Estimated Glomerular Filtration Rate as a Prognostic Factor in Urothelial Carcinoma of the Upper Urinary Tract: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
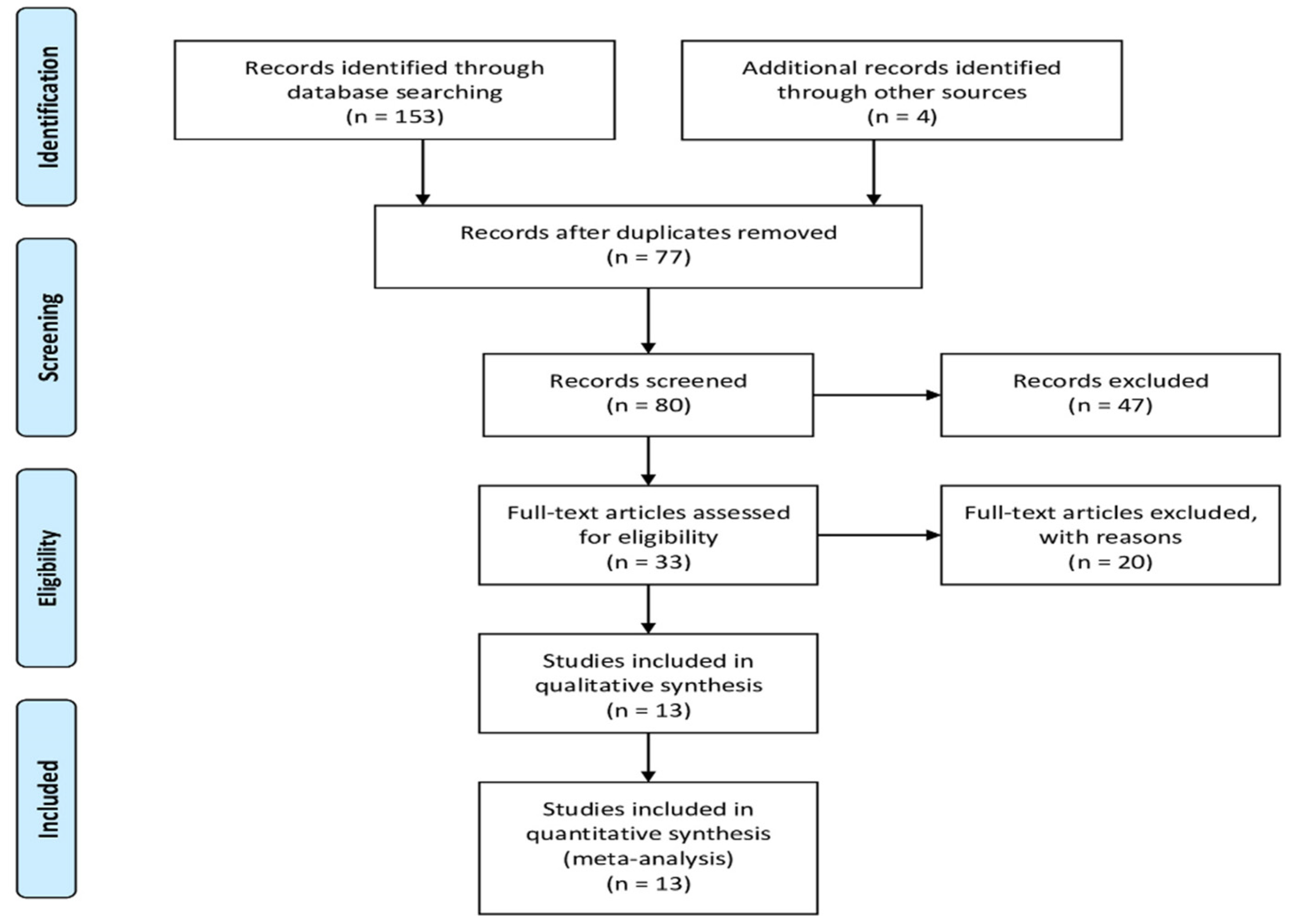
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Quality Assessments
2.5. Definition of Survival
2.6. Statistical Analysis
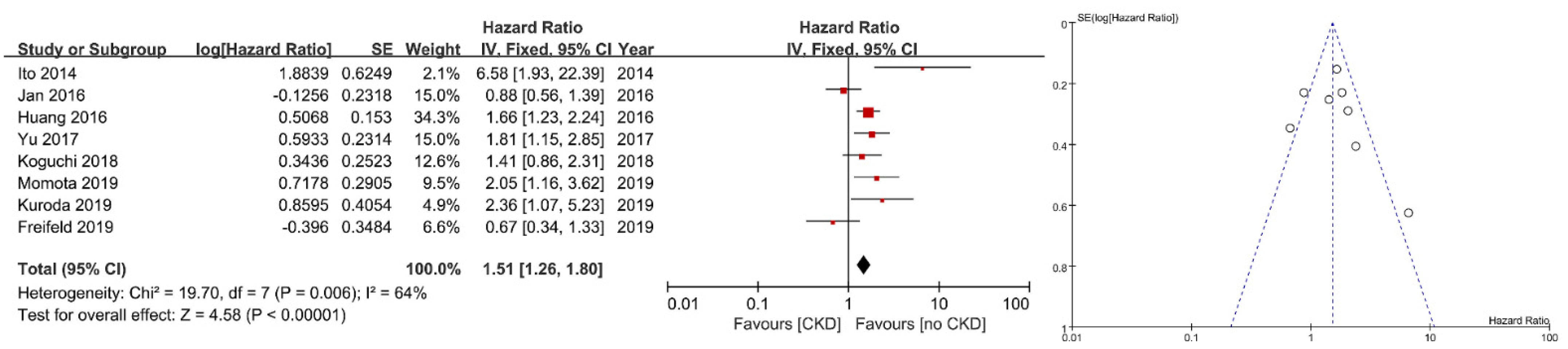
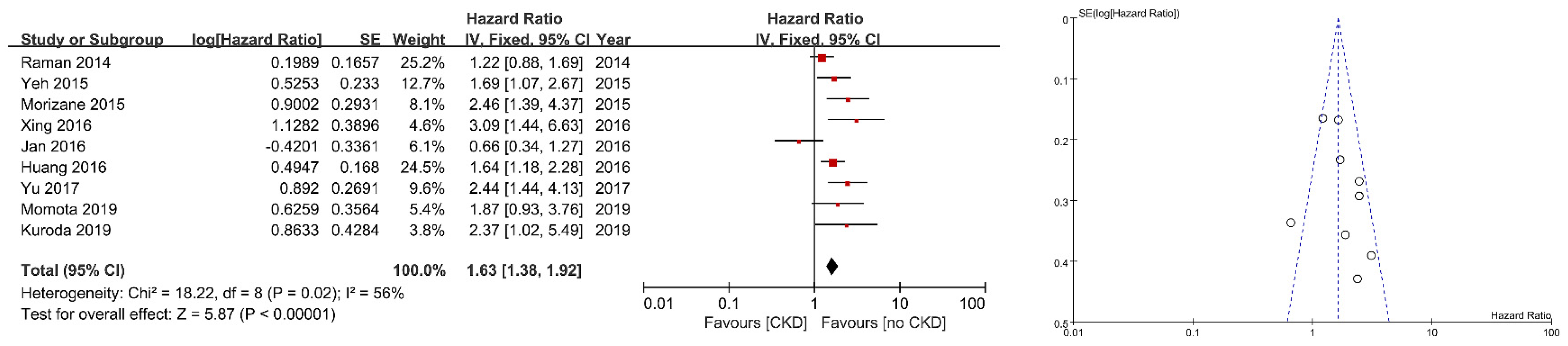
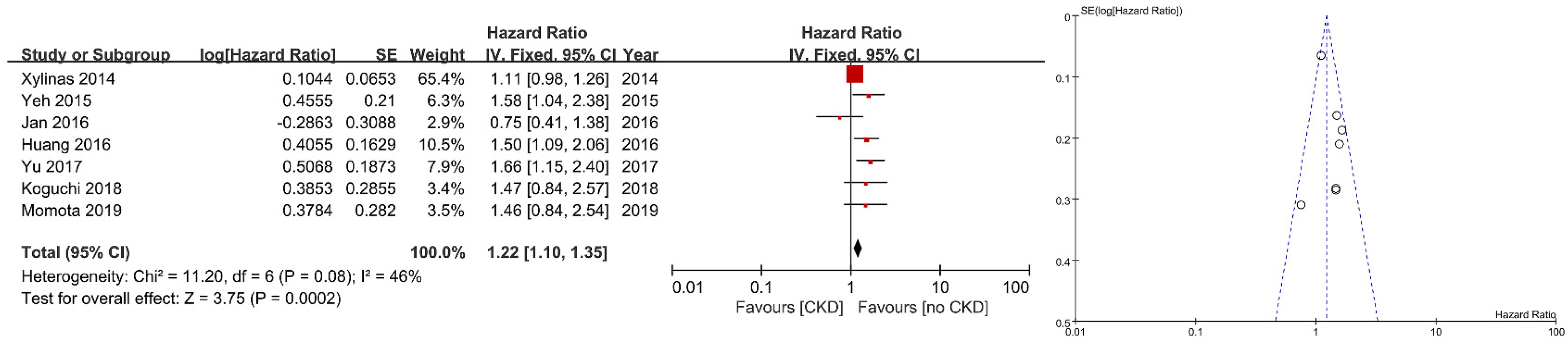
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Munoz, J.J.; Ellison, L.M. Upper tract urothelial neoplasms: Incidence and survival during the last 2 decades. J. Urol. 2000, 164, 1523–1525. [Google Scholar] [CrossRef]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [Green Version]
- Raman, J.D.; Messer, J.; Sielatycki, J.A.; Hollenbeak, C.S. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973–2005. BJU Int. 2011, 107, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Rouprêt, M.; Babjuk, M.; Comperat, E.; Zigeuner, R.; Sylvester, R.; Burger, M.; Cowan, N.; Böhle, A.; Van Rhijn, B.W.; Kaasinen, E. European guidelines on upper tract urothelial carcinomas: 2013 update. Eur. Urol. 2013, 63, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Rouprêt, M.; Zigeuner, R.; Palou, J.; Boehle, A.; Kaasinen, E.; Sylvester, R.; Babjuk, M.; Oosterlinck, W. European guidelines for the diagnosis and management of upper urinary tract urothelial cell carcinomas: 2011 update. Eur. Urol. 2011, 59, 584–594. [Google Scholar] [CrossRef]
- Hall, M.C.; Womack, S.; Sagalowsky, A.I.; Carmody, T.; Erickstad, M.D.; Roehrborn, C.G. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: A 30-year experience in 252 patients. Urology 1998, 52, 594–601. [Google Scholar] [CrossRef]
