Safety and Efficacy of Intermediate- and Therapeutic-Dose Anticoagulation for Hospitalised Patients with COVID-19: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Criteria for Considering Studies for This Review
- Therapeutic-dose versus standard thromboprophylaxis (low- or intermediate-dose anticoagulation);
- Intermediate-dose versus low-dose anticoagulation.
- All-cause mortality at day 28, day 60, time-to-event, and at hospital discharge;
- Clinical status at day 28, day 60, and up to the longest follow-up, including:
- ∘
- Worsening of clinical status: participants with clinical deterioration (e.g., new need for invasive mechanical ventilation) or death;
- ∘
- Improvement of clinical status: participants discharged alive. Participants should be discharged without clinical deterioration or death.
- Any thrombotic event or death within 28 days;
- Any thrombotic event within 28 days;
- Quality of life, including fatigue and neurological status, assessed with standardised scales (e.g., WHOQOL-100) at up to 7 days, up to 28 days, and longest follow-up available;
- Serious adverse events during the study period, defined as number of participants with any event;
- Adverse events (any grade) during the study period, defined as number of participants with any event;
- Major bleeding (ISTH criteria [15]) during the study period.
2.2. Search Methods for Identification of Studies
2.3. Data Collection and Analyses
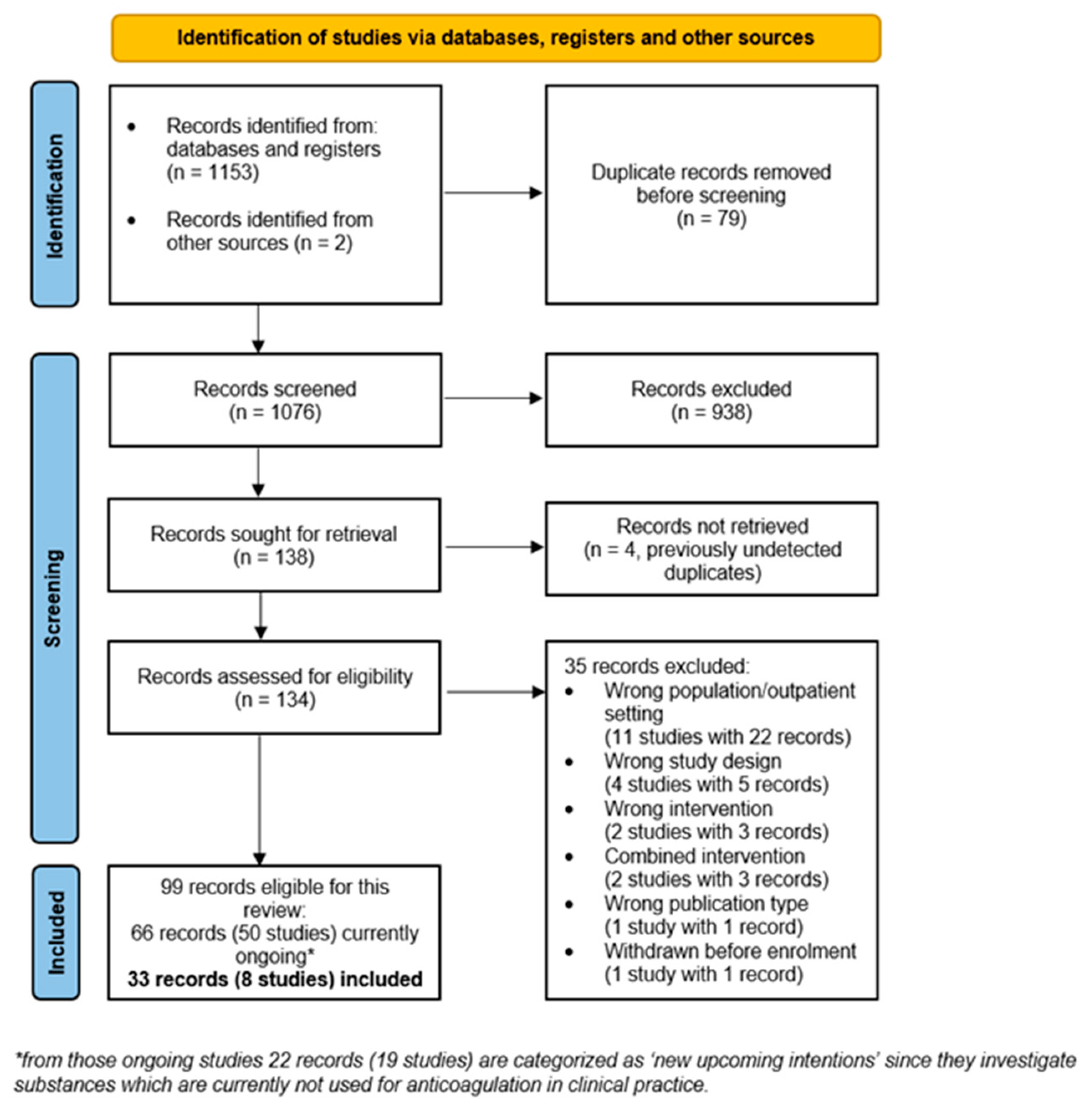
3. Results
3.1. Study Characteristics
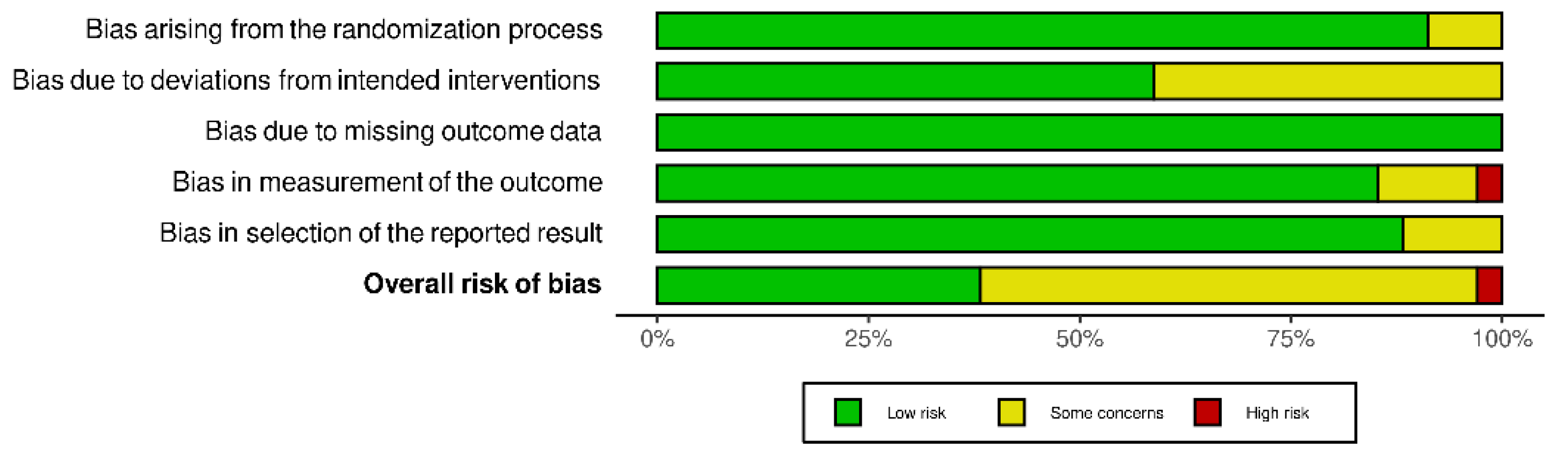
3.2. Risk of Bias
3.3. Intermediate-Dose Anticoagulation
3.4. Therapeutic-Dose Anticoagulation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Synowiec, A.; Szczepanski, A.; Barreto-Duran, E.; Lie, L.K.; Pyrc, K. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): A Systemic Infection. Clin. Microbiol. Rev. 2021, 34, e00133-20. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal Coagulation Parameters Are Associated with Poor Prognosis in Patients with Novel Coronavirus Pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [Green Version]
- Varikasuvu, S.R.; Varshney, S.; Dutt, N.; Munikumar, M.; Asfahan, S.; Kulkarni, P.P.; Gupta, P. D-Dimer, Disease Severity, and Deaths (3D-Study) in Patients with COVID-19: A Systematic Review and Meta-Analysis of 100 Studies. Sci. Rep. 2021, 11, 21888. [Google Scholar] [CrossRef]
- AWMF S3-Leitlinie 003/00: Prophylaxe der venösen Thromboembolie (VTE). Reg Nr 003/001. 2015. Available online: www.awmf.org/uploads/tx_szleitlinien/003–001l_S3_VTE-Prophylaxe_2015–12.pdf (accessed on 15 November 2021).
