Sarcopenia’s Prognostic Impact on Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection and Data Extraction
2.3. Study Quality Assessments
2.4. Statistical Analysis
3. Results
3.1. Search Results and Study Population Characteristics
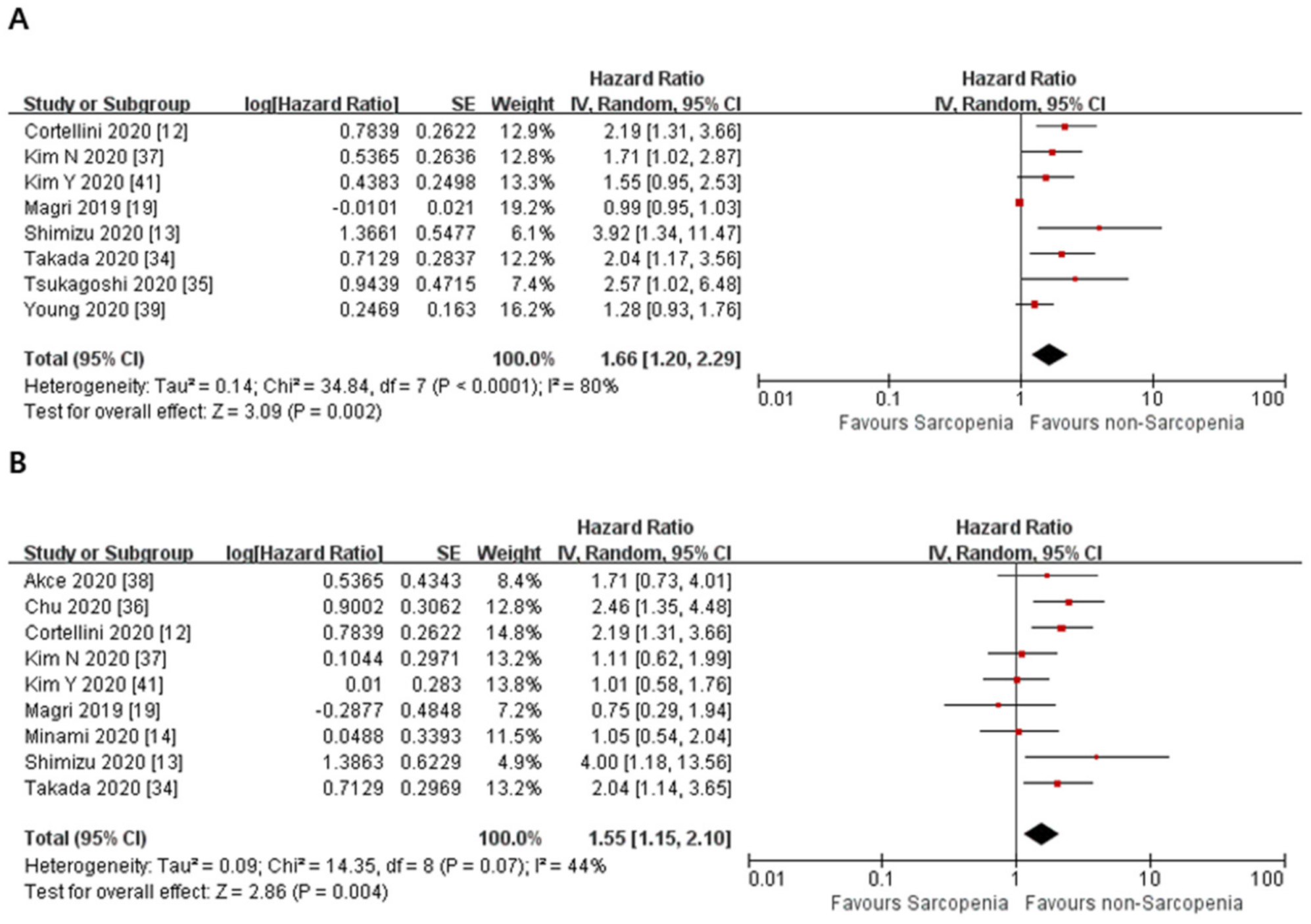
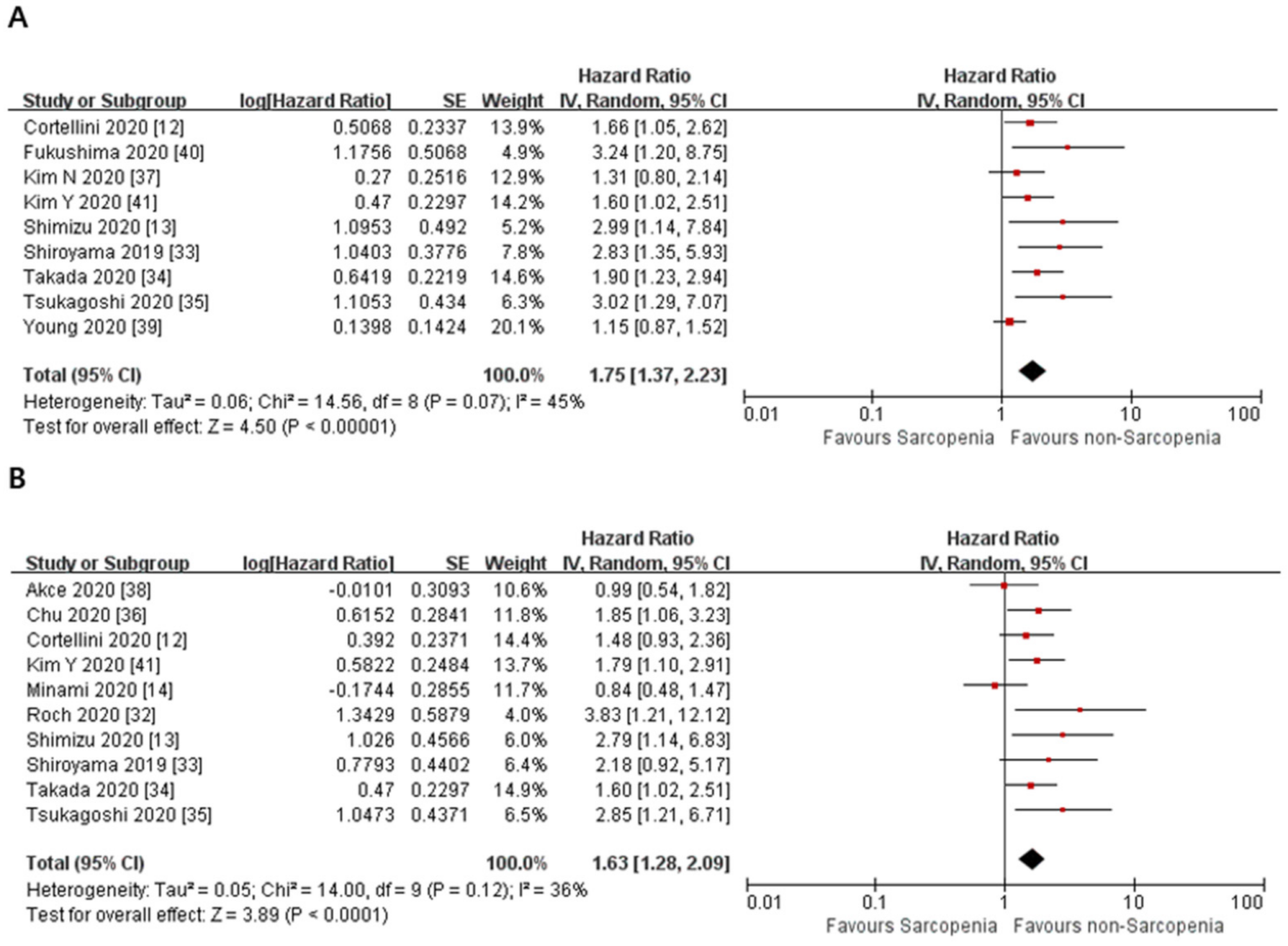
3.2. Primary Outcome
3.3. Subgroup Analyses
3.4. Quality Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef]
- Trejo-Avila, M.; Bozada-Gutiérrez, K.; Valenzuela-Salazar, C.; Herrera-Esquivel, J.; Moreno-Portillo, M. Sarcopenia predicts worse postoperative outcomes and decreased survival rates in patients with colorectal cancer: A systematic review and meta-analysis. Int. J. Colorectal Dis. 2021, 36, 1077–1096. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Gu, C.; Gan, S.; Li, Y.; Xiang, S.; Gong, L.; Chan, L.C.; Wang, S. Sarcopenia as a predictor of postoperative outcomes after urologic oncology surgery: A systematic review and meta-analysis. Urol. Oncol. 2020, 38, 560–573. [Google Scholar] [CrossRef]
- van Rijn-Dekker, M.I.; van den Bosch, L.; van den Hoek, J.G.M.; Bijl, H.P.; van Aken, E.S.M.; van der Hoorn, A.; Oosting, S.F.; Halmos, G.B.; Witjes, M.J.H.; van der Laan, H.P.; et al. Impact of sarcopenia on survival and late toxicity in head and neck cancer patients treated with radiotherapy. Radiother. Oncol. 