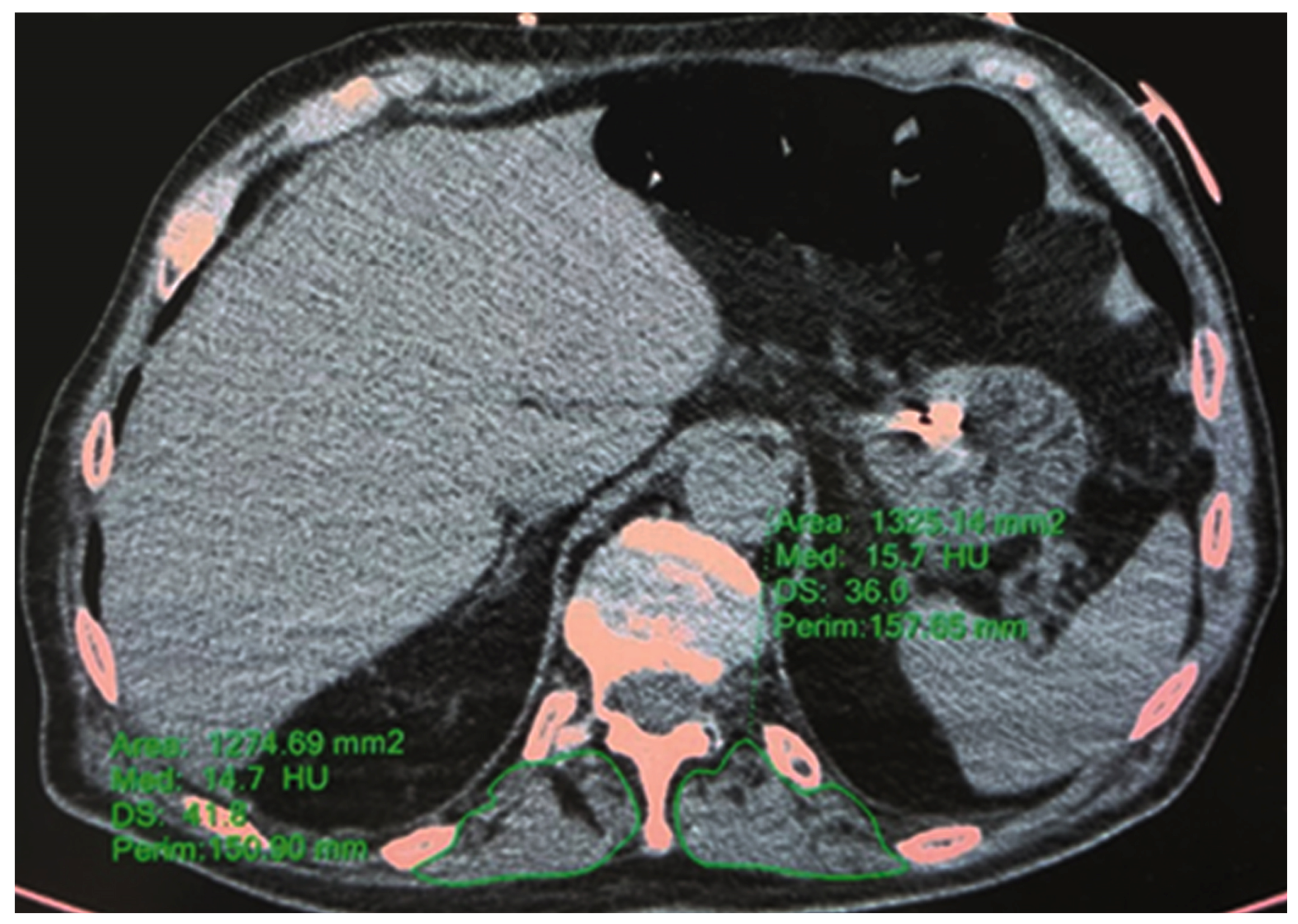
2.3.2. HRCT—T12 Paravertebral Muscle Area and Density
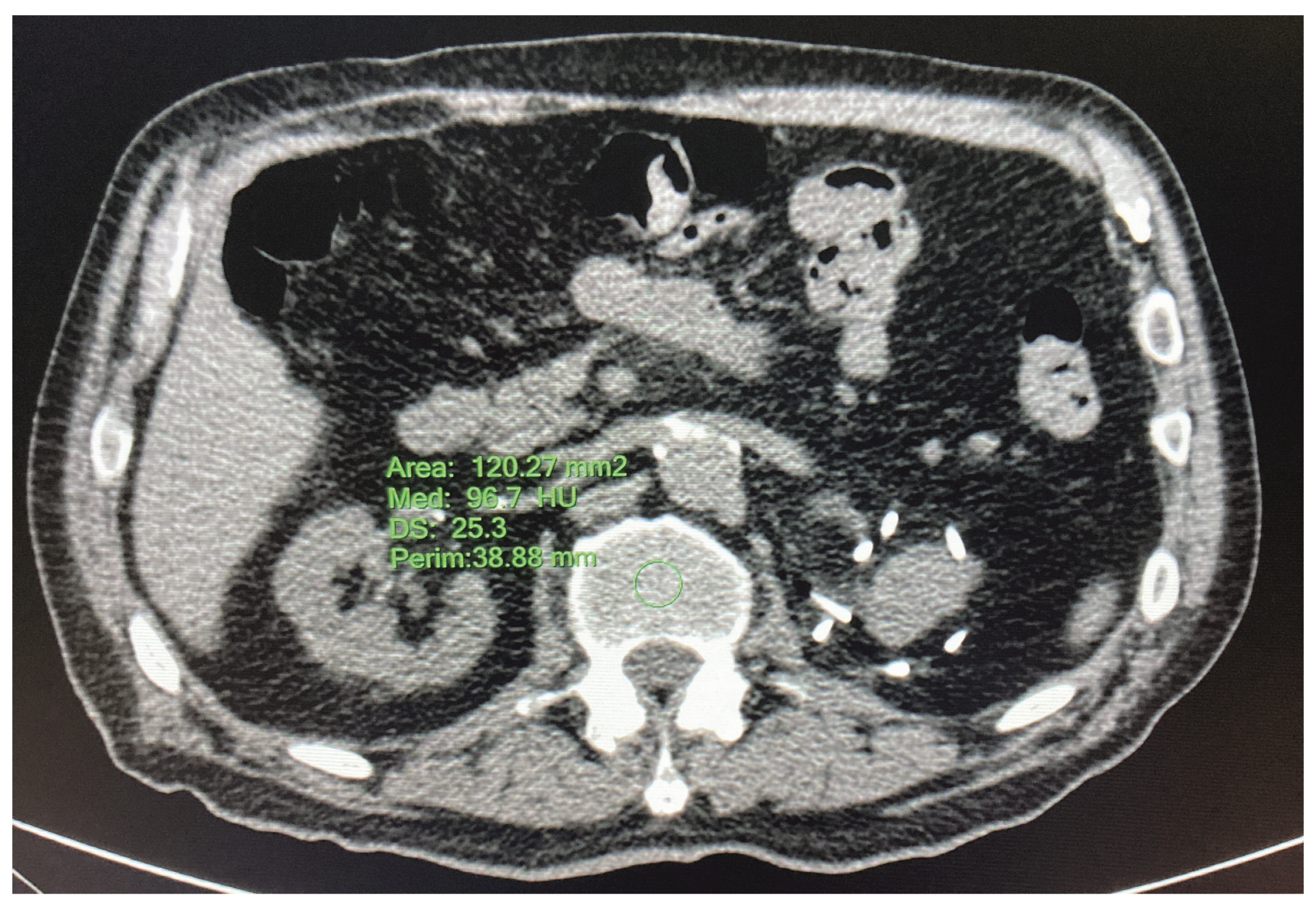
2.3.3. HRCT—L1 Bone Mineral Density
2.4. Statistical Analysis
3. Results
3.1. Baseline Features of the Patients
3.2. Association between DTAC Score, Bone Density, Muscle Density and Muscle Area
3.3. Association between Death and DTAC Score, Bone Density, Muscle Density and Muscle Area
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Mitnitski, A. Frailty in Relation to the Accumulation of Deficits. J. Gerontol. Ser. A 2007, 62, 722–727. [Google Scholar] [CrossRef] [Green Version]
- Dent, E.; Martin, F.C.; Bergman, H.; Woo, J.; Romero-Ortuno, R.; Walston, J.D. Management of frailty: Opportunities, challenges, and future directions. Lancet 2019, 394, 1376–1386. [Google Scholar] [CrossRef]
- Miles, A.; Webb, T.E.; Mcloughlin, B.C.; Mannan, I.; Rather, A.; Knopp, P.; Davis, D. Outcomes from COVID-19 across the range of frailty: Excess mortality in fitter older people. Eur. Geriatr. Med. 2020, 11, 851–855. [Google Scholar] [CrossRef]
- Owen, R.K.; Conroy, S.P.; Taub, N.; Jones, W.; Bryden, D.; Pareek, M.; Faull, C.; Abrams, K.R.; Davis, D.; Banerjee, J. Comparing associations between frailty and mortality in hospitalised older adults with or without COVID-19 infection: A retrospective observational study using electronic health records. Age Ageing 2020, 50, 307–316. [Google Scholar] [CrossRef]
- Aw, D.; Woodrow, L.; Ogliari, G.; Harwood, R. Association of frailty with mortality in older inpatients with Covid-19: A cohort study. Age Ageing 2020, 49, 915–922. [Google Scholar] [CrossRef]
- Bellelli, G.; Rebora, P.; Valsecchi, M.G.; Bonfanti, P.; Citerio, G.; COVID-19 Monza Team Members. Frailty index predicts poor outcome in COVID-19 patients. Intensiv. Care Med. 2020, 46, 1634–1636. [Google Scholar] [CrossRef] [PubMed]