- Schünemann, H.J.; Cushman, M.; Burnett, A.E.; Kahn, S.R.; Beyer-Westendorf, J.; Spencer, F.A.; Rezende, S.M.; Zakai, N.A.; Bauer, K.A.; Dentali, F.; et al. American Society of Hematology 2018 Guidelines for Management of Venous Thromboembolism: Prophylaxis for Hospitalized and Nonhospitalized Medical Patients. Blood Adv. 2018, 2, 3198–3225. [Google Scholar] [CrossRef]
- Słomka, A.; Kowalewski, M.; Żekanowska, E. Hemostasis in Coronavirus Disease 2019—Lesson from Viscoelastic Methods: A Systematic Review. Thromb. Haemost. 2021, 121, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Al-Ani, F.; Chehade, S.; Lazo-Langner, A. Thrombosis Risk Associated with COVID-19 Infection. A Scoping Review. Thromb. Res. 2020, 192, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Rote Liste Fachinfo Service. Available online: https://www.fachinfo.de/ (accessed on 15 November 2021).
- National Institute for Health and Care Excellence. COVID-19 Rapid Guideline: Reducing the Risk of Venous Thromboembolism in over 16s with COVID-19. In NICE Guideline 186; NICE: London, UK, 2020; Available online: https://www.nice.org.uk/guidance/ng186/ (accessed on 13 November 2021).
- Core Outcome set Developers’ Response to COVID-19. Available online: www.comet-initiative.org/Studies/Details/1538 (accessed on 13 November 2021).
- Marshall, J.C.; Murthy, S.; Diaz, J.; Adhikari, N.K.; Angus, D.C.; Arabi, Y.M.; Baillie, K.; Bauer, M.; Berry, S.; Blackwood, B.; et al. A Minimal Common Outcome Measure Set for COVID-19 Clinical Research. Lancet Infect. Dis. 2020, 20, e192–e197. [Google Scholar] [CrossRef]
- Kreuzberger, N.; Hirsch, C.; Chai, K.L.; Tomlinson, E.; Khosravi, Z.; Popp, M.; Neidhardt, M.; Piechotta, V.; Salomon, S.; Valk, S.J.; et al. SARS-CoV-2-Neutralising Monoclonal Antibodies for Treatment of COVID-19. Cochrane Database Syst. Rev. 2021, 9. [Google Scholar] [CrossRef]
- Popp, M.; Stegemann, M.; Riemer, M.; Metzendorf, M.-I.; Romero, C.S.; Mikolajewska, A.; Kranke, P.; Meybohm, P.; Skoetz, N.; Weibel, S. Antibiotics for the Treatment of COVID-19. Cochrane Database Syst. Rev. 2021, 2021. [Google Scholar] [CrossRef]
- Schulman, S.; Kearon, C.; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of Major Bleeding in Clinical Investigations of Antihemostatic Medicinal Products in Non-Surgical Patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Chapter 5: Collecting Data. In Cochrane Handbook for Systematic Reviews of Interventions Version 61; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2020; Available online: training.cochrane.org/handbook (accessed on 12 November 2021).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.S.J.; Page, M.J.; Elbers, R.G.; Sterne Jacobson, J.R. Chapter 8: Assessing Risk of Bias in a Randomized Trial. In Cochrane Handbook for Systematic; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G.; on Behalf of the Cochrane Statistical Methods Group. Chapter 10: Analysing Data and Undertaking Meta-Analyses. In Cochrane Handbook for Systematic Reviews of Interventions Version 61 (Updated September 2020); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2020; Available online: training.cochrane.org/handbook (accessed on 12 November 2021).
- Review Manager Web (RevManWeb). Version 3.11.1. 2021. The Cochrane Collaboration. 2021. Available online: www.revman.cochrane.org (accessed on 12 November 2021).
- Schünemann, H.J.; Vist, G.E.; Glasziou, P.; Akl, E.A.; Skoetz, N.; Guyatt, G.H. Chapter 14: Completing ‘Summary of Findings’ Tables and Grading the Certainty of the Evidence. In Cochrane Handbook for Systematic Reviews of Interventions Version 62 (Updated February 2021); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2021; Available online: www.training.cochrane.org/handbook (accessed on 12 November 2021).