2020, 147, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Olson, B.; Edwards, J.; Stone, L.; Jiang, A.; Zhu, X.; Holland, J.; Li, R.; Andersen, P.; Krasnow, S.; Marks, D.L.; et al. Association of Sarcopenia with Oncologic Outcomes of Primary Surgery or Definitive Radiotherapy Among Patients with Localized Oropharyngeal Squamous Cell Carcinoma. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 714–722. [Google Scholar] [CrossRef]
- Lee, C.M.; Kang, J. Prognostic impact of myosteatosis in patients with colorectal cancer: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2020, 11, 1270–1282. [Google Scholar] [CrossRef] [PubMed]
- Aleixo, G.F.P.; Shachar, S.S.; Nyrop, K.A.; Muss, H.B.; Malpica, L.; Williams, G.R. Myosteatosis and prognosis in cancer: Systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2020, 145, 102839. [Google Scholar] [CrossRef]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Cristescu, R.; Mogg, R.; Ayers, M.; Albright, A.; Murphy, E.; Yearley, J.; Sher, X.; Liu, X.Q.; Lu, H.; Nebozhyn, M.; et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 2018, 362, 6411. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Lee, K.H.; Kang, J.; Borcoman, E.; Saada-Bouzid, E.; Kronbichler, A.; Hong, S.H.; de Rezende, L.F.M.; Ogino, S.; Keum, N.; et al. Hyperprogressive Disease during Anti-PD-1 (PDCD1) / PD-L1 (CD274) Therapy: A Systematic Review and Meta-Analysis. Cancers 2019, 11, 1699. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Bozzetti, F.; Palumbo, P.; Brocco, D.; Di Marino, P.; Tinari, N.; De Tursi, M.; Agostinelli, V.; Patruno, L.; Valdesi, C.; et al. Weighing the role of skeletal muscle mass and muscle density in cancer patients receiving PD-1/PD-L1 checkpoint inhibitors: A multicenter real-life study. Sci. Rep. 2020, 10, 1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, T.; Miyake, M.; Hori, S.; Ichikawa, K.; Omori, C.; Iemura, Y.; Owari, T.; Itami, Y.; Nakai, Y.; Anai, S.; et al. Clinical Impact of Sarcopenia and Inflammatory/Nutritional Markers in Patients with Unresectable Metastatic Urothelial Carcinoma Treated with Pembrolizumab. Diagnostics 2020, 10, 310. [Google Scholar] [CrossRef] [PubMed]
- Minami, S.; Ihara, S.; Tanaka, T.; Komuta, K. Sarcopenia and Visceral Adiposity Did Not Affect Efficacy of Immune-Checkpoint Inhibitor Monotherapy for Pretreated Patients with Advanced Non-Small Cell Lung Cancer. World J. Oncol. 2020, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, L.; Xu, S. Sarcopenia affects clinical efficacy of immune checkpoint inhibitors in non-small cell lung cancer patients: A systematic review and meta-analysis. Int. Immunopharmacol. 2020, 88, 106907. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 11 November 2021).