- De Smet, R.; Mellaerts, B.; Vandewinckele, H.; Lybeert, P.; Frans, E.; Ombelet, S.; Lemahieu, W.; Symons, R.; Ho, E.; Frans, J.; et al. Frailty and Mortality in Hospitalized Older Adults With COVID-19: Retrospective Observational Study. J. Am. Med Dir. Assoc. 2020, 21, 928–932. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, J.; Carter, B.; Vilches-Moraga, A.; Quinn, T.J.; Braude, P.; Verduri, A.; Pearce, L.; Stechman, M.; Short, R.; Price, A.; et al. The effect of frailty on survival in patients with COVID-19 (COPE): A multicentre, European, observational cohort study. Lancet Public Health 2020, 5, e444–e451. [Google Scholar] [CrossRef] [PubMed]
- Graña, C.; Ghosn, L.; Evrenoglou, T.; Jarde, A.; Minozzi, S.; Bergman, H.; Buckley, B.S.; Probyn, K.; Villanueva, G.; Henschke, N.; et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst. Rev. 2022, 12, CD015477. [Google Scholar] [CrossRef]
- Mathieu, E.; Ritchie, H.; Ortiz-Ospina, E.; Roser, M.; Hasell, J.; Appel, C.; Giattino, C.; Rodés-Guirao, L. A global database of COVID-19 vaccinations. Nat. Hum. Behav. 2021, 5, 947–953. [Google Scholar] [CrossRef]
- Sablerolles, R.S.G.; Lafeber, M.; Kempen, J.A.L.V.; A van de Loo, B.P.; Boersma, E.; Rietdijk, W.J.R.; A Polinder-Bos, H.; Mooijaart, S.P.; van der Kuy, H.; Versmissen, J.; et al. Association between Clinical Frailty Scale score and hospital mortality in adult patients with COVID-19 (COMET): An international, multicentre, retrospective, observational cohort study. Lancet Health Longev. 2021, 2, e163–e170. [Google Scholar] [CrossRef] [PubMed]
- Battisti, S.; Pedone, C.; Napoli, N.; Russo, E.; Agnoletti, V.; Nigra, S.G.; Dengo, C.; Mughetti, M.; Conte, C.; Pozzilli, P.; et al. Computed Tomography Highlights Increased Visceral Adiposity Associated With Critical Illness in COVID-19. Diabetes Care 2020, 43, e129–e130. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Albano, D.; Cozzi, A.; Messina, C.; Arioli, R.; Bnà, C.; Bruno, A.; Carbonaro, L.A.; Carriero, A.; Carriero, S.; et al. CT-derived Chest Muscle Metrics for Outcome Prediction in Patients with COVID-19. Radiology 2021, 300, E328–E336. [Google Scholar] [CrossRef]
- Shaw, L.J.; Raggi, P.; Berman, D.S.; Callister, T.Q. Coronary artery calcium as a measure of biologic age. Atherosclerosis 2006, 188, 112–119. [Google Scholar] [CrossRef]
