The Role of Magnetic Resonance Imaging to Inform Clinical Decision-Making in Acute Spinal Cord Injury: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. KQ1: Diagnostic Accuracy of MRI
3.2. KQ2: Frequency of Abnormal Findings
3.2.1. Ligamentous Injury
3.2.2. Disc Injury/Herniation
3.2.3. Cord Compression
3.2.4. Epidural Hematoma
3.2.5. Fracture
3.2.6. Intramedullary Lesions in SCIWORA
3.3. KQ3: Influence of MRI on Clinical Decision-Making
3.3.1. If Surgery Is Required
3.3.2. Surgical Approach
3.3.3. When to Operate
3.3.4. Need for Instrumentation
3.3.5. Which Levels to Decompress
3.3.6. Need for Re-Operation after Surgery
3.4. KQ4: When to Perform MRI
3.5. KQ5: Frequency of Adverse Events When Performing MRI
3.6. KQ6: Effect of MRI on Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Wyndaele, M.; Wyndaele, J.J. Incidence, prevalence and epidemiology of spinal cord injury: What learns a worldwide literature survey? Spinal Cord 2006, 44, 523–529. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Vaccaro, A.; Wilson, J.R.; Singh, A.; Cadotte, D.W.; Harrop, J.S.; Aarabi, B.; Shaffrey, C.; Dvorak, M.; Fisher, C.; et al. Early versus Delayed Decompression for Traumatic Cervical Spinal Cord Injury: Results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). PLoS ONE 2012, 7, e32037. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Tetreault, L.A.; Wilson, J.R.; Kwon, B.K.; Burns, A.S.; Martin, A.R.; Hawryluk, G.; Harrop, J.S. A Clinical Practice Guideline for the Management of Acute Spinal Cord Injury: Introduction, Rationale, and Scope. Glob. Spine J. 2017, 7, 84s–94s. [Google Scholar] [CrossRef] [PubMed]
- Hadley, M.N.; Walters, B.C.; Grabb, P.A.; Oyesiku, N.M.; Przybylski, G.J.; Resnick, D.K.; Ryken, T.C. Guidelines for management of acute cervical spinal injuries. Introduction. Neurosurgery 2002, 50, S1. [Google Scholar]
- Walters, B.C.; Hadley, M.N.; Hurlbert, R.J.; Aarabi, B.; Dhall, S.S.; Gelb, D.E.; Harrigan, M.R.; Rozelle, C.J.; Ryken, T.C.; Theodore, N. Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery 2013, 60, 82–91. [Google Scholar] [CrossRef]
- Ryken, T.C.; Hadley, M.N.; Walters, B.C.; Aarabi, B.; Dhall, S.S.; Gelb, D.E.; Hurlbert, R.J.; Rozzelle, C.J.; Theodore, N. Radiographic assessment. Neurosurgery 2013, 72 (Suppl. 2), 54–72. [Google Scholar] [CrossRef] [Green Version]
- Kurpad, S.N.; Martin, A.R.; Tetreault, L.A.; Fischer, D.J.; Skelly, A.C.; Mikulis, D.; Flanders, A.E.; Aarabi, B.; Mroz, T.; Tsai, E.C.; et al. Impact of baseline magnetic resonance imaging on neurologic, functional, and safety outcomes in patients with acute traumatic spinal cord injury. Glob. Spine J. 2017, 7, 151S–174S. [Google Scholar]
- Bozzo, A.; Marcoux, J.; Radhakrishna, M.; Pelletier, J.; Goulet, B. The role of magnetic resonance imaging in the management of acute spinal cord injury. J. Neurotrauma 2011, 28, 1401–1411. [Google Scholar] [PubMed] [Green Version]
- Fehlings, M.G.; Martin, A.R.; Tetreault, L.; Aarabi, B.; Anderson, P.; Arnold, P.M.; Broke, D.; Burns, A.; Chiba, K.; Hawryluk, G.; et al. A Clinical Practice Guideline for the Management of Patients with Acute Spinal Cord Injury: Recommendations on the Role of Baseline Magnetic Resonance Imaging in Clinical Decision Making and Outcome Prediction. Glob. Spine J. 2017, 7, 221S–230S. [Google Scholar]
- Papadopoulos, S.M.; Selden, N.R.; Quint, D.J.; Patel, N.; Gillespie, B.; Grube, S. Immediate spinal cord decompression for cervical spinal cord injury: Feasibility and outcome. J. Trauma 2002, 52, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 6.2; Cochrane: Oxford, UK, 2021. [Google Scholar]
- Edelman, R.R. The History of MR Imaging as Seen through the Pages of Radiology. Radiology 2014, 273, S181–S200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2013. [Google Scholar]
- National Heart, Lung, and Blood Institute. National Institute of Health: Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies; National Heart, Lung, and Blood Institute: Bethesda, MD, USA, 2014.
- Krappinger, D.; Lindtner, R.A.; Zegg, M.J.; Henninger, B.; Kaser, V.; Spicher, A.; Schmid, R. Spondylotic traumatic central cord syndrome: A hidden discoligamentous injury? Eur. Spine J. 2019, 28, 434–441. [Google Scholar] [CrossRef] [Green Version]
- Henninger, B.; Kaser, V.; Ostermann, S.; Spicher, A.; Zegg, M.; Schmid, R.; Kremser, C.; Krappinger, D. Cervical Disc and Ligamentous Injury in Hyperextension Trauma: MRI and Intraoperative Correlation. J. Neuroimaging 2020, 30, 104–109. [Google Scholar] [CrossRef]
- Zhu, F.; Yao, S.; Ren, Z.; Telemacque, D.; Qu, Y.; Chen, K.; Yang, F.; Zeng, L.; Guo, X. Early durotomy with duroplasty for severe adult spinal cord injury without radiographic abnormality: A novel concept and method of surgical decompression. Eur. Spine J. 2019, 28, 2275–2282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, T.; Ueta, T.; Mori, E.; Yugue, I.; Kawano, O.; Takao, T.; Sakai, H.; Okada, S.; Shiba, K. Soft-tissue damage and segmental instability in adult patients with cervical spinal cord injury without major bone injury. Spine 2012, 37, E1560–E1566. [Google Scholar] [CrossRef]
- Mirvis, S.E.; Geisler, F.H.; Jelinek, J.J.; Joslyn, J.N.; Gellad, F. Acute cervical spine trauma: Evaluation with 1.5-T MR imaging. Radiology 1988, 166, 807–816. [Google Scholar]
- Bao, Y.; Zhong, X.; Zhu, W.; Chen, Y.; Zhou, L.; Dai, X.; Liao, J.; Li, Z.; Hu, K.; Bei, K.; et al. Feasibility and Safety of Cervical Kinematic Magnetic Resonance Imaging in Patients with Cervical Spinal Cord Injury without Fracture and Dislocation. Orthop. Surg. 2020, 12, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Kalfas, I.; Wilberger, J.; Goldberg, A.; Prostko, E.R. Magnetic resonance imaging in acute spinal cord trauma. Neurosurgery 1988, 23, 295–299. [Google Scholar] [CrossRef]
- Como, J.J.; Samia, H.; Nemunaitis, G.A.; Jain, V.; Anderson, J.S.; Malangoni, M.A.; Claridge, J.A. The misapplication of the term spinal cord injury without radiographic abnormality (SCIWORA) in adults. J. Trauma Acute Care Surg. 2012, 73, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Hendey, G.W.; Wolfson, A.B.; Mower, W.R.; Hoffman, J.R. Spinal cord injury without radiographic abnormality: Results of the National Emergency X-Radiography Utilization Study in blunt cervical trauma. J. Trauma 2002, 53, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Maung, A.A.; Johnson, D.C.; Barre, K.; Peponis, T.; Mesar, T.; Velmahos, G.C.; McGrail, D.; Kasotakis, G.; Gross, R.I.; Rosenblatt, M.S.; et al. Cervical spine MRI in patients with negative CT: A prospective, multicenter study of the Research Consortium of New England Centers for Trauma (ReCONECT). J. Trauma Acute Care Surg. 2017, 82, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Song, K.J.; Kim, G.H.; Lee, K.B. The efficacy of the modified classification system of soft tissue injury in extension injury of the lower cervical spine. Spine 2008, 33, E488–E493. [Google Scholar] [CrossRef]