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [Green Version]
- Magri, V.; Gottfried, T.; Di Segni, M.; Urban, D.; Peled, M.; Daher, S.; Stoff, R.; Bar, J.; Onn, A. Correlation of body composition by computerized tomography and metabolic parameters with survival of nivolumab-treated lung cancer patients. Cancer Manag. Res. 2019, 11, 8201–8207. [Google Scholar] [CrossRef] [Green Version]
- Loosen, S.H.; van den Bosch, V.; Gorgulho, J.; Schulze-Hagen, M.; Kandler, J.; Jördens, M.S.; Tacke, F.; Loberg, C.; Antoch, G.; Brümmendorf, T.; et al. Progressive Sarcopenia Correlates with Poor Response and Outcome to Immune Checkpoint Inhibitor Therapy. J. Clin. Med. 2021, 10, 1361. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, N.; Uchino, J.; Hirai, S.; Katayama, Y.; Yoshimura, A.; Okura, N.; Tanimura, K.; Harita, S.; Imabayashi, T.; Chihara, Y.; et al. Association of Sarcopenia with and Efficacy of Anti-PD-1/PD-L1 Therapy in Non-Small-Cell Lung Cancer. J. Clin. Med. 2019, 8, 450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrova, M.P.; Donev, I.S.; Radanova, M.A.; Eneva, M.I.; Dimitrova, E.G.; Valchev, G.N.; Minchev, V.T.; Taushanova, M.S.; Boneva, M.V.; Karanikolova, T.S.; et al. Sarcopenia and high NLR are associated with the development of hyperprogressive disease after second-line pembrolizumab in patients with non-small-cell lung cancer. Clin. Exp. Immunol. 2020, 202, 353–362. [Google Scholar] [CrossRef]
- Nishioka, N.; Naito, T.; Notsu, A.; Mori, K.; Kodama, H.; Miyawaki, T.; Mamesaya, N.; Kobayashi, H.; Omori, S.; Wakuda, K.; et al. Unfavorable impact of decreased muscle quality on the efficacy of immunotherapy for advanced non-small cell lung cancer. Cancer Med. 2021, 10, 247–256. [Google Scholar] [CrossRef]
- Bilen, M.A.; Martini, D.J.; Liu, Y.; Shabto, J.M.; Brown, J.T.; Williams, M.; Khan, A.I.; Speak, A.; Lewis, C.; Collins, H.; et al. Combined Effect of Sarcopenia and Systemic Inflammation on Survival in Patients with Advanced Stage Cancer Treated with Immunotherapy. Oncologist 2020, 25, e528–e535. [Google Scholar] [CrossRef] [Green Version]
- Qayyum, A.; Bhosale, P.; Aslam, R.; Avritscher, R.; Ma, J.; Pagel, M.D.; Sun, J.; Mohamed, Y.; Rashid, A.; Beretta, L.; et al. Effect of sarcopenia on systemic targeted therapy response in patients with advanced hepatocellular carcinoma. Abdom. Radiol. 2021, 46, 1008–1015. [Google Scholar] [CrossRef]
- Cortellini, A.; Verna, L.; Porzio, G.; Bozzetti, F.; Palumbo, P.; Masciocchi, C.; Cannita, K.; Parisi, A.; Brocco, D.; Tinari, N.; et al. Predictive value of skeletal muscle mass for immunotherapy with nivolumab in non-small cell lung cancer patients: A “hypothesis-generator” preliminary report. Thorac. Cancer 2019, 10, 347–351. [Google Scholar] [CrossRef]
- Daly, L.E.; Power, D.G.; O’Reilly, Á.; Donnellan, P.; Cushen, S.J.; O’Sullivan, K.; Twomey, M.; Woodlock, D.P.; Redmond, H.P.; Ryan, A.M. The impact of body composition parameters on ipilimumab toxicity and survival in patients with metastatic melanoma. Br. J. Cancer 2017, 116, 310–317. [Google Scholar] [CrossRef]
- Heidelberger, V.; Goldwasser, F.; Kramkimel, N.; Jouinot, A.; Huillard, O.; Boudou-Rouquette, P.; Chanal, J.; Arrondeau, J.; Franck, N.; Alexandre, J. Sarcopenic overweight is associated with early acute limiting toxicity of anti-PD1 checkpoint inhibitors in melanoma patients. Investig. New Drugs 2017, 35, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Hihara, J.; Tokumoto, N.; Kohashi, T.; Hara, T.; Shimbara, K.; Takahashi, S. Association between skeletal muscle loss and the response to nivolumab immunotherapy in advanced gastric cancer patients. Int. J. Clin. Oncol. 2021, 26, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.B.; Ravichandran, S.; Rushing, C.; Beasley, G.M.; Hanks, B.A.; Jung, S.H.; Salama, A.; Ho, L.; Mosca, P.J. Higher BMI, But Not Sarcopenia, Is Associated With Pembrolizumab-related Toxicity in Patients With Advanced Melanoma. Anticancer Res. 2020, 40, 5245–5254. [Google Scholar] [CrossRef]