- Ito, K.; Kuroda, K.; Asakuma, J.; Hamada, S.; Tachi, K.; Tasaki, S.; Sato, A.; Horiguchi, A.; Seguchi, K.; Asano, T. Preoperative risk factors for extraurothelial recurrence in patients with ureteral cancer treated with radical nephroureterectomy. J. Urol. 2014, 191, 1685–1692. [Google Scholar] [CrossRef]
- Kondo, T.; Nakazawa, H.; Ito, F.; Hashimoto, Y.; Toma, H.; Tanabe, K. Impact of the extent of regional lymphadenectomy on the survival of patients with urothelial carcinoma of the upper urinary tract. J. Urol. 2007, 178, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Tanabe, K. The role of lymph node dissection in the management of urothelial carcinoma of the upper urinary tract. Int. J. Clin. Oncol. 2011, 16, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Roscigno, M.; Cozzarini, C.; Bertini, R.; Scattoni, V.; Freschi, M.; Da Pozzo, L.F.; Briganti, A.; Gallina, A.; Capitanio, U.; Colombo, R. Prognostic value of lymph node dissection in patients with muscle-invasive transitional cell carcinoma of the upper urinary tract. Eur. Urol. 2008, 53, 794–802. [Google Scholar] [CrossRef]
- Raman, J.D.; Lin, Y.K.; Kaag, M.; Atkinson, T.; Crispen, P.; Wille, M.; Smith, N.; Hockenberry, M.; Guzzo, T.; Peyronnet, B.; et al. High rates of advanced disease, complications, and decline of renal function after radical nephroureterectomy. Urol. Oncol. 2014, 32, 47.e9–47.e14. [Google Scholar] [CrossRef]
- Saito, K.; Kawakami, S.; Ohtsuka, Y.; Fujii, Y.; Masuda, H.; Kumagai, J.; Kobayashi, T.; Kageyama, Y.; Kihara, K. The impact of preoperative serum C-reactive protein on the prognosis of patients with upper urinary tract urothelial carcinoma treated surgically. BJU Int. 2007, 100, 269–273. [Google Scholar] [CrossRef]
