Efficacy and Safety of Tranexamic Acid in Shoulder Arthroscopic Surgery: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Quality Appraisal and Assessment of Risk of Bias
2.4. Data Collection and Abstraction
2.5. Meta-Analysis Methods and Subgroup Analysis
3. Results
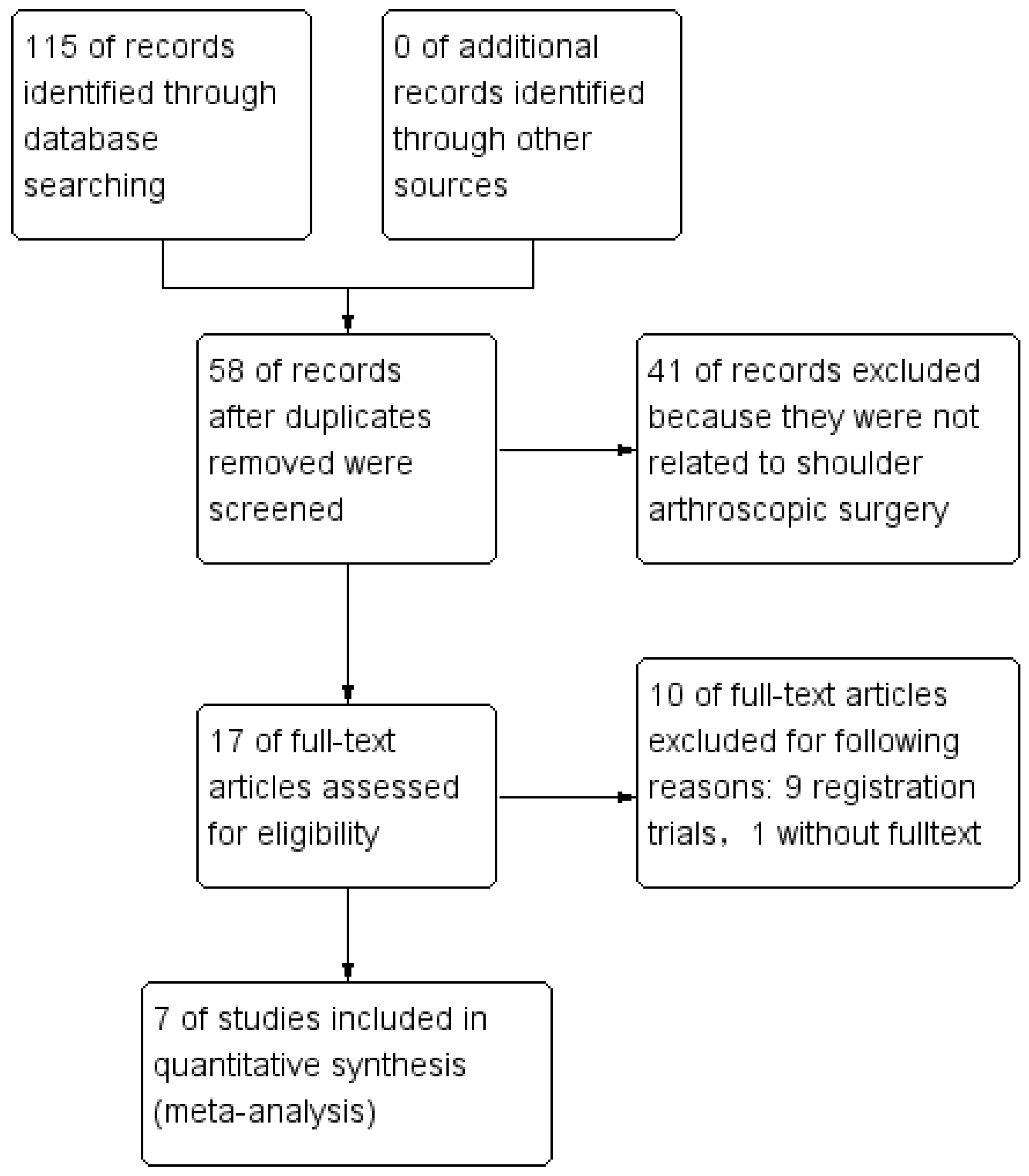
3.1. Study Selection
3.2. Search Results
3.3. Quality Appraisal and Risk of Bias
3.4. Primary Outcomes
Visual Clarity
3.5. VAS 1 Day Postoperational
3.6. Blood Loss
3.7. Secondary Outcomes
Operation Time
3.8. Irrigation Amount Used
3.9. Postoperative Shoulder Swelling
3.10. The Need for Pressure Increase and MAP
3.11. Adverse and Functional Outcomes
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, H.H.; Wu, W.T.; Tsai, I.C.; Chang, K.V. Does Suprascapular Nerve Release Provide Additional Benefits for Rotator Cuff Repair: A Systematic Review and Meta-Analysis. J. Shoulder Elbow Surg. 2022, 31, 2121–2430. [Google Scholar] [CrossRef] [PubMed]
- Walker-Santiago, R.; Maldonado, D.R.; Domb, B.G.; Lall, A.C. Fundamentals of Arthroscopy Fluid Management and Strategies to Safely Improve Visualization. J. Am. Acad. Orthop. Surg. 2021, 29, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Gruson, K.I.; Lo, Y.; Stallone, S.; Qawasmi, F.; Lee, S.; Shah, P. A Comparison of Operative Time and Intraoperative Blood Volume Loss Between Stemless and Short-stem Anatomic Total Shoulder Arthroplasty: A Single Institution’s Experience. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2022, 6, e22.00141. [Google Scholar] [CrossRef] [PubMed]
- Van Montfoort, D.O.; van Kampen, P.M.; Huijsmans, P.E. Epinephrine Diluted Saline-Irrigation Fluid in Arthroscopic Shoulder Surgery: A Significant Improvement of Clarity of Visual Field and Shortening of Total Operation Time. A Randomized Controlled Trial. Arthroscopy 2016, 32, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.A.; Wyrobek, J.A.; Butwick, A.J.; Pivalizza, E.G.; Hare, G.M.T.; Mazer, C.D.; Goobie, S.M. Update on Applications and Limitations of Perioperative Tranexamic Acid. Anesth. Analg. 2022, 135, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.K.; Lee, J.Y.; Tay, A.; Kuster, M. Intra-articular versus intravenous administration of tranexamic acid in lower limb total arthroplasty: A systematic review and meta-analysis of randomised clinical trials. Eur. J. Orthop. Surg. Traumatol. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Donovan, R.L.; Varma, J.R.; Whitehouse, M.R.; Blom, A.W.; Kunutsor, S.K. Tranexamic acid use to decrease blood loss in primary shoulder and elbow replacement: A systematic review and meta-analysis. J. Orthop. 2021, 24, 239–247. [Google Scholar] [CrossRef]