- Thomas, I.C.; Thompson, C.A.; Yang, M.; Allison, M.A.; Forbang, N.I.; Michos, E.D.; McClelland, R.L.; Budoff, M.J.; Criqui, M.H. Thoracic Aorta Calcification and Noncardiovascular Disease–Related Mortality. Arter. Thromb. Vasc. Biol. 2018, 38, 1926–1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuro-O, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Yang, C.-H.; Kuo, W.-T.; Chen, C.-Y. Evaluation of Anti-aging Compounds Using the Promoters of Elastin and Fibrillin-1 Genes Combined with a Secreted Alkaline Phosphatase Reporter in Normal Human Fibroblasts. Curr. Pharm. Biotechnol. 2015, 16, 1053–1062. [Google Scholar] [CrossRef]
- Bhimraj, A.; Morgan, R.L.; Shumaker, A.H.; Lavergne, V.; Baden, L.; Cheng, V.C.-C.; Edwards, K.M.; Gandhi, R.; Muller, W.J.; O’Horo, J.C.; et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with (COVID-19). Clin. Infect. Dis. 2020, 27, ciaa478. [Google Scholar] [CrossRef]
- Nishimura, J.M.; Ansari, A.Z.; D’Souza, D.M.; Moffatt-Bruce, S.D.; Merritt, R.E.; Kneuertz, P.J. Computed Tomography-Assessed Skeletal Muscle Mass as a Predictor of Outcomes in Lung Cancer Surgery. Ann. Thorac. Surg. 2019, 108, 1555–1564. [Google Scholar] [CrossRef]
- Looijaard, W.G.P.M.; Dekker, I.M.; Stapel, S.N.; Girbes, A.R.J.; Twisk, J.W.R.; Straaten, H.M.O.-V.; Weijs, P.J.M. Skeletal muscle quality as assessed by CT-derived skeletal muscle density is associated with 6-month mortality in mechanically ventilated critically ill patients. Crit. Care 2016, 20, 386. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Ma, H.; Du, G.; Fan, D.; Liu, J.; Wang, X.; Zhang, W.; Liu, B.; Yin, F. Low Skeletal Muscle Area at the T12 Paravertebral Level as a Prognostic Marker for Community-Acquired Pneumonia. Acad. Radiol. 2022, 29, e205–e210. [Google Scholar] [CrossRef]