- Cheng, X.; Ni, B.; Liu, Q.; Chen, J.; Guan, H.; Guo, Q. Clinical and radiological outcomes of spinal cord injury without radiologic evidence of trauma with cervical disc herniation. Arch. Orthop. Trauma Surg. 2013, 133, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Aarabi, B.; Alexander, M.; Mirvis, S.E.; Shanmuganathan, K.; Chesler, D.; Maulucci, C.; Iguchi, M.; Aresco, C.; Blacklock, T. Predictors of outcome in acute traumatic central cord syndrome due to spinal stenosis. J. Neurosurg. Spine 2011, 14, 122–130. [Google Scholar] [CrossRef]
- Doran, S.E.; Papadopoulos, S.M.; Ducker, T.B.; Lillehei, K.O. Magnetic resonance imaging documentation of coexistent traumatic locked facets of the cervical spine and disc herniation. J. Neurosurg. 1993, 79, 341–345. [Google Scholar] [PubMed]
- Darsaut, T.E.; Ashforth, R.; Bhargava, R.; Broad, R.; Emery, D.; Kortbeek, F.; Lambert, R.; Lavoie, M.; Mahood, J.; MacDowell, I.; et al. A pilot study of magnetic resonance imaging-guided closed reduction of cervical spine fractures. Spine 2006, 31, 2085–2090. [Google Scholar] [CrossRef]
- Gupta, S.K.; Rajeev, K.; Khosla, V.K.; Sharma, B.S.; Paramjit; Mathuriya, S.N.; Pathak, A.; Tewari, M.K.; Kumar, A. Spinal cord injury without radiographic abnormality in adults. Spinal Cord 1999, 37, 726–729. [Google Scholar] [PubMed]
- Sharma, S.; Singh, M.; Wani, I.H.; Sharma, S.; Sharma, N.; Singh, D. Adult Spinal Cord Injury without Radiographic Abnormalities (SCIWORA): Clinical and Radiological Correlations. J. Clin. Med. Res. 2009, 1, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Koyanagi, I.; Iwasaki, Y.; Hida, K.; Imamura, H.; Fujimoto, S.; Akino, M. Acute cervical cord injury associated with ossification of the posterior longitudinal ligament. Neurosurgery 2003, 53, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Asan, Z. Spinal Cord Injury without Radiological Abnormality in Adults: Clinical and Radiological Discordance. World Neurosurg. 2018, 114, e1147–e1151. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, Q.; Zhao, J.; Yu, H.; Ma, X.; Wang, L. Early MRI finding in adult spinal cord injury without radiologic abnormalities does not correlate with the neurological outcome: A retrospective study. Spinal Cord 2015, 53, 750–753. [Google Scholar] [CrossRef] [Green Version]
- Ghanta, M.K.; Smith, L.M.; Polin, R.S.; Marr, A.B.; Spires, W.V. An analysis of Eastern Association for the Surgery of Trauma practice guidelines for cervical spine evaluation in a series of patients with multiple imaging techniques. Am. Surg. 2002, 68, 563–567. [Google Scholar] [PubMed]
- Fehlings, M.G.; Rao, S.C.; Tator, C.H.; Skaf, G.; Arnold, P.; Benzel, E.; Dickman, C.; Cuddy, B.; Green, B.; Hitchon, P.; et al. The optimal radiologic method for assessing spinal canal compromise and cord compression in patients with cervical spinal cord injury. Part II: Results of a multicenter study. Spine 1999, 24, 605–613. [Google Scholar] [CrossRef]
- D’Souza, M.M.; Choudhary, A.; Poonia, M.; Kumar, P.; Khushu, S. Diffusion tensor MR imaging in spinal cord injury. Injury 2017, 48, 880–884. [Google Scholar] [CrossRef]
- Machino, M.; Yukawa, Y.; Ito, K.; Nakashima, H.; Kanbara, S.; Morita, D.; Kato, F. Can magnetic resonance imaging reflect the prognosis in patients of cervical spinal cord injury without radiographic abnormality? Spine 2011, 36, E1568–E1572. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.; Rajagopal, K.; Ramesh, A. Cervical spinal cord injury with and without the radiographical evidence of trauma–A retrospective comparative study in adults. J. Clin. Diagn. Res. 2010, 4, 2183–2189. [Google Scholar]
- Tewari, M.K.; Gifti, D.S.; Singh, P.; Khosla, V.K.; Mathuriya, S.N.; Gupta, S.K.; Pathak, A. Diagnosis and prognostication of adult spinal cord injury without radiographic abnormality using magnetic resonance imaging: Analysis of 40 patients. Surg. Neurol. 2005, 63, 204–209. [Google Scholar] [CrossRef]
- Vaccaro, A.R.; Falatyn, S.P.; Flanders, A.E.; Balderston, R.A.; Northrup, B.E.; Cotler, J.M. Magnetic resonance evaluation of the intervertebral disc, spinal ligaments, and spinal cord before and after closed traction reduction of cervical spine dislocations. Spine 1999, 24, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Selden, N.R.; Quint, D.J.; Patel, N.; d’Arcy, H.S.; Papadopoulos, S.M. Emergency magnetic resonance imaging of cervical spinal cord injuries: Clinical correlation and prognosis. Neurosurgery 1999, 44, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Boese, C.K.; Müller, D.; Bröer, R.; Eysel, P.; Krischek, B.; Lehmann, H.C.; Lechler, P. Spinal cord injury without radiographic abnormality (SCIWORA) in adults: MRI type predicts early neurologic outcome. Spinal Cord 2016, 54, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Ryu, R.C.; Kim, T.T.; Alban, R.F.; Margulies, D.R.; Ley, E.J.; Barmparas, G. Is magnetic resonance imaging becoming the new computed tomography for cervical spine clearance? Trends in magnetic resonance imaging utilization at a Level I trauma center. J. Trauma Acute Care Surg. 2020, 89, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Aarabi, B.; Olexa, J.; Chryssikos, T.; Galvagno, S.M.; Hersh, D.S.; Wessell, A.; Sansur, C.; Schwartzbauer, G.; Crandall, K.; Shanmuganathan, K.; et al. Extent of Spinal Cord Decompression in Motor Complete (American Spinal Injury Association Impairment Scale Grades A and B) Traumatic Spinal Cord Injury Patients: Post-Operative Magnetic Resonance Imaging Analysis of Standard Operative Approaches. J. Neurotrauma 2019, 36, 862–876. [Google Scholar] [CrossRef]
- Badhiwala, J.H.; Wilson, J.R.; Witiw, C.D.; Harrop, J.S.; Vaccaro, A.R.; Aarabi, B.; Grossman, R.G.; Geisler, F.H.; Fehlings, M.G. The influence of timing of surgical decompression for acute spinal cord injury: A pooled analysis of individual patient data. Lancet Neurol. 2021, 20, 117–126. [Google Scholar] [CrossRef]
- Masterson, K. A New Spinal Cord Injury Treatment is Getting Patients Back on Their Feet. 2018. Available online: https://www.ucsf.edu/news/2018/09/411471/new-spinal-cord-injury-treatment-getting-patients-back-their-feet (accessed on 10 October 2021).