- Pecold, J.; Al-Jeabory, M.; Krupowies, M.; Manka, E.; Smereka, A.; Ladny, J.R.; Szarpak, L. Tranexamic Acid for Shoulder Arthroplasty: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 11, 48. [Google Scholar] [CrossRef]
- Hurley, E.T.; Fat, D.L.; Pauzenberger, L.; Mullett, H. Tranexamic acid for the Latarjet procedure: A randomized controlled trial. J. Shoulder Elb. Surg. 2020, 29, 882–885. [Google Scholar] [CrossRef]
- Johns, W.L.; Walley, K.C.; Hammoud, S.; Gonzalez, T.A.; Ciccotti, M.G.; Patel, N.K. Tranexamic Acid in Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2021, 49, 4030–4041. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Hong, C.-K.; Hsu, K.-L.; Kuan, F.-C.; Chen, Y.; Yeh, M.-L.; Su, W.-R. Intravenous Administration of Tranexamic Acid Significantly Improved Clarity of the Visual Field in Arthroscopic Shoulder Surgery. A Prospective, Double-Blind, and Randomized Controlled Trial. Arthrosc. -J. Arthrosc. Relat. Surg. 2020, 36, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Ersin, M.; Demirel, M.; Büget, M.; Edipoğlu, İ.S.; Atalar, A.C.; Erşen, A. The effect of intravenous tranexamic acid on visual clarity during arthroscopic rotator cuff repair: A randomized, double-blinded, placebo-controlled pilot study. Acta Orthop. Traumatol. Turc. 2020, 54, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Cenatiempo, M.; Distefano, M.; Tucci, R.; Di Bella, L.; Chiaracane, G.; Marotta, T. Preoperative Systemic Administration of Acid Tranexamic to Reduce Intraoperative Bleeding in Arthroscopic Rotator Cuff Repair. J. Shoulder Elb. Surg. 2021, 30, e464. [Google Scholar] [CrossRef]
- Mackenzie, S.P.; Spasojevic, M.; Smith, M.; Mattern, O.; Piggott, R.P.; Patel, S.S.; Bedaiwy, N.; Cass, B.; Young, A. The effect of single-dose, preoperative intravenous tranexamic acid on early postoperative pain scores after rotator cuff repair: A double-blind, randomized controlled trial. J. Shoulder Elb. Surg. 2022, 31, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Kajita, Y.; Iwahori, Y.; Harada, Y. Tranexamic acid administration for arthroscopic rotator cuff repair: A prospective, double-blind, randomized controlled trial. J. Orthop. Sci. 2021, in press. [CrossRef] [PubMed]
- Vrabel, M. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Rev. Espaola De Nutr. Hum. Y Dietética 2009, 18, e123. [Google Scholar]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Wiley-Blackwell: NewYork, NY, USA, 2008. [Google Scholar]