- Hermann, D.M.; Lehmann, N.; Gronewold, J.; Bauer, M.; Mahabadi, A.A.; Weimar, C.; Berger, K.; Moebus, S.; Jöckel, K.-H.; Erbel, R.; et al. Thoracic aortic calcification is associated with incident stroke in the general population in addition to established risk factors. Eur. Heart J.-Cardiovasc. Imaging 2015, 16, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendrickson, N.R.; Pickhardt, P.J.; Del Rio, A.M.; Rosas, H.G.; Anderson, P.A. Bone Mineral Density T-Scores Derived from CT At-tenuation Numbers (Hounsfield Units): Clinical Utility and Correlation with Dual-energy X-ray Absorptiometry. Iowa Orthop. J. 2018, 38, 25–31. [Google Scholar] [PubMed]
- Pickhardt, P.J.; Pooler, B.D.; Lauder, T.; del Rio, A.M.; Bruce, R.J.; Binkley, N. Opportunistic Screening for Osteoporosis Using Abdominal Computed Tomography Scans Obtained for Other Indications. Ann. Intern. Med. 2013, 158, 588–595. [Google Scholar] [CrossRef] [Green Version]
- Koenker, R.; Chernozhukov, V.; He, X.; Peng, L. Handbook of Quantile Regression; CRC Press: Boca Raton, FL, USA, 2018; 463p. [Google Scholar]
- Machado, J.A.; Parente, P.; Santos Silva, J. QREG2: Stata Module to Perform Quantile Regression with Robust and Clustered Standard Errors; 2021; Available online: https://ideas.repec.org/c/boc/bocode/s457369.html (accessed on 12 March 2023).
- Royston, P.; Sauerbrei, W. Multivariable Model—Building: A Pragmatic Approach to Regression Analysis Based on Fractional Polyno-Mials for Modelling Continuous Variables; John Wiley: Chichester, UK, 2008. [Google Scholar]
- Heinze, G.; Puhr, R. Bias-reduced and separation-proof conditional logistic regression with small or sparse data sets. Stat. Med. 2010, 29, 770–777. [Google Scholar] [CrossRef]
- Puhr, R.; Heinze, G.; Nold, M.; Lusa, L.; Geroldinger, A. Firth’s logistic regression with rare events: Accurate effect estimates and predictions? Stat. Med. 2017, 36, 2302–2317. [Google Scholar] [CrossRef]
- Tan, L.; Wang, Q.; Zhang, D.; Ding, J.; Huang, Q.; Tang, Y.-Q.; Wang, Q.; Miao, H. Lymphopenia predicts disease severity of COVID-19: A descriptive and predictive study. Signal Transduct. Target. Ther. 2020, 5, 33. [Google Scholar] [CrossRef]