- Hirsch, L.; Bellesoeur, A.; Boudou-Rouquette, P.; Arrondeau, J.; Thomas-Schoemann, A.; Kirchgesner, J.; Gervais, C.; Jouinot, A.; Chapron, J.; Giraud, F.; et al. The impact of body composition parameters on severe toxicity of nivolumab. Eur. J. Cancer 2020, 124, 170–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roch, B.; Coffy, A.; Jean-Baptiste, S.; Palaysi, E.; Daures, J.P.; Pujol, J.L.; Bommart, S. Cachexia-sarcopenia as a determinant of disease control rate and survival in non-small lung cancer patients receiving immune-checkpoint inhibitors. Lung Cancer 2020, 143, 19–26. [Google Scholar] [CrossRef]
- Shiroyama, T.; Nagatomo, I.; Koyama, S.; Hirata, H.; Nishida, S.; Miyake, K.; Fukushima, K.; Shirai, Y.; Mitsui, Y.; Takata, S.; et al. Impact of sarcopenia in patients with advanced non-small cell lung cancer treated with PD-1 inhibitors: A preliminary retrospective study. Sci. Rep. 2019, 9, 2447. [Google Scholar] [CrossRef] [Green Version]
- Takada, K.; Yoneshima, Y.; Tanaka, K.; Okamoto, I.; Shimokawa, M.; Wakasu, S.; Takamori, S.; Toyokawa, G.; Oba, T.; Osoegawa, A.; et al. Clinical impact of skeletal muscle area in patients with non-small cell lung cancer treated with anti-PD-1 inhibitors. J. Cancer Res. Clin. Oncol. 2020, 146, 1217–1225. [Google Scholar] [CrossRef]
- Tsukagoshi, M.; Yokobori, T.; Yajima, T.; Maeno, T.; Shimizu, K.; Mogi, A.; Araki, K.; Harimoto, N.; Shirabe, K.; Kaira, K. Skeletal muscle mass predicts the outcome of nivolumab treatment for non-small cell lung cancer. Medicine 2020, 99, e19059. [Google Scholar] [CrossRef]
- Akce, M.; Liu, Y.; Zakka, K.; Martini, D.J.; Draper, A.; Alese, O.B.; Shaib, W.L.; Wu, C.; Wedd, J.P.; Sellers, M.T.; et al. Impact of Sarcopenia, BMI, and Inflammatory Biomarkers on Survival in Advanced Hepatocellular Carcinoma Treated with Anti-PD-1 Antibody. Am. J. Clin. Oncol. 2021, 44, 74–81. [Google Scholar] [CrossRef]
- Kim, N.; Yu, J.I.; Park, H.C.; Yoo, G.S.; Choi, C.; Hong, J.Y.; Lim, H.Y.; Lee, J.; Choi, M.S.; Lee, J.E.; et al. Incorporating sarcopenia and inflammation with radiation therapy in patients with hepatocellular carcinoma treated with nivolumab. Cancer Immunol. Immunother. 2020, 70, 1593–1603. [Google Scholar] [CrossRef]
- Chu, M.P.; Li, Y.; Ghosh, S.; Sass, S.; Smylie, M.; Walker, J.; Sawyer, M.B. Body composition is prognostic and predictive of ipilimumab activity in metastatic melanoma. J. Cachexia Sarcopenia Muscle 2020, 11, 748–755. [Google Scholar] [CrossRef]
- Young, A.C.; Quach, H.T.; Song, H.; Davis, E.J.; Moslehi, J.J.; Ye, F.; Williams, G.R.; Johnson, D.B. Impact of body composition on outcomes from anti-PD1 +/- anti-CTLA-4 treatment in melanoma. J. Immunother. Cancer 2020, 8, e000821. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, H.; Fukuda, S.; Moriyama, S.; Uehara, S.; Yasuda, Y.; Tanaka, H.; Yoshida, S.; Yokoyama, M.; Matsuoka, Y.; Fujii, Y. Impact of sarcopenia on the efficacy of pembrolizumab in patients with advanced urothelial carcinoma: A preliminary report. Anticancer Drugs 2020, 31, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.Y.; Lee, J.; Jeong, W.K.; Kim, S.T.; Kim, J.H.; Hong, J.Y.; Kang, W.K.; Kim, K.M.; Sohn, I.; Choi, D. Prognostic significance of sarcopenia in microsatellite-stable gastric cancer patients treated with programmed death-1 inhibitors. Gastric Cancer 2021, 24, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A.; Makarov, V.; Merghoub, T.; Yuan, J.; Zaretsky, J.M.; Desrichard, A.; Walsh, L.A.; Postow, M.A.; Wong, P.; Ho, T.S.; et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014, 371, 2189–2199. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Kronbichler, A.; Eisenhut, M.; Hong, S.H.; van der Vliet, H.J.; Kang, J.; Shin, J.I.; Gamerith, G. Tumor Mutational Burden and Efficacy of Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancers 2019, 11, 1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, J.K.; de Souto Barreto, P.; Fougère, B.; Rolland, Y.; Vellas, B.; Cesari, M. Chronic inflammation and sarcopenia: A regenerative cell therapy perspective. Exp. Gerontol. 2018, 103, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J. T cell exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef]