- Bikdeli, B.; Talasaz, A.H.; Rashidi, F.; Bakhshandeh, H.; Rafiee, F.; Rezaeifar, P.; Baghizadeh, E.; Matin, S.; Jamalkhani, S.; Tahamtan, O.; et al. Intermediate-Dose versus Standard-Dose Prophylactic Anticoagulation in Patients with COVID-19 Admitted to the Intensive Care Unit: 90-Day Results from the INSPIRATION Randomized Trial. Thromb. Haemost. 2020, 196, 382–394. [Google Scholar]
- The REMAP-CAP Investigators; ACTIV-4a Investigators; Goligher, E.C.; Bradbury, C.A.; McVerry, B.J.; Lawler, R.P.; Berger, J.S.; Gong, M.N.; Carrier, M.; Reynolds, H.R.; et al. Therapeutic Anticoagulation with Heparin in Critically Ill Patients with Covid-19. N. Engl. J. Med. 2021, 385, 777–789. [Google Scholar] [PubMed]
- The ATTACC, ACTIV-4a, and REMAP-CAP Investigators; Lawler, P.R.; Goligher, E.C.; Berger, J.S.; Neal, M.D.; McVerry, B.J.; Nicolau, J.C.; Gong, M.N.; Carrier, M.; Rosenson, R.S. Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 385, 790–802. [Google Scholar] [PubMed]
- Lemos, A.C.B.; do Espirito Santo, D.A.; Salvetti, M.C.; Gilio, R.N.; Agra, L.B.; Pazin-Filho, A.; Miranda, C.H. Therapeutic versus Prophylactic Anticoagulation for Severe COVID-19: A Randomized Phase II Clinical Trial (HESACOVID). Thromb. Res. 2020, 196, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.D.; de Barros, E.; Silva, P.G.M.; Furtado, R.H.M.; Macedo, A.V.S.; Bronhara, B.; Damiani, L.P.; Barbosa, L.M.; de Aveiro Morata, J.; Ramacciotti, E.; et al. Therapeutic versus Prophylactic Anticoagulation for Patients Admitted to Hospital with COVID-19 and Elevated D-Dimer Concentration (ACTION): An Open-Label, Multicentre, Randomised, Controlled Trial. Lancet 2021, 397, 2253–2263. [Google Scholar] [CrossRef]
- Perepu, U.S.; Chambers, I.; Wahab, A.; Ten Eyck, P.; Wu, C.; Dayal, S.; Sutamtewagul, G.; Bailey, S.R.; Rosenstein, L.J.; Lentz, S.R. Standard Prophylactic versus Intermediate Dose Enoxaparin in Adults with Severe COVID-19: A Multi-Center, Open-Label, Randomized Controlled Trial. J. Thromb. Haemost. 2021, 19, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Sadeghipour, P.; Talasaz, A.H.; Rashidi, F.; Sharif-Kashani, B.; Beigmohammadi, M.T.; Farrokhpour, M. Effect of Intermediate-Dose vs. Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial. JAMA 2021, 325, 1620–1630. [Google Scholar] [PubMed]
- Sholzberg, M.; Tang, G.H.; Rahhal, H.; AlHamzah, M.; Kreuziger, L.B.; Áinle, F.N.; Alomran, F.; Alayed, K.; Alsheef, M.; AlSumait, F.; et al. Heparin for Moderately Ill Patients with COVID-19. medRxiv 2021, 21259351. [Google Scholar]
- Spyropoulos, A.C.; Goldin, M.; Giannis, D.; Diab, W.; Wang, J.; Khanijo, S.; Mignatti, A.; Gianos, E.; Cohen, M.; Sharifova, G.; et al. Efficacy and Safety of Therapeutic-Dose Heparin vs. Standard Prophylactic or Intermediate-Dose Heparins for Thromboprophylaxis in High-Risk Hospitalized Patients With COVID-19: The HEP-COVID Randomized Clinical Trial. JAMA Intern. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Levy, J.H.; Warkentin, T.E.; Thachil, J.; Van Der Poll, T.; Levi, M. Diagnosis and Management of Sepsis-Induced Coagulopathy and Disseminated Intravascular Coagulation. J. Thromb. Haemost. 2019, 17, 1989–1994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-Bias VISualization (Robvis): An R Package and Shiny Web App for Visualizing Risk-of-Bias Assessments. Res. Synth. Methods 2020. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant Treatment Is Associated with Decreased Mortality in Severe Coronavirus Disease 2019 Patients with Coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Lachant, D.J.; Lachant, N.A.; Kouides, P.; Rappaport, S.; Prasad, P.; White, R.J. Chronic Therapeutic Anticoagulation Is Associated with Decreased Thrombotic Complications in SARS-CoV-2 Infection. J. Thromb. Haemost. 2020, 18, 2640–2645. [Google Scholar] [CrossRef] [PubMed]
- Parisi, R.; Costanzo, S.; Di Castelnuovo, A.; de Gaetano, G.; Donati, M.B.; Iacoviello, L. Different Anticoagulant Regimens, Mortality, and Bleeding in Hospitalized Patients with COVID-19: A Systematic Review and an Updated Meta-Analysis. Semin. Thromb. Hemost. 2021, 47, 372–391. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Warkentin, T.E.; Thachil, J.; Levi, M.; Levy, J.H. Proposal of the Definition for COVID-19-Associated Coagulopathy. J. Clin. Med. 2021, 10, 191. [Google Scholar] [CrossRef]
- EMA/617633/2020—Xarelto (Rivaroxaban): An Overview of Xarelto and Why It Is Authorised in the EU European Medicines Agency. Available online: https://www.ema.europa.eu/en/documents/overview/xarelto-epar-medicine-overview_en.pdf (accessed on 15 November 2021).