- Pieralice, S.; Vigevano, F.; Del Toro, R.; Napoli, N.; Maddaloni, E. Lifestyle Management of Diabetes: Implications for the Bone-Vascular Axis. Curr. Diabetes Rep. 2018, 18, 84. [Google Scholar] [CrossRef]
- Fadini, G.P.; Rattazzi, M.; Matsumoto, T.; Asahara, T.; Khosla, S. Emerging Role of Circulating Calcifying Cells in the Bone-Vascular Axis. Circulation 2012, 125, 2772–2781. [Google Scholar] [CrossRef] [Green Version]
- Giannini, F.; Toselli, M.; Palmisano, A.; Cereda, A.; Vignale, D.; Leone, R.; Nicoletti, V.; Gnasso, C.; Monello, A.; Manfrini, M.; et al. Coronary and total thoracic calcium scores predict mortality and provides pathophysiologic insights in COVID-19 patients. J. Cardiovasc. Comput. Tomogr. 2021, 15, 421–430. [Google Scholar] [CrossRef]
- Abramowitz, Y.; Jilaihawi, H.; Chakravarty, T.; Mack, M.J.; Makkar, R.R. Porcelain Aorta. Circulation 2015, 131, 827–836. [Google Scholar] [CrossRef] [Green Version]
- Nuti, R.; Brandi, M.L.; Checchia, G.; Di Munno, O.; Dominguez, L.; Falaschi, P.; Fiore, C.E.; Iolascon, G.; Maggi, S.; Michieli, R.; et al. Guidelines for the management of osteoporosis and fragility fractures. Intern. Emerg. Med. 2019, 14, 85–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirschfeld, H.P.; Kinsella, R.; Duque, G. Osteosarcopenia: Where bone, muscle, and fat collide. Osteoporos. Int. 2017, 28, 2781–2790. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yin, L.; Zhao, Y.; Su, Y.; Sun, W.; Chen, S.; Liu, Y.; Yang, M.; Yu, A.; Guglielmi, G.; et al. Muscle Density, but Not Size, Correlates Well With Muscle Strength and Physical Performance. J. Am. Med. Dir. Assoc. 2020, 22, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Ormsbee, M.J.; Prado, C.M.; Ilich, J.Z.; Purcell, S.; Siervo, M.; Folsom, A.; Panton, L. Osteosarcopenic obesity: The role of bone, muscle, and fat on health. J. Cachex-Sarcopenia Muscle 2014, 5, 183–192. [Google Scholar] [CrossRef]
| Male Sex | 41 (51%) |
| Age (years) | 79 (73; 85) |
| Age (years/10) | |
| 6 | 11 (14%) |
| 7 | 34 (42%) |
| 8 | 29 (36%) |
| 9 | 6 (8%) |
| White blood cells (109/L) | 6.69 (4.96; 9.65) |
| Lymphocytes (109/L) | 0.76 (0.55; 1.13) |