- Martin, A.R.; Aleksanderek, I.; Cohen-Adad, J.; Tarmohamed, Z.; Tetreault, L.; Smith, N.; Cadotte, D.W.; Crawley, A.; Ginsberg, H.; Mikulis, D.J.; et al. Translating state-of-the-art spinal cord MRI techniques to clinical use: A systematic review of clinical studies utilizing DTI, MT, MWF, MRS, and fMRI. NeuroImage 2016, 10, 192–238. [Google Scholar] [CrossRef] [Green Version]


| Key Questions (KQ) |
|---|
| KQ1: What is the diagnostic accuracy of MRI to detect the following features that are likely to alter clinical management in patients with acute SCI? |
|
| KQ2: What is the frequency of abnormal MRI findings (from KQ1) in patients with acute SCI? |
| KQ3: How often does obtaining an MRI alter clinical decision-making in acute SCI?? |
|
| KQ4: When should MRI be performed in acute SCI? |
|
| KQ5: What is the frequency of adverse events when performing MRI in acute SCI patients? |
| KQ6: How does obtaining an MRI (compared with not obtaining MRI) affect neurological, functional, and health-related quality of life outcomes? |
| Inclusion | Exclusion | |
|---|---|---|
| Patient | ||
| Adult human population (≥16 years old) Studies that include patients in the acute phase of SCI (within 7 days of injury) | Pediatric population (age < 16) | |
| Intervention | ||
| MRI scan within 7 days of injury to inform one or more clinical decisions | MRI purely for prognosis | |
| Outcome | ||
| Addresses one or more key questions in Table 1 | ||
| Comparison | ||
| MRI vs. no MRI MRI vs. CT No comparison (MRI alone) | ||
| Study Design | ||
| Studies designed to assess the detection of a specific imaging feature and/or its relationship to alter decision-making or outcomes | Review articles Opinions Case reports or series < 10 patients Animal or biomechanical studies |
| Citation | Disease | Sample Size | Age (Years) | SCI Level | Sequence | Field Strength | Injury | Comparison | Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|
| Ligamentous Injury | ||||||||||
| Maeda et al., 2012 | SCIWORA, hyperextension injury | n = 88 | Mean: 64, range: 33–89 | Cervical | NS | NS | ALL injury | Instability on flexion/extension radiographs | Unstable: 23/28 (sensitivity: 0.82) Stable: 39/60 (specificity: 0.65) | |
| Krappinger et al., 2019 ** | SCIWORA, CCS, hyperextension injury | n = 23 | Mean: 62.7, range: 38–87 | Cervical | T1W, T2W, STIR | 1.5T | ALL injury PLL injury | Intraoperative findings Intraoperative findings | Radiologist On-Call Specialized MRI Radiologist | Injured: 15/22 patients (sensitivity: 0.68), 15/25 segments (sensitivity: 0.60) Uninjured: 0, denominator NS (specificity: 1.0) Injured: 19/22 patients (sensitivity: 0.86), 22/25 segments (sensitivity: 0.88) Uninjured: 0, denominator NS (specificity: 1.0) 100% agreement, no injury in 23/23 patients |
| Henninger et al., 2020 ** | SCIWORA, hyperextension injury | n = 21 | Mean: 62, range: 38–87 | Cervical | T1W, STIR | 1.5T | ALL injury PLL injury | Intraoperative findings Intraoperative findings | STIR T2 Any sequence Any sequence | 88% agreement 61% agreement 88% agreement PLL injured: 1/2 (sensitivity: 0.50) |
| Fracture | ||||||||||
| Mirvis et al., 1988 * | SCI | n = 21 | Mean: 42.5, range: 17–66 | Cervical | T1W, T2W | 1.5T | Fracture | CT myelography | Fracture: 2/5 (sensitivity: 0.40) No fracture: 14/14 (specificity: 1.0) | |
| Disc Injury/Herniation | ||||||||||
| Kalfas et al., 1988 * | SCI | n = 62 | NS | Cervical (n = 40), Thoracic (n = 17), Lumbar (n = 5) | T1W, T2W | 0.5T | Disc herniation with cord compression | Intraoperative findings | 2/2 (sensitivity: 1.0) | |
| Maeda et al., 2012 | SCIWORA, hyperextension injury | n = 88 | Mean: 64, range: 33–89 | Cervical | NS | NS | Intervertebral disc injury | Instability on flexion/extension radiographs | Unstable: 18/28 (sensitivity: 0.64) Stable: 41/60 (specificity: 0.68) | |
| Bao et al., 2020 | SCIWORA | n = 16 | Mean: 51.1, range: 30–73 | Cervical | T1W, T2W | 3.0T | Intervertebral disc injury | Intraoperative findings | T2W | 5/5 (sensitivity: 1.0) |
| Henninger et al., 2020 ** | SCIWORA, hyperextension injury | n = 21 | Mean: 62, range: 38–87 | Cervical | T1W, STIR | 1.5T | Intervertebral disc injury | Intraoperative findings | STIR T2W Any sequence | 88% agreement 61% agreement 79% agreement |
| Cord Contusion/Edema | ||||||||||
| Zhu et al., 2019 | SCIWORA | n = 16 | Mean: 47.5, range: 22–65 | Cervical | T2W | NS | Hemorrhage, contusion, or edema | Intraoperative findings | MRI | 100% (16/16) |
| Citation | Disease State | Sample Size | Age (Years) | SCI Level | Sequence | Field Strength | Injury | Outcome |
|---|---|---|---|---|---|---|---|---|
| Ligamentous Injury | ||||||||
| Vaccaro et al., 1999 | SCI, facet dislocation | n = 11 | Mean: 46, range: 17–84 | Cervical | T1W, T2W | 1.5T | ALL injury PLL injury | 73% (8/11) 45% (5/11) |
| Ghanta et al., 2002 * | SCIWORA | n = 13 (subgroup) | Mean: 28.5, range: 0.4–78 | Cervical | NS | NS | Ligamentous injury | 0% (0/13) |
| Hendey et al., 2002 | SCIWORA | n = 27 | Median: 42, range: 21–89 | Cervical | NS | NS | Ligamentous injury | 11% (3/27) |
| Koyanagi et al., 2003 | SCIWORA in OPLL | n = 28 | Mean: 63.0, range: 45–78 | Cervical | T2W | NS | Paravertebral soft tissue injury | 43% (12/28) |
| Song et al., 2008 | SCIWORA, hyperextension injury | n = 27 | Mean: 54.1, range: 21–72 | Cervical | T1W, T2W | 1.5T | ALL injury or avulsion of cartilaginous endplate PLL injury Ligamentum flavum or interspinous injury | 100% (27/27) 100% (27/27) 70% (19/27) |