- Bayram, E.; Yildirm, C.; Erturk, A.K.; Yilmaz, M.; Atlihan, D. Comparison of the efficacy of irrigation with epinephrine or tranexamic acid on visual clarity during arthroscopic rotator cuff repair: A double-blind, randomized-controlled study. Jt. Dis. Relat. Surg. 2021, 32, 115–121. [Google Scholar] [CrossRef]
- Gao, H.-L.; Zhang, J.-C.; He, Y.; Zhai, W.-T.; Xiao, L.-B.; Shi, Q. Clinical study on the control of intra-articular hemorrhage by tranexamic acid after shoulder arthroscopy. Zhongguo Gu Shang = China J. Orthop. Traumatol. 2020, 33, 238–241. [Google Scholar]
- Nicholson, T.A.; Kirsch, J.; Churchill, R.; Abboud, J.A.; Lazarus, M.D.; Namdari, S. The effect of tranexamic acid for visualization on pump pressure and visualization during arthroscopic rotator cuff repair: A blinded, randomized controlled trial. In Proceedings of the AAOS American Academy of Orthopaedic Surgeons, 2021 Annual Meeting 2021, San Diego, CA, USA, 31 August–3 September 2021. [Google Scholar]
- Le, B.T.; Wu, X.L.; Lam, P.H.; Murrell, G.A. Factors predicting rotator cuff retears: An analysis of 1000 consecutive rotator cuff repairs. Am. J. Sports Med. 2014, 42, 1134–1142. [Google Scholar] [CrossRef]
- Tctr, Subacromial Injection of Tranexamic Acid Improves Early Postoperative Range of Motion in Arthroscopic Rotator Cuff Repair: A Prospective Randomized Controlled Trial. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=TCTR202105300022021 (accessed on 20 October 2022).
- Nct, Arthroscopic Rotator Cuff Repair Performed with Intraarticular Tranexamic Acid Could It Provide Improved Visual Clarity and Less Post-Operative Pain? A Prospective, Double Blind, Randomized Study of 64 Patients. Available online: https://clinicaltrials.gov/show/NCT054532662022 (accessed on 20 October 2022).
- Nct, The Aim of This Study is to Examine the Effect of Intravenously Administered Tranexamic Acid (TXA) on the Visual Clarity, Perioperative Hemorrhage, Duration and Early Postoperative Course of Shoulder Arthroscopy in Beach Chair Position. Available online: https://clinicaltrials.gov/show/NCT053976522022 (accessed on 20 October 2022).
- Nct, Effect on Post-operative Pain of Tranexamic Acid Injection During Shoulder Surgery. Available online: https://clinicaltrials.gov/show/NCT053029862022 (accessed on 20 October 2022).
- Nct, Does Tranexamic Acid Improve Visualization During Arthroscopic Rotator Cuff Repair. Available online: https://clinicaltrials.gov/show/NCT048653802021 (accessed on 20 October 2022).
- Nct, Tranexamic Acid to Improve Arthroscopic Visualization in Shoulder Surgery: RCT. Available online: https://clinicaltrials.gov/show/NCT045944082020 (accessed on 20 October 2022).