| Neutrophils (109/L) | 5.64 (3.80; 8.32) |
| Platelets (109/L) | 210 (156; 279) |
| Hemoglobin (g/dL) | 12 (11; 13) |
| Prothrombin time (INR) * | 1.1 (1.0; 1.2) |
| ALT (U/L) ** | 21 (14; 34) |
| Total bilirubin (mg/dL) * | 0.4 (0.3; 0.6) |
| LDH (U/L) ** | 325 (274; 401) |
| C-reactive protein (mg/dL) | 79 (40; 123) |
| DTA calcium score (Agatston units) | 984 (51; 2991) |
| Ln DTA calcium score (Agatston units) | 7 (4; 8) |
| Muscle density T12 (HU) | 20 (4; 29) |
| Muscle area (cm2) | 2820 (2234; 3191) |
| Bone density L1 (HU) | 86 (63; 117) |
| ln DTA calcium score | |||
|---|---|---|---|
| M1a | M1b | M1c | |
| Bone density L1 (HU) | −0.02 ** [−0.04 to −0.01] | −0.00 [−0.02 to 0.02] | 0.00 [−0.02 to 0.03] |
| Age (years)/10 | 2.12 *** [0.90 to 3.33] | 2.28 ** [0.81 to 3.74] | |
| Male sex | −0.56 [−2.35 to 1.23] | ||
| Muscle density T12 (HU) | |||
| Intercept | 8.71 *** [7.54 to 9.88] | −10.64 [−21.84 to 0.56] | −12.25 [−25.55 to 1.04] |
| ln DTA calcium score | |||
| M2a | M2b | M2c | |
| Age (years)/10 | 1.89 ** [0.78 to 3.01] | 1.91 ** [0.72 to 3.11] | |
| Male sex | −0.40 [−1.93 to 1.13] | ||
| Muscle density T12 (HU) | −0.03 * [−0.06 to −0.001] | −0.01 [−0.05 to 0.03] | −0.01 [−0.04 to 0.03] |
| Intercept | 7.15 *** [6.45 to 7.85] | −9.01 [−18.50 to 0.48] | −8.92 [−18.99 to 1.15] |
| T12 Muscle density | |||
| M3a | M3b | M3c | |
| Bone density L1 (HU) | 0.13 * [0.02 to 0.24] | 0.08 [−0.11 to 0.26] | −0.03 [−0.15 to 0.10] |
| Age (years)/10 | −5.74 [−15.77 to 4.30] | −10.89 ** [−18.87 to −2.90] | |
| Male sex | 11.05 * [2.25 to 19.86] | ||
| Intercept | 7.93 [−3.12 to 18.98] | 56.77 [−33.29 to 146.83] | 98.58 ** [29.27 to 167.89] |
| T12 Muscle area | |||
| M4a | M4b | M4c | |
| Bone density L1 (HU) | 1.37 [−3.91 to 6.65] | 1.23 [−4.45 to 6.90] | −3.49 [−9.08 to 2.10] |
| Age (years)/10 | −133.60 [−429.37 to 162.17] | −255.17 [−514.86 to 4.52] | |
| Male sex | 696.00 ** [276.35 to 1115.65] | ||
| Intercept | 2715.30 *** [2198.35 to 3232.26] | 3700.20 ** [1184.68 to 6215.72] | 4745.28 *** [2526.68 to 6963.89] |
| M1a | M1b | M1c | |
|---|---|---|---|