- Beurskens, D.M.H.; Huckriede, J.P.; Schrijver, R.; Hemker, H.C.; Reutelingsperger, C.P.; Nicolaes, G.A.F. The Anticoagulant and Nonanticoagulant Properties of Heparin. Thromb. Haemost. 2020, 120, 1371–1383. [Google Scholar] [CrossRef]
- Kapoor, M.; Panda, P.K.; Saini, L.K.; Bahurupi, Y.A. A Retrospective Analysis of DIC Score and SIC Score in Prediction of COVID-19 Severity. medRxiv 2021, 21259369. [Google Scholar]
- Carfora, V.; Spiniello, G.; Ricciolino, R.; Di Mauro, M.; Migliaccio, M.G.; Mottola, F.F.; Verde, N.; Coppola, N.; Vanvitelli COVID-19 Group. Anticoagulant Treatment in COVID-19: A Narrative Review. J. Thromb. Thrombolysis 2021, 51, 642–648. [Google Scholar]
| Study Reference | Study Design | Setting and Patient Status | Randomised Patients | Intervention | Comparator | Selected Outcomes |
|---|---|---|---|---|---|---|
| INSPIRATION, [22,28] | RCT, open-label, multi-centre | ICU WHO 5–9, with 45% WHO 5 | 600 | Intermediate-dose anticoagulation A with enoxaparin 1 mg/kg OD sc for 30 days; weight and CrCI adjusted | Standard thromboprophylaxis with enoxaparin 40 mg OD; weight and CrCI adjusted | 30-day mortality, 90-day mortality, any venous thrombotic events, any venous thrombotic events or death, major bleeding |
| Perepu-2021 [27] | RCT, multi-centre, open-label | Hospitalised + mod. ISTH Overt DIC score ≥3 + ICU, WHO 5–9, no details on respiratory status reported | 173 | Intermediate-dose anticoagulation A with enoxaparin 1 mg/kg sc OD until hospital discharge; weight and CrCI adjusted | Standard thromboprophylaxis with enoxaparin 40 mg sc OD until hospital discharge or extended beyond, weight and CrCI adjusted | 30-day mortality, any venous thrombotic events, major bleeding |
| HESACOVID, [25] | RCT, open-label, single centre | ICU WHO ≥ 7 | 20 | Therapeutic-dose anticoagulation A with enoxaparin 1 mg/kg sc BID for at least 96 h and up to 14 days | Standard thromboprophylaxis with enoxaparin 40 mg OD; weight and CrCI adjusted | 28-day mortality, in-hospital mortality, any thrombotic event |
| ACTION [26] | RCT, multi-centre, open-label | Hospitalised/ ICU + ↑ D-Dimer, WHO 4–9, with 85% WHO 4–5 | 614 | Therapeutic-dose anticoagulation A with rivaroxaban 20 mg po OD (280 patients, 90%) for 30 days | Standard thromboprophylaxis with enoxaparin 40 mg sc OD, continued until or extended beyond hospital discharge; weight and CrCI adjusted | 30-day mortality, survival until hospital discharge (30 days), any thrombotic event, any thrombotic event or death, major bleeding |
| RAPID 2021 [29] | RCT, multi-centre, open-label | Hospitalised + ↑ D-Dimer, WHO 4–5, with 6% WHO 6 | 465 | Therapeutic-dose anticoagulation A Enoxaparin 1 mg/kg sc BID; weight and CrCI adjusted | Standard thromboprophylaxis with enoxaparin 40 mg OD, weight and CrCl adjusted | All-cause mortality, venous thrombotic events, major bleeding |