- Nct, The Effect of Intravenous Tranexamic Acid and Interscalene Block Applied on Shoulder Arthroscopy. Available online: https://clinicaltrials.gov/show/NCT044192462020 (accessed on 20 October 2022).
- Kct, Prospective Trials to Validate the Effect of Intravenous Tranexamic Acid on Visual Clarity during Arthroscopic Shoulder Surgery in Patients under Interscalene Brachial Plexus Block. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=KCT00064502021 (accessed on 20 October 2022).
- Chi, C.I. Tranexamic Acid Decreases Blood Loss during Shoulder Arthroscopy. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR-INR-170137082017 (accessed on 20 October 2022).
- Avery, D.M., 3rd; Gibson, B.W.; Carolan, G.F. Surgeon-rated visualization in shoulder arthroscopy: A randomized blinded controlled trial comparing irrigation fluid with and without epinephrine. Arthroscopy 2015, 31, 12–18. [Google Scholar] [CrossRef]
- Kuo, L.T.; Chen, C.L.; Yu, P.A.; Hsu, W.H.; Chi, C.C.; Yoo, J.C. Epinephrine in irrigation fluid for visual clarity in arthroscopic shoulder surgery: A systematic review and meta-analysis. Int. Orthop. 2018, 42, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Changjun, C.; Xin, Z.; Yue, L.; Chengcheng, Z.; Qiuru, W.; Qianhao, L.; Pengde, K. Tranexamic acid attenuates early post-operative systemic inflammatory response and nutritional loss and avoids reduction of fibrinogen in total hip arthroplasty within an enhanced recovery after surgery pathway. Int. Orthop. 2021, 45, 2811–2818. [Google Scholar] [CrossRef] [PubMed]
- Hartland, A.W.; Teoh, K.H.; Rashid, M.S. Clinical Effectiveness of Intraoperative Tranexamic Acid Use in Shoulder Surgery: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2021, 49, 3145–3154. [Google Scholar] [CrossRef] [PubMed]
- Haratian, A.; Shelby, T.; Hasan, L.K.; Bolia, I.K.; Weber, A.E.; Petrigliano, F.A. Utilization of Tranexamic Acid in Surgical Orthopaedic Practice: Indications and Current Considerations. Orthop. Res. Rev. 2021, 13, 187–199. [Google Scholar] [CrossRef]
- Albano, D.; Chianca, V.; Zappia, M.; Russo, R.; Romano, S.; Sconfienza, L.M.; Di Pietto, F. Imaging of Usual and Unusual Complication of Rotator Cuff Repair. J. Comput. Assist Tomogr. 2019, 43, 359–366. [Google Scholar] [CrossRef]
- Denard, P.J.; Lädermann, A.; Burkhart, S.S. Prevention and management of stiffness after arthroscopic rotator cuff repair: Systematic review and implications for rotator cuff healing. Arthroscopy 2011, 27, 842–848. [Google Scholar] [CrossRef]






| Included Study, Year | Level of Evidence | Country | Procedure | Group (No.) | Administration | Anesthesia Measured | Postoperative Rehabilitation | Pain Control | Measured Outcomes | Complications | Follow-Up | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TXA | Control | |||||||||||
| Liu et al., 2019 [11] | II | China | RCR | 1 g (37) | Saline (35) | Intravenously 10 min before surgery | General anesthesia combined with anecho-guided interscalene plexus block | Protected with an arm abduction brace | Postoperative analgesia (tramadol/acetaminophen 37.5 mg/325 mg 4 times daily), morphine 4 mg subcutaneously, or ketorolac 30 mg intramuscularly were given if necessary | Visual clarity, analgesic usage, blood loss, operative time, inpatient duration, associated comorbidities, VAS pain score on postoperative day 1, and postoperative shoulder swelling | No thromboembolic adverse effects, wound complications, or infections noted in either group | 12 weeks |
| Ersin et al., 2020 [12] | II | Turkey | RCR | 10 mg/kg (32) | Saline (28) | Intravenously 20 min before surgery | General anesthesia | NM | NM | Visual clarity, operation time, irrigation amount used, and the need for pressure increase | NM | NM |