| Ln DTA calcium score (Agatston units) | 1.480 * [1.022,2.145] | 1.225 [0.833,1.802] | 1.206 [0.832,1.750] |
| Age (years)/10 | 3.177 * [1.201,8.406] | 3.066 * [1.178,7.978] | |
| Male sex | 0.747 [0.227,2.460] | ||
| M2a | M2b | M2c | |
| Age (years)/10 | 3.530 ** [1.361,9.154] | 3.464 ** [1.362,8.811] | |
| Male sex | 0.849 [0.249,2.898] | ||
| Bone density L1 (HU) | 0.981* [0.966,0.996] | 0.990 [0.973,1.008] | 0.991 [0.974,1.009] |
| M2a | M2b | M2c | |
| Age (years)/10 | 4.023 ** [1.566,10.335] | 3.926 ** [1.561,9.873] | |
| Male sex | 0.755 [0.228,2.492] | ||
| Muscle density T12 (HU) | 0.973 * [0.948,0.999] | 0.992 [0.965,1.021] | 0.994 [0.966,1.022] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Toro, R.; Palmese, F.; Feletti, F.; Zani, G.; Minguzzi, M.T.; Maddaloni, E.; Napoli, N.; Bedogni, G.; Domenicali, M. Relationship between Muscle Mass, Bone Density and Vascular Calcifications in Elderly People with SARS-CoV-2 Pneumonia. J. Clin. Med. 2023, 12, 2372. https://doi.org/10.3390/jcm12062372
Del Toro R, Palmese F, Feletti F, Zani G, Minguzzi MT, Maddaloni E, Napoli N, Bedogni G, Domenicali M. Relationship between Muscle Mass, Bone Density and Vascular Calcifications in Elderly People with SARS-CoV-2 Pneumonia. Journal of Clinical Medicine. 2023; 12(6):2372. https://doi.org/10.3390/jcm12062372
Chicago/Turabian StyleDel Toro, Rossella, Francesco Palmese, Francesco Feletti, Gianluca Zani, Maria Teresa Minguzzi, Ernesto Maddaloni, Nicola Napoli, Giorgio Bedogni, and Marco Domenicali. 2023. "Relationship between Muscle Mass, Bone Density and Vascular Calcifications in Elderly People with SARS-CoV-2 Pneumonia" Journal of Clinical Medicine 12, no. 6: 2372. https://doi.org/10.3390/jcm12062372
APA StyleDel Toro, R., Palmese, F., Feletti, F., Zani, G., Minguzzi, M. T., Maddaloni, E., Napoli, N., Bedogni, G., & Domenicali, M. (2023). Relationship between Muscle Mass, Bone Density and Vascular Calcifications in Elderly People with SARS-CoV-2 Pneumonia. Journal of Clinical Medicine, 12(6), 2372. https://doi.org/10.3390/jcm12062372