- Nelke, C.; Dziewas, R.; Minnerup, J.; Meuth, S.G.; Ruck, T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine 2019, 49, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Schnyder, S.; Handschin, C. Skeletal muscle as an endocrine organ: PGC-1α, myokines and exercise. Bone 2015, 80, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Nardone, O.M.; de Sire, R.; Petito, V.; Testa, A.; Villani, G.; Scaldaferri, F.; Castiglione, F. Inflammatory Bowel Diseases and Sarcopenia: The Role of Inflammation and Gut Microbiota in the Development of Muscle Failure. Front. Immunol. 2021, 12, 2783. [Google Scholar] [CrossRef]
- Roviello, G.; Iannone, L.F.; Bersanelli, M.; Mini, E.; Catalano, M. The gut microbiome and efficacy of cancer immunotherapy. Pharmacol. Ther. 2021, 107973, Epub ahead of printing. [Google Scholar] [CrossRef]
- Guzman-Prado, Y.; Ben Shimol, J.; Samson, O. Sarcopenia and the risk of adverse events in patients treated with immune checkpoint inhibitors: A systematic review. Cancer Immunol. Immunother. 2021, 70, 2771–2780. [Google Scholar] [CrossRef]
- Rutten, I.J.G.; Ubachs, J.; Kruitwagen, R.; Beets-Tan, R.G.H.; Olde Damink, S.W.M.; Van Gorp, T. Psoas muscle area is not representative of total skeletal muscle area in the assessment of sarcopenia in ovarian cancer. J. Cachexia Sarcopenia Muscle 2017, 8, 630–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, K.I.; Doleman, B.; Scott, S.; Lund, J.N.; Williams, J.P. Simple psoas cross-sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications. Colorectal Dis. 2015, 17, O20–O26. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Author, Year | Country | Cancer | Stage | Time Point of CT Exam a | ICI Type | Measurement of Sarcopenia | Cut-Off Value of Sarcopenia b | No. of Patients | Median Age of Patients | No. of Sarcopenia(%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Minami 2020 [14] | Japan | NSCLC | Advanced | 90 days | Nivolumab, Pembrolizumab, Atezolizumab | PMI | Male:6.36, Female:3.92 | 74 | 70 | 53(71) |
| Magri 2019 [19] | Italy | NSCLC | Stage IV | 10 weeks | Nivolumab | SMI | NA | 46 | 65 | NA |
| Roch 2020 [32] | France | NSCLC | Metastatic | NA | Nivolumab, Pembrolizumab | SMI | Male: 52.4, Female: 38.5 | 142 | 64 | 92(66) |
| Shiroyama 2019 [33] | Japan | NSCLC | Advanced | 90 days | Nivolumab, Pembrolizumab | PMI | Male:6.36, Female:3.92 | 42 | 71 | 22(52) |
| Takada 2020 [34] | Japan | NSCLC | Stage III, IV/Recurred | NA | Nivolumab, Pembrolizumab | SMI | Male: 25.63, Female: 21.73 | 103 | 67 | 51(49) |
| Tsukagoshi 2020 [35] | Japan | NSCLC | Stage III, IV | 30 days | Nivolumab | PMI | Male:6.36, Female:3.92 | 30 | 67 | 13(43) |
| Akce 2020 [36] | USA | HCC | Advanced | 2 months | Anti PD-1, Anti-PD-1 + others(not specified) | SMI | Male: 43, Female: 39 | 57 | 66 | 28(49) |
| Kim N 2020 [37] | Korea | HCC | Advanced | NA | Nivolumab | SMI | Male: 42, Female: 38 | 102 | 61 | 23(23) |
| Chu 2020 [38] | Canada | Melanoma | Metastatic/ advanced | 30 days | Ipilimumab | SMI | Male: 43(52 c), Female: 41 | 97 | 56 | NA |
| Young 2020 [39] | USA | Melanoma | Metastatic/ advanced | 6 months | Nivolumab, Pembrolizumab, Atezolizumab, Ipilimunab + nivolumab | SMI | Male: 43(52 c), Female: 41 | 287 | 63 | 154(54) |
| Shimizu 2020 [13] | Japan | Urothelial carcinoma | Metastatic/ advanced | NA | Pembrolizumab | PMI | Male:6.36, Female:3.92 | 27 | 73 | 15(56) |