| Mahmood et al., 2010 * | SCIWORA, SCI | SCIWORA: n = 25, SCI: n = 25 | Mean: 45, range: 12–64 | Cervical | T1W, T2W | 0.5T | SCIWORA: ALL injury SCIWORA: PLL injury SCIWORA: interspinous ligament injury SCIWORA: ligamentum flavum injury SCIWORA: supraspinous ligament injury SCI: ALL injury SCI: PLL injury SCI: interspinous ligament injury SCI: ligamentum flavum injury SCI: supraspinous ligament injury | 8% (2/25) 4% (1/25) 8% (2/25) 4% (1/25) 20% (5/25) 88% (22/25) 48% (12/25) 80% (20/25) 80% (20/25) 64% (16/25) |
| Aarabi et al., 2011 | SCI, CCS | n = 42 | Mean: 58.3, range: 32–87 | Cervical | T2W, STIR | NS | ALL injury | 36% (15/42) |
| Como et al., 2012 | SCIWORA | n = 24 | Mean: 60.5, range: 34–83 | Cervical | T1W, T2W | 1.5T | Ligamentous injury | 29% (7/24) |
| Maeda et al., 2012 | SCIWORA, hyperextension injury | n = 88 | Mean: 64, range: 33–89 | Cervical | NS | NS | ALL injury | 50% (44/88) |
| Cheng et al., 2013 | SCIWORA | n = 70 | Mean: 57.7, range: 36–79 | Cervical | T1W, T2W | NS | ALL injury (among patients with disc herniation) | 8% (2/26) |
| Maung et al., 2017 * | SCIWORA | n = 123 | NS | Cervical | NS | NS | Ligamentous injury | 19% (23/123) |
| Krappinger et al., 2019 ** | SCIWORA (CCS), hyperextension injury | n = 23 | Mean: 62.7, range: 38–87 | Cervical | T1W, T2W, STIR | 1.5T | ALL injury PLL injury | 96% (22/23) 0% (0/23) |
| Henninger et al., 2020 ** | SCIWORA, hyperextension injury | n = 21 | Mean: 62, range: 38–87 | Cervical | T1W, STIR | 1.5T | ALL injury Facet capsule injury | 100% (21/21) 48% (10/21) |
| Meta-analysis *** | SCIWORA SCI (including SCIWORA) | n = 404 n = 482 | Range: 0.4–89 Range: 0.4–89 | Cervical Cervical | Any ligamentous injury Any ligamentous injury | 36% (145/404), I2 = 0.94, p < 0.001 39% (190/483), I2 = 0.93, p < 0.001 | ||
| Disc Injury or Herniation | ||||||||
| Kalfas et al., 1988 * | SCI | n = 62 | NS | Cervical (n = 40), Thoracic (n = 17), Lumbar (n = 5) | T1W, T2W | 0.5T | Disc herniation with cord compression | 3% (2/62) |
| Mirvis et al., 1988 | SCI | n = 21 | Mean: 42.5, range: 17–66 | Cervical | T1W, T2W | 1.5T | Disc herniation | 57% (12/21) 37% (7/19) on CT Myelography |
| Doran et al., 1993 | SCI, facet dislocation | n = 12 | Mean: 34.1, range: 18–59 | Cervical | NS | NS | Disc herniation with cord compression Disc bulge or herniation | 83% (10/12) 100% (12/12) |
| Gupta et al., 1999 | SCIWORA | n = 15 | Range: 20–60 | Cervical | NS | NS | Disc herniation | 40% (6/15) |
| Selden et al., 1999 | SCI | n = 55 | Mean: 29.2, range: 2–92 | Cervical | T1W, T2W | 1.5T | Disc herniation | 42% (23/55) |
| Vaccaro et al., 1999 | SCI, facet dislocation | n = 11 | Mean: 46, range: 17–84 | Cervical | T1W, T2W | 1.5T | Disc herniation | Pre-Reduction:18% (2/11) Post-Reduction: 45% (5/11) |
| Ghanta et al., 2002 * | SCIWORA | n = 13 (subgroup) | Mean: 28.5, range: 0.4–78 | Cervical | NS | NS | Disc herniation | 15% (2/13) |
| Hendey et al., 2002 | SCIWORA | n = 27 | Median: 42, range: 21–89 | Cervical | NS | NS | Disc herniation | 48% (13/27) |
| Tewari et al., 2004 | SCIWORA | n = 40 | Mean: 42.1, range: 16–70 | Cervical | T1W, T2W | NS | Disc herniation | 38% (15/40) |
| Darsaut et al., 2006 | SCI, fracture-dislocation | n = 17 | Mean: 40.2, range: 19–78 | Cervical | T1W, T2W | 1.5T | Disc injury Disc herniation Disc herniation | Pre-Traction: 88% (15/17) Pre-Traction: 24% (4/17) Post-Traction: 0% (0/17) |
| Song et al., 2008 | SCIWORA, hyperextension injury | n = 27 (subgroup) | Mean: 54.1, range: 21–72 | Cervical (Lower) | T1W, T2W | 1.5T | Disc herniation | 100% (27/27) |
| Sharma et al., 2009 | SCIWORA | n = 12 | Mean: 38.66, range: 22–58 | Cervical | T1W, T2W | NS | Disc herniation | 17% (2/12) |
| Mahmood et al., 2010 * | SCIWORA, SCI | SCIWORA: n = 25, SCI: n = 25 | Mean: 45, range: 12–64 | Cervical | T1W, T2W | 0.5T | SCIWORA: disc injury SCIWORA: disc herniation SCI: disc injury SCI: disc herniation | 16% (4/25) 44% (11/25) 40% (10/25) 16% (4/25) |
| Maeda et al., 2012 | SCIWORA | n = 88 | Mean: 64, range: 33–89 | Cervical | NS | NS | Disc injury | 42% (37/88) |
| Cheng et al., 2013 | SCIWORA | n = 70 | Mean: 57.7, range: 36–79 | Cervical | T1W, T2W | NS | Disc herniation | 37% (26/70) |
| Maung et al., 2017 * | SCIWORA | n = 123 | NS | Cervical | NS | NS | Disc injury | 4% (5/123) |
| Meta-analysis | SCIWORA SCI (including SCIWORA) | n = 400 n = 577 | Mixed | SCIWORA: disc injury SCIWORA: disc herniation SCI: disc injury SCI: disc herniation SCI: Disc herniation with cord compression | 20% (46/230), I2 = 0.96, p < 0.001 45% (102/229), I2 = 0.84, p < 0.001 26% (71/278), I2 = 0.95, p < 0.001 43% (159/370), I2 = 0.83, p < 0.001 16% (12/74), I2 = 0.98, p < 0.001 | |||
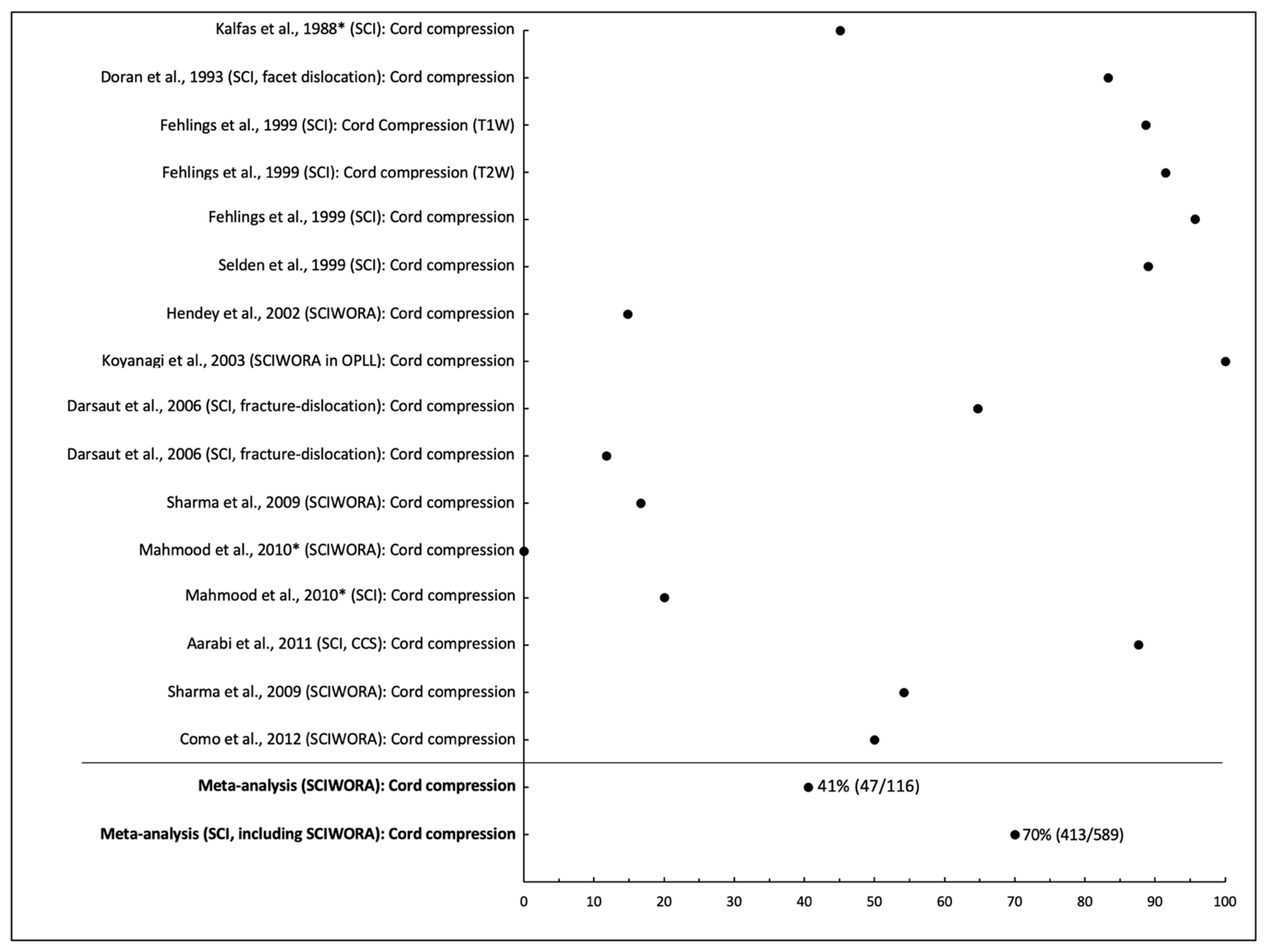
| Cord Compression | ||||||||
| Kalfas et al., 1988 * | SCI | n = 62 | NS | Cervical (n = 40), Thoracic (n = 17), Lumbar (n = 5) | T1W, T2W | 0.5T | Cord compression | 45% (28/62) |
| Doran et al., 1993 | SCI, facet dislocation | n = 12 (subgroup) | Mean: 34.1, range: 18–59 | Cervical | NS | NS | Cord compression | 83% (10/12) |
| Fehlings et al., 1999 | SCI | n = 71 | Mean: 39.7, range: 17–96 | Sub-axial (C3-T1) | T1W, T2W | NS | Cord compression | T1W: 89% (63/71) T2W: 92% (65/71) Either: 96% (68/71) |
| Selden et al., 1999 | SCI | n = 55 | Mean: 29.2, range: 2–92 | Cervical | T1W, T2W | 1.5T | Cord compression | 89% (49/55) |
| Hendey et al., 2002 | SCIWORA | n = 27 | Median: 42, range: 21–89 | Cervical | NS | NS | Cord compression | 15% (4/27) |
| Koyanagi et al., 2003 | SCIWORA in OPLL | n = 28 | Mean: 63.0, range: 45–78 | Cervical | T2W | NS | Cord compression | 100% (28/28) |
| Darsaut et al., 2006 | SCI, fracture-dislocation | n = 17 | Mean: 40.2, range: 19–78 | Sub-axial (C3-T1) | T1W, T2W | 1.5T | Cord compression | Pre-Traction: 65% (11/17) Post-Traction 6% (2/17) |
| Sharma et al., 2009 | SCIWORA | n = 12 | Mean: 38.66, range: 22–58 | Cervical | T1W, T2W | NS | Cord compression | 16% (2/12) |