| ATTACC, ACTIV-4a, REMAP-CAP Non-critically ill [24] | RCT, open-label, Bayesian, adaptive, multiplatform | Hospitalised WHO 4–5, with 5% WHO 6–7 | 2244 | Therapeutic-dose anticoagulation A (79.6%) with enoxaparin 1 mg/kg sc minus 10% BID, weight and CrCl adjusted | Standard low- or intermediate-dose thromboprophylaxis with 78.7 %: enoxaparin, 9.6%: dalteparin; Low-dose: 71.7%, intermediate-dose: 26.5% subtherapeutic-dose: 0.8% therapeutic-dose: 0.9% | In-hospital mortality, clinical worsening: intubation or death, clinical improvement: discharged without receiving organ support, any thrombotic event, any thrombotic event or death, major bleeding |
| ATTAC, ACTIV-4a, REMAP-CAP Critically ill [23] | RCT, open-label, Bayesian, adaptive, multiplatform | ICU WHO 6–9, 1.5% WHO 4–5 | 1207 | Therapeutic-dose anticoagulation A (77.6%) with enoxaparin 1 mg/kg minus 10% BID, weight and CrCl adjusted | Standard low- or intermediate-dose thromboprophylaxis with 52.1%: enoxaparin, 32.8%: dalteparin; Low-dose: 40.4%, Intermediate-dose: 51.7% Subtherapeutic-dose: 1.8% Therapeutic-dose: 6.1% | In-hospital mortality, any thrombotic event, any thrombotic event or death, major bleeding |
| HEP-COVID 2021 [30] | RCT, multi-center, open-label | Hospitalised + ↑ D-Dimer or ISTH SIC score ≥ 4, WHO 5–7, with 77% WHO 5, both strata reported for some outcomes | 257 | Therapeutic-dose anticoagulation A with enoxaparin 1 mg/kg sc BID, or 40 mg sc OD/BID weight and CrCI adjusted, until hospital discharge | Standard thromboprophylaxis with enoxaparin 40 mg sc OD/BID weight and CrCI adjusted, until hospital discharge | All-cause mortality, any thromboembolic event, any thromboembolic event or death, major bleeding |
| Outcome | Study Population * | Risk Ratio (M–H, Random, 95% CI) | Risk Ratio (M–H, Fixed, 95% CI) | Heterogeneity | Certainty of Evidence |
|---|---|---|---|---|---|
| All-cause mortality at 30 days | Pooled effect, mixed population (WHO 4–9), 763 participants, 2 studies [22,27,28] | 0.98 (0.74, 1.32) | 1.01 (0.84, 1.21) | Tau2 = 0.02; Chi2 = 1.28, df = 1 (p = 0.26); I2 = 22% | Low-certainty evidence due to serious risk of bias and imprecision |
| All-cause mortality at 90 days | Mixed population (WHO 4–9), 590 participants, 1 study [22,28] | 1.07 (0.89, 1.28) | 1.07 (0.89, 1.28) | NA | Low-certainty evidence due to serious risk of bias and imprecision |
| Any thrombotic event or death up to 30 days | Mixed population (WHO 4–9), 590 participants, 1 study [22,28] | 1.03 (0.86, 1.24) | 1.03 (0.86, 1.24) | NA | Low-certainty evidence due to serious risk of bias and imprecision |
| Any venous thrombotic event up to 30 days | Pooled effect, mixed population (WHO 4–9), 763 participants, 2 studies [22,27,28] | 0.99 (0.51, 1.96) | 0.99 (0.50, 1.95) | Chi2 = 0.13, df = 1 (p = 0.72); I2 = 0% | Low-certainty evidence due to serious risk of bias and imprecision |