| Fukushima 2020 [40] | Japan | Urothelial carcinoma | Advanced | 30 days | Pembrolizumab | SMI | Male: 43(52 c), Female: 41 | 28 | 74 | 19(68) |
| Kim Y 2020 [41] | Korea | Gastric cancer | Metastatic | 3 months | Nivolumab, Pembrolizumab | SMI | Male: 49, Female: 31 | 149 | 57 | 79(53) |
| Cortellini 2020 [12] | Italy | NSCLC, Melanoma, RCC, others | Advanced | 90 days | Pembrolizumab, Nivolumab, Atezolizumab, and others | SMI | Male: 48.4(50.2 c), Female: 36.9(59.6 c) | 100 | 66 | 50(50) |
| Selection | Comparability | Outcome | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Author, Year | Representativeness of the Exposed Cohort | Selection of the Non-Exposed Cohort | Ascertainment of Exposure | Demonstration That Outcome of Interest Was Not Present at Start of Study | Comparability of Cohorts on the Basis of the Design or Analysis a | Assessment of Outcome | Was Follow-Up Long Enough for Outcomes to Occur b | Adequacy of Follow Up of Cohorts c | |
| Young 2020 [39] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Minami 2020 [14] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Shimizu 2020 [13] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Shiroyama 2019 [33] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Kim Y 2020 [41] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Cortellini 2020 [12] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Roch 2020 [32] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Fukushima 2020 [40] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Takada 2020 [34] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Akce 2020 [36] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Chu 2020 [38] | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 6 |
| Kim N 2020 [37] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Magri 2019 [19] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Tsukagoshi 2020 [35] | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 5 |
| Certainty Assessment | Effect | Certainty | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Relative (95% CI) | ||
| Overall survival in univariate analysis | |||||||||
| 8 | observational studies | serious a | not serious b | not serious | not serious | none | HR 1.66 (1.20 to 2.29) | ⨁◯◯◯ VERY LOW | |
| Overall survival with adjusted HRs | |||||||||
| 9 | observational studies | serious a | not serious | serious c | not serious | none | HR 1.55 (1.15 to 2.10) | ⨁◯◯◯ VERY LOW | |
| Progression free survival in univariate analysis | |||||||||
| 9 | observational studies | serious a | not serious | not serious | not serious | none | HR 1.75 (1.37 to 2.23) | ⨁◯◯◯ VERY LOW | |
| Progression free survival with adjusted HRs | |||||||||
| 10 | observational studies | serious a | not serious | serious c | not serious | none | HR 1.63 (1.28 to 2.09) | ⨁◯◯◯ VERY LOW | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Kim, N.W.; Kim, J.Y.; Lee, J.H.; Noh, J.H.; Lee, H.; Jeong, J.W.; Lee, S.; Kang, J. Sarcopenia’s Prognostic Impact on Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 5329. https://doi.org/10.3390/jcm10225329
Lee D, Kim NW, Kim JY, Lee JH, Noh JH, Lee H, Jeong JW, Lee S, Kang J. Sarcopenia’s Prognostic Impact on Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021; 10(22):5329. https://doi.org/10.3390/jcm10225329
Chicago/Turabian StyleLee, Donggun, Na Won Kim, Jong Yeob Kim, Joo Hyung Lee, Ji Hyun Noh, Haejun Lee, Jin Woon Jeong, Seungeun Lee, and Jeonghyun Kang. 2021. "Sarcopenia’s Prognostic Impact on Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 10, no. 22: 5329. https://doi.org/10.3390/jcm10225329
APA StyleLee, D., Kim, N. W., Kim, J. Y., Lee, J. H., Noh, J. H., Lee, H., Jeong, J. W., Lee, S., & Kang, J. (2021). Sarcopenia’s Prognostic Impact on Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 10(22), 5329. https://doi.org/10.3390/jcm10225329