| Mahmood et al., 2010 * | SCIWORA, SCI | SCIWORA: n = 25, SCI: n = 25 | Mean: 45, range: 12–64 | Cervical | T1W, T2W | 0.5T | SCIWORA: cord compression SCI: cord compression | 0% (0/25) 20% (5/25) |
| Aarabi et al., 2011 | SCI, CCS | n = 211 | Mean: 58.3, range: 32–87 | Cervical | T2W, STIR | NS | Cord compression | 88% (185/211) |
| Como et al., 2012 | SCIWORA | n = 24 | Mean: 60.5, range: 34–83 | Cervical | T1W, T2W | 1.5T | Cord compression | 54% (13/24) |
| D’Souza et al., 2017 | SCI | n = 20 | Mean: 35.95, range: 17–54 | Cervical | T1W, T2W | 3T | Cord compression | 50% (10/20) |
| Meta-analysis | SCIWORA SCI (including SCIWORA) | n = 116 n = 589 | Range: 17–96 | Mixed | Cord compression Cord compression | 41% (47/116), I2 = 0.94, p < 0.001 70% (413/589), I2 = 0.95, p < 0.001 | ||
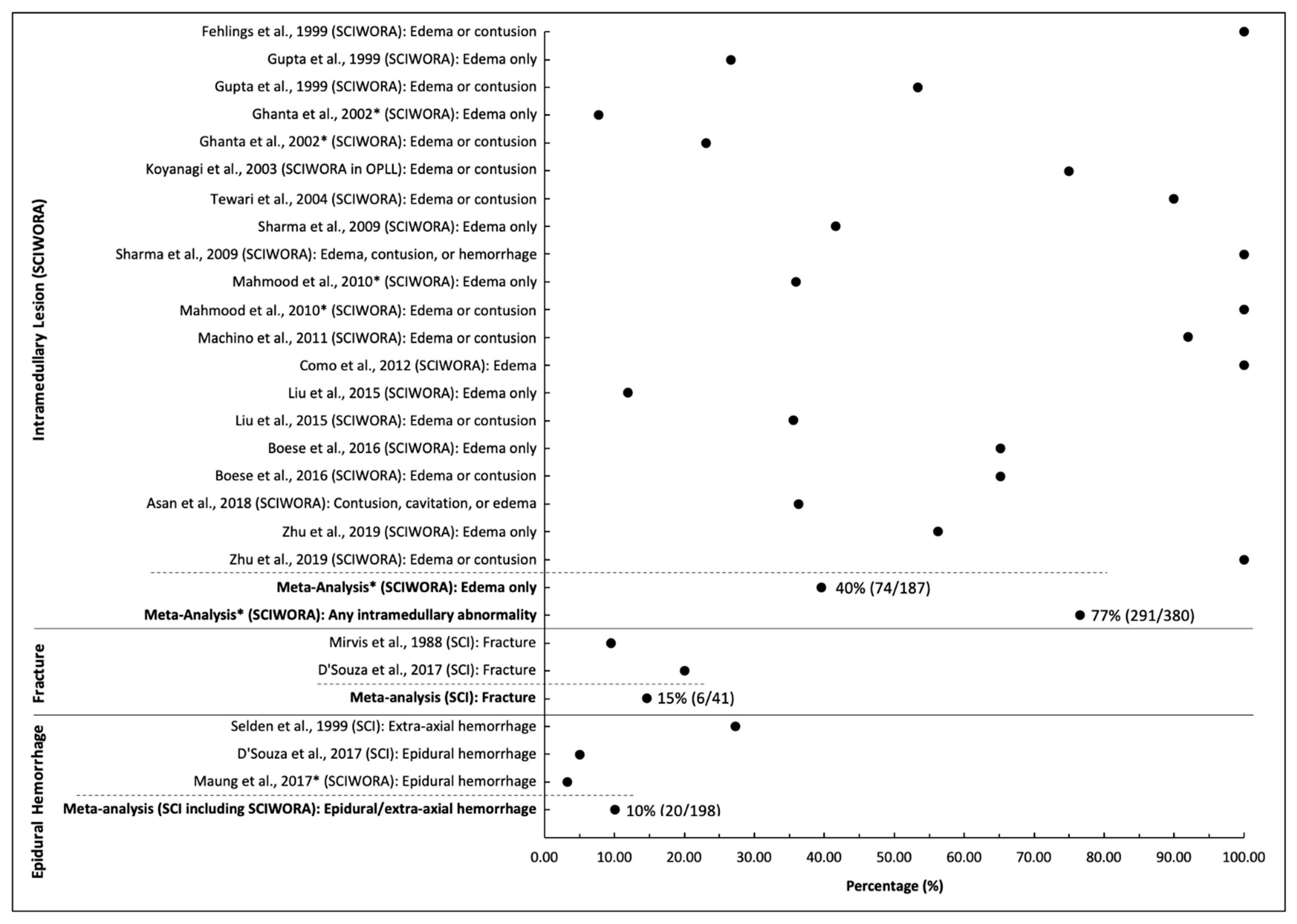
| Epidural Hematoma | ||||||||
| Selden et al., 1999 | SCI | n = 55 | Mean: 29.2, range: 2–92 | Cervical | T1W, T2W | 1.5T | Extra-axial hemorrhage | 27% (15/55) |
| D’Souza et al., 2017 | SCI | n = 20 | Mean: 35.95, range: 17–54 | Cervical | T1W, T2W, DTI | 3T | Epidural hemorrhage | 5% (1/20) |
| Maung et al., 2017 * | SCIWORA | n = 123 | NS | Cervical | NS | NS | Epidural hemorrhage | 3% (4/123) |
| Meta-analysis | SCI (including SCIWORA) | n = 143 | Range: 17–54 | Cervical | Epidural/extra-axial hemorrhage | 10% (20/198), I2 = 0.92, p < 0.001 | ||
| Fracture | ||||||||
| Mirvis et al., 1988 | SCI | n = 21 | Mean: 42.5, range: 17–66 | Cervical | T1W, T2W | 1.5T | Fracture | 10% (2/21) |
| D’Souza et al., 2017 | SCI | n = 20 | Mean: 35.95, range: 17–54 | Cervical | T1W, T2W, DTI | 3T | Fracture | 20% (4/20) |
| Meta-analysis | SCI | n = 41 | Range: 17–66 | Cervical | Fracture | 15% (6/41), I2 = 0, p = 0.61 | ||
| Intramedullary Lesion (SCIWORA) | ||||||||
| Fehlings et al., 1999 | SCIWORA | n = 14 (subgroup) | Mean: 39.7, range: 17–96 | Sub-axial (C3-T1) | T2W | NS | Edema or contusion | 100% (14/14) |
| Gupta et al., 1999 | SCIWORA | n = 15 | Range: 20–60 | Cervical | NS | NS | Edema only Edema or contusion | 27% (4/15) 53% (8/15) |
| Ghanta et al., 2002 * | SCIWORA | n = 13 (subgroup) | Mean: 28.5, range: 0.4–78 | Cervical | NS | NS | Edema only Edema or contusion | 8% (1/13) 23% (3/13) |
| Koyanagi et al., 2003 | SCIWORA in OPLL | n = 28 | Mean: 63.0, range: 45–78 | Cervical | T2W | NS | Edema or contusion | 75% (21/28) |
| Tewari et al., 2004 | SCIWORA | n = 40 | Mean: 42.1, range: 16–70 | Cervical | T1W, T2W | NS | Edema or contusion | 90% (36/40) |
| Sharma et al., 2009 | SCIWORA | n = 12 | Mean: 38.66, range: 22–58 | Cervical | T1W, T2W | NS | Edema only Edema, contusion, or hemorrhage | 42% (5/12) 100% (12/12) |
| Mahmood et al., 2010 * | SCIWORA | n = 25 (subgroup) | Mean: 45, range: 12–64 | Cervical | T1W, T2W | 0.5T | Edema only Edema or contusion | 36% (9/25) 100% (25/25) |
| Machino et al., 2011 | SCIWORA | n = 100 | Mean: 55, range: 16–87 | Cervical | T2W | 1.5T | Edema or contusion | 92% (92/100) |
| Como et al., 2012 | SCIWORA | n = 24 | Mean: 60.5, range: 34–83 | Cervical | T1W, T2W | 1.5T | Edema | 100% (24/24) |
| Liu et al., 2015 | SCIWORA | n = 59 | Mean: 41.1, range: 21–68 | Cervical (n = 19), Thoracic (n = 40) | NS | 3T | Edema only Edema or contusion | 12% (7/59) 36% (21/59) |
| Boese et al., 2016 | SCIWORA | n = 23 | Mean: 53.7, range: 22–80 | Cervical | T1W, T2W | 1.5T | Edema only Edema or contusion | 65% (15/23) 65% (15/23) |
| Asan et al., 2018 | SCIWORA | n = 11 | Range: 28–81 | Cervical (n = 7), Thoracic (n = 4) | NS | NS | Contusion, cavitation, or edema | 36% (4/11) |
| Zhu et al., 2019 | SCIWORA | n = 16 | Mean: 47.5, range: 22–65 | Cervical | T2W | NS | Edema only Edema or contusion | 56% (9/16) 100% (16/16) |
| Meta-Analysis * | SCIWORA | n = 380 | Range: 12–87 | Cervical (n = 336), Thoracic (n = 44) | Edema only Any intramedullary abnormality | 40% (74/187), I2 = 0.90, p < 0.001 77% (291/380), I2 = 0.91, p < 0.001 | ||
| Citation | Disease State | Sample Size | Age (Years) | SCI Level | Sequence | Field Strength | MRI Finding and Change in Decision-Making | Outcome |
|---|---|---|---|---|---|---|---|---|
| If Surgery Is Required | ||||||||
| Kalfas et al., 1988 | SCI | n = 62 | NS | Cervical (n = 40), Thoracic (n = 17), Lumbar (n = 5) | T1W, T2W | 0.5T | 2 patients had cord compression due to acute disc herniation leading to anterior surgery | 3% (2/62) |
| Mirvis et al., 1988 | SCI | n = 21 | Mean: 42.5, range: 17–66 | Cervical | T1W, T2W | 1.5T | 3 patients with disc herniation were managed with anterior decompression | 14% (3/21) |
| Doran et al., 1993 | SCI | n = 12 (subgroup) | Mean: 34.1, range: 18–59 | Cervical | NS | NS | 10 patients with frank disc herniation and severe cord compression were managed with anterior cervical discectomy | 83% (10/12) |
| Gupta et al., 1999 | SCIWORA | n = 15 | Range: 20–60 | Cervical | NS | NS | 6 patients had intervertebral disc prolapse, all underwent anterior surgery | 40% (6/15) |
| Selden et al., 1999 ** | SCI | n = 55 | Mean: 29.2, range: 2–92 | Cervical | T1W, T2W | 1.5T | Among 18 patients with bilateral dislocated facets, acute disc herniation in 10/18 led to anterior surgery Among 26 patients who underwent successful closed reduction, ongoing cord compression in 13/26 led to surgery | 56% (10/55) 50% (13/26) |
| Ghanta et al., 2002 * | SCIWORA | n = 13 (subgroup) | Mean: 28.5, range: 0.4–78 | Cervical | NS | NS | 1 patient with disc herniation was managed with anterior decompression | 8% (1/13) |
| Papadopoulos et al., 2002 ** | SCI | n = 66 | Mean: 32, range: 2–92 | Cervical | T1W, T2W | 1.5T | 34 patients had cord compression leading to emergent surgery | 51% (34/66) |