| Major bleeding up to 28 days | Pooled effect, mixed population (WHO 4–9), 763 participants, 2 studies [22,27,28] | 1.48 (0.53, 4.15) | 1.49 (0.53, 4.14) | Tau2 = 0.00; Chi2 = 0.23, df = 1 (p = 0.63); I2 = 0% | Low-certainty evidence due to serious risk of bias and imprecision |
| Outcome | Study Population * | Risk Ratio (M–H, Random, 95% CI) | Risk Ratio (M–H, Fixed, 95% CI) | Heterogeneity | Certainty of Evidence |
|---|---|---|---|---|---|
| All-cause mortality (28 days) | Moderately diseased population (WHO 4–5), 465 participants, 1 study [29] | 0.23 (0.08, 0.67) | 0.23 (0.08, 0.67) | NA | Low-certainty evidence due to very serious imprecision |
| Severely diseased population (WHO 6–9), 20 participants, 1 study [25] | 0.33 (0.04, 2.69) | 0.33 (0.04, 2.69) | NA | Very low-certainty evidence due to risk of bias and very serious imprecision | |
| Mixed population (WHO 4–9), 867 participants, 2 studies [26,30] | 1.07 (0.56, 2.03) | 1.08 (0.77, 1.51) | Tau2 = 0.16; Chi2 = 3.54, df = 1 (p = 0.06); I2 = 72% | Low-certainty evidence due to serious heterogeneity and imprecision | |
| Pooled effect, mixed population (WHO 4–9), 1352 participants, 4 studies [25,26,29,30] | 0.68 (0.32, 1.45) | 0.85 (0.62, 1.16) | Tau2 = 0.38; Chi2 = 11.47, df = 3 (p = 0.009); I2 = 74% | Low-certainty evidence due to serious heterogeneity and imprecision | |
| All-cause mortality in hospital | Pooled effect, mixed population (WHO 4–9), 3344 participants, 3 studies [23,24,25] | 0.97 (0.79, 1.19) | 0.99 (0.86, 1.13) | Tau2 = 0.01; Chi2 = 2.78, df = 2 (p = 0.25); I2 = 28% | Low-certainty evidence due to serious indirectness and risk of bias |
| Worsening of clinical status: Progression to intubation or death (28 days) | Moderately diseased population (WHO 4–5), 2231 participants, 1 study [24] | 0.90 (0.72, 1.14) | 0.90 (0.72, 1.14) | NA | Low-certainty evidence due to serious indirectness and risk of bias |
| Worsening of clinical status: Progression to any mechanical ventilation or death (28 days) | Moderately diseased population (WHO 4–5), 465 participants, 1 study [29] | 0.63 (0.39, 1.02) | 0.63 (0.39, 1.02) | NA | Low-certainty evidence due to very serious imprecision |
| Improvement of clinical status: participants discharged alive without clinical deterioration or death at 28 days | Mixed population (WHO 4–9), 614 participants, 1 study [26] | 0.96 (0.90, 1.02) | 0.96 (0.90, 1.02) | NA | High-certainty evidence |
| Improvement of clinical status: survival until hospital discharge without receiving organ support | Moderately diseased population (WHO 4–5), 2219 participants, 1 study [24] | 1.05 (1.00, 1.10) | 1.05 (1.00, 1.10) | NA | Low-certainty evidence due to serious indirectness and risk of bias |
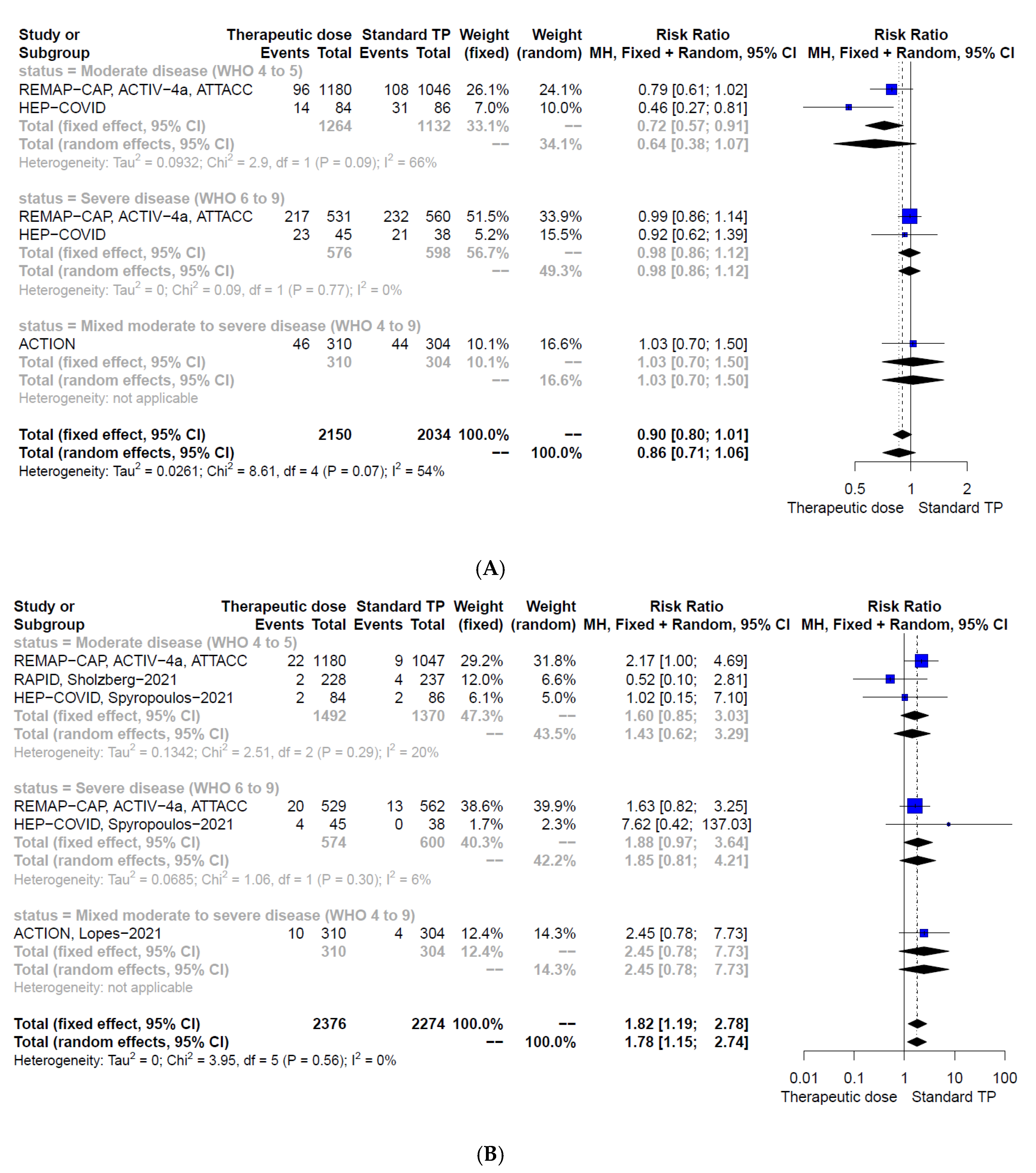