| Gao et al., 2020 [19] | II | China | RCR | 0.5 g in 10-mL Saline (30) | Saline (30) | Intra-articularly inject in to the shoulder joint and subacromial space | General anesthesia | Fixed with Shoulder arm strap suspension | NM | Hb reduction and shoulder swelling | 6 cases in the TXA group and 2 cases in the placebo group occurred subcutaneous bloody ecchymosis | 1 week |
| Takahashi et al., 2021 [15] | I | Japan | RCR | 1 g (33) | Saline (33) | Intravenously 10 min before surgery | General anesthesia | Immobilized for 4 weeks, dflexion and relaxation of the muscles on the day after surgery, strengthen exercises of the rotator cuff and scapular stabilizers at 6 weeks. Rehabilitation was performed at least 3 months after surgery. Full return to sports or heavy labor was allowed after 6 months. | 1 g acetaminophen intravenously every 6 h for 24 h postoperatively | Visual clarity, VAS pain scores, blood loss, operative time | NM | 1 week |
| Bayram et al., 2021 [18] | I | Turkey | RCR | 0.42 mg per 1 L of saline (43) | 0.33 mg of epinephrine per 1 L of saline (47) | Add in irrigation fluid | General anesthesia | NM | NM | Visual clarity, total operating time, potential thrombotic or thromboembolic side effects, mean arterial pressure (MAP), and total amount of irrigation fluid used | No cardiac, thrombotic, or thromboembolic complications | NM |
| Mackenzie et al., 2022 [14] | I | Australia | RCR | 2 g (47) | Saline (42) | Preoperative and intravenously before the surgery | General anesthesia with the use of an interscalene nerve block using bupivacaine 0.75% | Immobilized in a sling for 4–6 weeks followed by a standardized physiotherapy-led protocol involving active assisted range of motion exercises from 6 weeks followed by a graduated strengthening regime from 12 weeks | NM | VAS pain score, American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, and constant scores and range of motion | 6 cases of frozen shoulder, 5 occurred in the placebo group and 1 in the TXA group, one of 3 patients with re-tears underwent reverse shoulder arthroplasty | 52 weeks |
| Nicholson et al., 2022 [20] | II | USA | RCR | 1 g (50) | No TXA (50) | Preoperative and intravenously before the surgery | A preoperative interscalene brachial plexus nerve block | NM | NM | Visualization, VAS pain scores, operative time, final pump pressure, number of increases in pump pressure, total amount of irrigation fluid utilized, MAP, blood pressure, and anesthesia medical interventions for blood pressure | NM | NM |
| Study | Study Design | Randomization | Concealment of Allocation | Double Blinding | Withdrawal and Dropout | Total |
|---|---|---|---|---|---|---|
| Bayram et al., 2021 [18] | RCT | 2 | 2 | 2 | 1 | 7 |
| Ersin et al., 2020 [12] | RCT | 2 | 1 | 2 | 1 | 6 |
| Gao et al., 2020 [19] | RCT | 2 | 1 | 1 | 1 | 5 |
| Liu et al., 2019 [11] | RCT | 2 | 2 | 2 | 1 | 7 |
| Mackenzie et al., 2022 [14] | RCT | 2 | 2 | 2 | 1 | 7 |
| Nicholson et al., 2022 [20] | RCT | 2 | 1 | 1 | 1 | 5 |
| Takahashi et al., 2021 [15] | RCT | 2 | 2 | 2 | 1 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Xiao, D.; Fu, W.; Cai, W.; Huang, X.; Li, Q.; Li, J. Efficacy and Safety of Tranexamic Acid in Shoulder Arthroscopic Surgery: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 6886. https://doi.org/10.3390/jcm11236886
Sun Y, Xiao D, Fu W, Cai W, Huang X, Li Q, Li J. Efficacy and Safety of Tranexamic Acid in Shoulder Arthroscopic Surgery: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(23):6886. https://doi.org/10.3390/jcm11236886
Chicago/Turabian StyleSun, Yiyuan, Dan Xiao, Weili Fu, Wufeng Cai, Xihao Huang, Qi Li, and Jian Li. 2022. "Efficacy and Safety of Tranexamic Acid in Shoulder Arthroscopic Surgery: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 23: 6886. https://doi.org/10.3390/jcm11236886
APA StyleSun, Y., Xiao, D., Fu, W., Cai, W., Huang, X., Li, Q., & Li, J. (2022). Efficacy and Safety of Tranexamic Acid in Shoulder Arthroscopic Surgery: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(23), 6886. https://doi.org/10.3390/jcm11236886