| Tewari et al., 2004 ** | SCIWORA | n = 40 | Mean: 42.1, range: 16–70 | Cervical | T1W, T2W | NS | 3 patients with disc herniation were managed with anterior decompression | 8% (3/40) |
| Sharma et al., 2009 | SCIWORA | n = 12 | Mean: 38.66, range: 22–58 | Cervical | T1W, T2W | NS | 2 patients had disc prolapse and underwent surgery due to this finding | 17% (2/12) |
| Machino et al., 2011 | SCIWORA | n = 100 | Mean: 55, range: 16–87 | Cervical | T2W | 1.5T | 100 patients had profound neurological deficits and cord compression requiring surgical decompression | 100% (100/100) |
| Como et al., 2012 | SCIWORA | n = 24 | Mean: 60.5, range: 34–83 | Cervical | T1W, T2W | 1.5T | 13 patients required operative decompression | 54% (13/24) |
| Boese et al., 2016 | SCIWORA | n = 23 | Mean: 53.7, range: 22–80 | Cervical | T1W, T2W | 1.5T | Only patients with both cord compression and intramedullary edema (classified as Type IIc) were considered for surgery, 8/15 of these underwent surgery | 35% (8/23) |
| Maung et al., 2017 | SCIWORA | n = 123 | NS | Cervical | NS | NS | 6 patients had MRI findings that led to surgical treatment (ligamentous injury, epidural hematoma) | 5% (6/123) |
| Bao et al., 2020 | SCIWORA | n = 16 | Mean: 51.1, range: 30–73 | Cervical | T1W, T2W | 3.0T | 10 patients received surgical treatment based upon neutral MRI results (cord compression, disc injury) and another 2 patients had surgery based on kinetic MRI showing instability | 75% (12/16) |
| Huang et al., 2020 * | SCI, SCIWORA | SCIWORA: n = 42, SCI: n = 12 | NS | Cervical | NS | 3T, 1.5T | 10 patients had MRI findings that led to surgical treatment (cord compression, ligamentous injury, disc herniation) | 19% (10/54) |
| Meta-analysis *** | SCI, SCIWORA | n = 611 | Mixed | Any finding leading to surgery | 36% (223/611), I2 = 0.96, p < 0.001 | |||
| Surgical Approach | ||||||||
| Kalfas et al., 1988 | SCI | n = 62 | NS | Cervical (n = 40), Thoracic (n = 17), Lumbar (n = 5) | T1W, T2W | 0.5T | 2 patients had cord compression due to acute disc herniation leading to anterior surgery | 3% (2/62) |
| Mirvis et al., 1988 | SCI | n = 21 | Mean: 42.5, range: 17–66 | Cervical | T1W, T2W | 1.5T | 3 patients with disc herniation were managed with anterior decompression | 14% (3/21) |
| Doran et al., 1993 | SCI | n = 12 (subgroup) | Mean: 34.1, range: 18–59 | Cervical | NS | NS | 10 patients with frank disc herniation and severe cord compression were managed with anterior cervical discectomy | 83% (10/12) |
| Selden et al., 1999 ** | SCI | n = 55 | Mean: 29.2, range: 2–92 | Cervical | T1W, T2W | 1.5T | Among 18 patients with bilateral dislocated facets, acute disc herniation in 10/18 led to anterior surgery | 18% (10/55) |
| Ghanta et al., 2002 * | SCIWORA | n = 13 (subgroup) | Mean: 28.5, range: 0.4–78 | Cervical | NS | NS | 1 patient with disc herniation was managed with anterior decompression | 8% (1/13) |
| Tewari et al., 2004 ** | SCIWORA | n = 40 | Mean: 42.1, range: 16–70 | Cervical | T1W, T2W | NS | 3 patients with disc herniation were managed with anterior decompression | 8% (3/40) |
| Sharma et al., 2009 | SCIWORA | n = 12 | Mean: 38.66, range: 22–58 | Cervical | T1W, T2W | NS | 2 patients had disc prolapse and underwent surgery due to this finding | 17% (2/12) |
| Aarabi et al., 2011 | SCIWORA, CCS | n = 211 | Mean: 58.3, range: 32–87 | Cervical | T2W, STIR | NS | Among 42 patients that required surgery, anterior approach was chosen in 28 due to anterior compression limited to 1–3 segments and/or kyphosis, while posterior was chosen in the remaining 14 | 20% (42/211) |
| Cheng et al., 2013 | SCIWORA | n = 70 | Mean: 57.7, range: 36–79 | Cervical | T1W, T2W | NS | Among 70 patients treated surgically, MRI findings dictated surgical approach: 45 underwent anterior surgery due to anterior cord compression (disc, osteophytes, or OPLL); the remaining 25 underwent posterior procedures | 100% (70/70) |
| Meta-analysis * | SCI, SCIWORA | n = 500 | Mixed | Any finding leading to difference in surgical approach | 29% (143/500), I2 = 0.97, p < 0.001 | |||
| When to Operate | ||||||||
| Selden et al., 1999 ** | SCI | n = 55 | Mean: 29.2, range: 2–92 | Cervical | T1W, T2W | 1.5T | 27 patients had cord compression leading to emergent surgery | 49% (27/55) |
| Papadopoulos et al., 2002 ** | SCI | n = 66 | Mean: 32, range: 2–92 | Cervical | T1W, T2W | 1.5T | 34 patients had cord compression leading to emergent surgery No cord compression in 32 patients after traction, allowing delayed surgery in 22 Total | 51% (34/66) 33% (22/66) 85% (56/66) |
| Darsaut et al., 2006 | SCI, fracture-dislocation | n = 17 | Mean: 40.2; range: 19–78 | Sub-axial (C3-T1) | T2W, T1W | 1.5T | Among 11 patients with cord compression pre-reduction, MRI showed decompression in 9/11, leading to delayed surgery | 53% (9/17) |
| Meta-analysis *** | SCI, SCIWORA | n = 83 | Mixed | Any finding leading to difference in surgical timing | 78% (65/83), I2 = 0.84, p = 0.01 | |||
| Need for Instrumentation | ||||||||
| Krappinger et al., 2019 ** | SCIWORA, CCS, hyperextension injury | n = 23 | Mean: 62.7, range: 38–87 | Cervical | T1W, T2W, STIR | 1.5T | Findings of cord edema in all 23 patients and ligamentous injury (suggesting segmental instability) in 19 patients (including instability at a different level in several patients) led to decompression and instrumented fusion | 100% (23/23) |
| Which Levels or How Many Levels to Decompress | ||||||||
| Krappinger et al., 2019 ** | SCIWORA, CCS, hyperextension injury | n = 23 | Mean: 62.7, range: 38–87 | Cervical | T1W, T2W, STIR | 1.5T | Findings of cord edema in all 23 patients and ligamentous injury (suggesting segmental instability) in 19 patients (including instability at a different level in several patients) led to decompression and instrumented fusion | 100% (23/23) |