| Any thrombotic event or death | Moderately diseased population (WHO 4–5), 2396 participants, 2 studies [24,30] | 0.64 (0.38, 1.07) | 0.72 (0.57, 0.91) | Chi2 = 2.90, df = 1 (p = 0.09); I2 = 66% | Low-certainty evidence due to serious risk of bias and indirectness/heterogeneity |
| Severely diseased population (WHO 6–9), 1174 participants, 2 studies [23,30] | 0.98 (0.86, 1.12) | 0.98 (0.86, 1.12) | Chi2 = 0.09, df = 1 (p = 0.77); I2 = 0% | Low-certainty evidence due to serious risk of bias and indirectness | |
| Mixed population (WHO 4–9), 614 participants, 1 study [26] | 1.03 (0.70, 1.50) | 1.03 (0.70, 1.50) | NA | Low-certainty evidence due to serious risk of bias and imprecision | |
| Pooled effect, mixed population (WHO 4–9), 4184 participants, 4 studies [23,24,26,30] | 0.86 (0.71, 1.06) | 0.90 (0.80, 1.01) | Chi2 = 8.61, df = 4 (p = 0.07); I2 = 54% | Low-certainty evidence due to serious risk of bias and indirectness/heterogeneity | |
| Any thrombotic event | Pooled effect, mixed population (WHO 4–9), 4669 participants, 6 studies [23,24,25,26,29,30] | 0.58 (0.45, 0.74) | 0.57 (0.45, 0.73) | Tau2 = 0.00; Chi2 = 4.68, df = 5 (p = 0.46); I2 = 0% | Moderate-certainty evidence due to serious risk of bias |
| Major bleeding at 28 days | Pooled effect, mixed population (WHO 4–9), 4650 participants, 5 studies [23,24,26,29,30] | 1.78 (1.15, 2.74) | 1.82 (1.19, 2.78) | Tau2 = 0.00; Chi2 = 3.95, df = 5 (p = 0.56); I2 = 0% | Low-certainty evidence due to serious indirectness and risk of bias |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, S.; Popp, M.; Schmid, B.; Stegemann, M.; Metzendorf, M.-I.; Kranke, P.; Meybohm, P.; Weibel, S. Safety and Efficacy of Intermediate- and Therapeutic-Dose Anticoagulation for Hospitalised Patients with COVID-19: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 57. https://doi.org/10.3390/jcm11010057
Reis S, Popp M, Schmid B, Stegemann M, Metzendorf M-I, Kranke P, Meybohm P, Weibel S. Safety and Efficacy of Intermediate- and Therapeutic-Dose Anticoagulation for Hospitalised Patients with COVID-19: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(1):57. https://doi.org/10.3390/jcm11010057
Chicago/Turabian StyleReis, Stefanie, Maria Popp, Benedikt Schmid, Miriam Stegemann, Maria-Inti Metzendorf, Peter Kranke, Patrick Meybohm, and Stephanie Weibel. 2022. "Safety and Efficacy of Intermediate- and Therapeutic-Dose Anticoagulation for Hospitalised Patients with COVID-19: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 1: 57. https://doi.org/10.3390/jcm11010057
APA StyleReis, S., Popp, M., Schmid, B., Stegemann, M., Metzendorf, M.-I., Kranke, P., Meybohm, P., & Weibel, S. (2022). Safety and Efficacy of Intermediate- and Therapeutic-Dose Anticoagulation for Hospitalised Patients with COVID-19: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(1), 57. https://doi.org/10.3390/jcm11010057