| Need for Re-operation After Surgery | ||||||||
| Aarabi et al., 2011 ** | SCIWORA, CCS | n = 211 | Mean: 58.3, range: 32–87 | Cervical | T2W, STIR | NS | Among 28 patients that underwent anterior surgery, post-operative MRI found ongoing cord compression in 11, leading to additional posterior surgery | 5% (11/211) |
| Aarabi et. al., 2019 ** | SCI | n = 184 | Mean: 43.5 | Cervical | T1W, STIR | NS | Ongoing cord compression after surgery (inadequate decompression), but rates of re-operation were not reported | 34% (63/184) |
| Citation | Disease State | Sample Size | Age (Years) | SCI Level | Sequence | Field Strength | Evidence Regarding Timing of MRI |
|---|---|---|---|---|---|---|---|
| Performance of MRI on Initial Assessment (Prior to Intervention)? | |||||||
| Kalfas et al., 1988 | SCI | n = 62 | NS | Cervical (n = 40), Thoracic (n = 17), Lumbar (n = 5) | T1W, T2W | 0.5T | Useful to detect disc herniation, cord compression (if to operate, surgical approach) |
| Doran et al., 1993 | SCI, facet dislocation | n = 12 | Mean: 34.1, range: 18–59 | Cervical | NS | NS | Useful to detect disc herniation, cord compression (if to operate, surgical approach) |
| Gupta et al., 1999 | SCIWORA | n = 15 | Range: 20–60 | Cervical | NS | NS | Useful to detect disc herniation, cord compression (if to operate, surgical approach) |
| Selden et al., 1999 ** | SCI | n = 55 | Mean: 29.2, range: 2–92 | Cervical | T1W, T2W | 1.5T | Useful to detect disc herniation, cord compression (if to operate vs. closed reduction, surgical approach, timing of surgery) |
| Vaccaro et al., 1999 | SCI, facet dislocation | n = 11 | Mean: 46, range: 17–84 | Cervical | T1W, T2W | 1.5T | Unclear if pre-reduction MRI has utility: 2 patients had disc herniations prior to closed reduction but did not deteriorate after reduction |
| Papadopoulos et al., 2002 ** | SCI | n = 66 | Mean: 32, range: 2–92 | Cervical | T1W, T2W | 1.5T | Useful to detect cord compression (if to operate, timing of surgery) |
| Darsaut et al., 2006 | SCI, fracture-dislocation | n = 17 | Mean: 40.2, range: 19–78 | Cervical | T1W, T2W | 1.5T | Unclear if pre-reduction MRI has utility: 11 patients had cord compression prior to traction/reduction but did not deteriorate after reduction |
| Sharma et al., 2009 | SCIWORA | n = 12 | Mean: 38.66, range: 22–58 | Cervical | T1W, T2W | NS | Useful to detect disc herniation, cord compression (if to operate, surgical approach) |
| Aarabi et al., 2011 | SCIWORA, CCS | n = 211 | Mean: 58.3, range: 32–87 | Cervical | T2W, STIR | NS | Useful to detect anterior cord compression (surgical approach) |
| Como et al., 2012 | SCIWORA | n = 24 | Mean: 60.5, range: 34–83 | Cervical | T1W, T2W | 1.5T | Useful to detect cord compression (if to operate) |
| Cheng et al., 2013 | SCIWORA | n = 70 | Mean: 57.7, range: 36–79 | Cervical | T1W, T2W | NS | Useful to detect anterior cord compression (surgical approach) |
| Boese et al., 2016 | SCIWORA | n = 23 | Mean: 53.7, range: 22–80 | Cervical | T1W, T2W | 1.5T | Useful to detect cord compression and edema (if to operate), but authors state “our results cannot provide guidance on therapeutic management” |
| Maung et al., 2017 | SCIWORA | n = 123 | NS | Cervical | NS | NS | Useful to detect ligamentous injury, epidural hematoma (if to operate) |
| Bao et al., 2020 | SCIWORA | n = 16 | Mean: 51.1, range: 30–73 | Cervical | T1W, T2W | 3.0T | Useful to detect cord compression, disc injury, instability (if to operate) |
| Huang et al., 2020 * | SCI, SCIWORA | SCIWORA: n = 42, SCI: n = 12 | NS | Cervical | NS | 3T, 1.5T | Useful to detect cord compression, ligamentous injury, disc herniation (if to operate) |
| Performance of MRI After Closed Reduction to Assess Decompression? | |||||||
| Selden et al., 1999 ** | SCI | n = 55 | Mean: 29.2, range: 2–92 | Cervical | T1W, T2W | 1.5T | Useful to detect post-reduction cord compression (if to operate) |
| Vaccaro et al., 1999 | SCI, facet dislocation | n = 11 | Mean: 46, range: 17–84 | Cervical | T1W, T2W | 1.5T | Unclear if post-reduction MRI has utility: 5 patients had disc herniations after closed reduction but did not deteriorate |
| Darsaut et al., 2006 | SCI, fracture-dislocation | n = 17 | Mean: 40.2, range: 19–78 | Cervical | T1W, T2W | 1.5T | Useful to detect post-reduction cord compression (if to operate) |
| Performance of MRI After Surgery to Assess Decompression? | |||||||
| Aarabi et al., 2011 ** | SCIWORA, CCS | n = 211 | Mean: 58.3, range: 32–87 | Cervical | T2W, STIR | NS | Useful to detect post-operative cord compression (if to re-operate) |
| Aarabi et al., 2019 ** | SCI | n = 184 | Mean: 43.5 | Cervical | T1W, STIR | NS | Useful to detect post-operative cord compression (if to re-operate), but rates of re-operation were not reported |
| Performance of MRI within a Specific Time Period (e.g., 24 h) | |||||||
| Aarabi et al., 2019 * | SCI | n = 184 | Mean: 43.5 | Cervical | T1W, STIR | NS | Time interval to pre-operative MRI did not differ between successfully (8.3 +/− 7.7 h) and unsuccessfully (8.6 +/− 8.7 h) decompressed patients |
| Citation | Disease | Sample Size | Age (Years) | SCI Level | Sequence | Field Strength | Activity/Imaging | Adverse Event | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Kalfas et al., 1988 * | SCI | n = 62 | NS | Cervical (n = 40) Thoracic (n = 17) Lumbar (n = 5) | T1W, T2W | 0.5T | MRI within first 36 h of injury | Any adverse event | 0% (0/62) |
| Selden et al., 1999 ** | SCI | n = 18 | Mean: 29.2, range: 2–92 | Cervical | T1W, T2W | 1.5T | MRI within first 21 h of injury | Any adverse event | 0% (0/55) |
| Papadopoulos et al., 2002 ** | SCI | n = 66 | Mean: 32, range: 2–92 | Cervical | T1W, T2W | 1.5T | Emergent MRI (average: 4.1 h) | Neurological deterioration | 0% (0/66) |
| Darsaut et al., 2006 | SCI, fracture-dislocation | n = 17 | Mean: 40.2, range: 19–78 | Cervical | T1W, T2W | 1.5T | MRI during closed reduction | Permanent neurological deterioration during reduction/MRI Burning sensation at pin sites | 0% (0/12) 0% (0/12) |
| Bao et al., 2020 | SCIWORA | n = 16 | Mean: 51.1, range: 30–73 | Cervical | T1W, T2W | 3.0T | Neutral, flexion, and extension MRI Neutral and flexion MRI | Neurological deterioration Neurological deterioration | 0% (0/14) 0% (0/2) |
| Meta-analysis *** | SCI | n = 156 | NS | Mixed | NS | NS | Any adverse event | 0% (0/156), I2 = 0, p = 1 |
| Citation | Disease State | Sample Size | Age (Years) | SCI Level | Sequence | Field Strength | Outcome | Imaging/Treatment Group | Result | |
|---|---|---|---|---|---|---|---|---|---|---|
| Improvement in Frankel Grade from Admission | ||||||||||
| Papadopoulos et al., 2002 ** | SCI | n = 91 | Mean: 32, range: 2–92 | Cervical | T1W, T2W | 1.5T | Any Frankel Grade improvement Grade A/B improvement to D/E | MRI-protocol Reference group MRI-protocol Reference group | 50% (30/66) 24% (6/25) 16% (8/50) 0% (0/20) | p < 0.006 p = 0.09 *** |
| Length of Stay | ||||||||||
| Papadopoulos et al., 2002 ** | SCI | n = 91 | Mean: 32, range: 2–92 | Cervical | T1W, T2W | 1.5T | ICU stay General care duration Rehabilitation duration Total length of stay | MRI-protocol Reference group MRI-protocol Reference group MRI-protocol Reference group MRI-protocol Reference group | 9.9 ± 1.7 days 23.8 ± 3.7 days 8.4 ± 1.7 days 9.3 ± 3.0 days 58.1 ± 5.6 days 66.0 ± 10.7 days 71.4 ± 5.9 days 99.9 ± 13.1 days | p < 0.001 p = 0.31 p = 0.47 p = 0.02 |
| Study | Year | Study Design | Risk of Bias | |
|---|---|---|---|---|
| 1 | Aarabi et al. | 2011 | Retrospective case series | Minimally low |
| 2 | Aarabi et al. | 2019 | Retrospective case series | Minimally low |
| 3 | Asan et al. | 2018 | Prospective case series | Moderately low |
| 4 | Bao et al. | 2020 | Retrospective case series | Moderately low |
| 5 | Boese et al. | 2016 | Retrospective case series | Moderately low |
| 6 | Cheng et al. | 2012 | Retrospective case series | Moderately low |
| 7 | Como et al. | 2012 | Retrospective case series | Moderately low |
| 8 | Darsaut et al. | 2006 | Prospective case series | Moderately low |
| 9 | Doran et al. | 1993 | Retrospective case series | High |
| 10 | D’Souza et al. | 2017 | Retrospective case control study | Moderately low |
| 11 | Fehlings et al. | 1999 | Retrospective case series | Moderately low |
| 12 | Ghanta et al. | 2002 | Retrospective case series | Moderately low |
| 13 | Gupta et al. | 1999 | Retrospective case series | Moderately low |
| 14 | Hendey et al. | 2002 | Retrospective case series | Moderately low |
| 15 | Henninger et al. | 2020 | Retrospective case series | Moderately low |
| 16 | Huang et al. | 2020 | Retrospective case series | Minimally low |
| 17 | Kalfas et al. | 1988 | Retrospective case series | Moderately low |
| 18 | Koyanagi et al. | 2003 | Retrospective case series | Moderately low |
| 19 | Krappinger et al. | 2019 | Retrospective case series | Minimally low |
| 20 | Liu et al. | 2015 | Retrospective case series | Minimally low |
| 21 | Machino et al. | 2019 | Retrospective case series | Minimally low |
| 22 | Maeda et al. | 2012 | Retrospective case series | Minimally low |
| 23 | Mahmood et al. | 2010 | Retrospective case control study | Moderately low |
| 24 | Maung et al. | 2016 | Retrospective case series | Moderately low |
| 25 | Mirvis et al. | 1988 | Retrospective case series | Moderately low |
| 26 | Papadopoulos et al. | 2002 | Prospective cohort study | High |
| 27 | Selden et al. | 1999 | Retrospective case series | Minimally low |
| 28 | Sharma et al. | 2009 | Retrospective case series | Minimally low |
| 29 | Song et al. | 2008 | Retrospective case series | Minimally low |
| 30 | Tewari et al. | 2005 | Retrospective case series | Minimally low |
| 31 | Vaccaro et al. | 1999 | Retrospective case series | Minimally low |
| 32 | Zhu et al. | 2019 | Retrospective case series | Minimally low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaffari-Rafi, A.; Peterson, C.; Leon-Rojas, J.E.; Tadokoro, N.; Lange, S.F.; Kaushal, M.; Tetreault, L.; Fehlings, M.G.; Martin, A.R. The Role of Magnetic Resonance Imaging to Inform Clinical Decision-Making in Acute Spinal Cord Injury: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 4948. https://doi.org/10.3390/jcm10214948
Ghaffari-Rafi A, Peterson C, Leon-Rojas JE, Tadokoro N, Lange SF, Kaushal M, Tetreault L, Fehlings MG, Martin AR. The Role of Magnetic Resonance Imaging to Inform Clinical Decision-Making in Acute Spinal Cord Injury: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021; 10(21):4948. https://doi.org/10.3390/jcm10214948
Chicago/Turabian StyleGhaffari-Rafi, Arash, Catherine Peterson, Jose E. Leon-Rojas, Nobuaki Tadokoro, Stefan F. Lange, Mayank Kaushal, Lindsay Tetreault, Michael G. Fehlings, and Allan R. Martin. 2021. "The Role of Magnetic Resonance Imaging to Inform Clinical Decision-Making in Acute Spinal Cord Injury: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 10, no. 21: 4948. https://doi.org/10.3390/jcm10214948
APA StyleGhaffari-Rafi, A., Peterson, C., Leon-Rojas, J. E., Tadokoro, N., Lange, S. F., Kaushal, M., Tetreault, L., Fehlings, M. G., & Martin, A. R. (2021). The Role of Magnetic Resonance Imaging to Inform Clinical Decision-Making in Acute Spinal Cord Injury: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 10(21), 4948. https://doi.org/10.3390/jcm10214948