- Kikuchi, E.; Margulis, V.; Karakiewicz, P.I.; Roscigno, M.; Mikami, S.; Lotan, Y.; Remzi, M.; Bolenz, C.; Langner, C.; Weizer, A. Lymphovascular invasion predicts clinical outcomes in patients with node-negative upper tract urothelial carcinoma. J. Clin. Oncol. 2009, 27, 612–618. [Google Scholar] [CrossRef]
- Margulis, V.; Shariat, S.F.; Matin, S.F.; Kamat, A.M.; Zigeuner, R.; Kikuchi, E.; Lotan, Y.; Weizer, A.; Raman, J.D.; Wood, C.G. Outcomes of radical nephroureterectomy: A series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer 2009, 115, 1224–1233. [Google Scholar] [CrossRef]
- Zigeuner, R.; Shariat, S.F.; Margulis, V.; Karakiewicz, P.I.; Roscigno, M.; Weizer, A.; Kikuchi, E.; Remzi, M.; Raman, J.D.; Bolenz, C. Tumour necrosis is an indicator of aggressive biology in patients with urothelial carcinoma of the upper urinary tract. Eur. Urol. 2010, 57, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Rouprêt, M.; Babjuk, M.; Compérat, E.; Zigeuner, R.; Sylvester, R.J.; Burger, M.; Cowan, N.C.; Böhle, A.; Van Rhijn, B.W.; Kaasinen, E.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Cell Carcinoma: 2015 Update. Eur. Urol. 2015, 68, 868–879. [Google Scholar] [CrossRef]
- Favaretto, R.L.; Shariat, S.F.; Chade, D.C.; Godoy, G.; Adamy, A.; Kaag, M.; Bochner, B.H.; Coleman, J.; Dalbagni, G. The effect of tumor location on prognosis in patients treated with radical nephroureterectomy at Memorial Sloan-Kettering Cancer Center. Eur. Urol. 2010, 58, 574–580. [Google Scholar] [CrossRef] [Green Version]
- Otto, W.; Shariat, S.F.; Fritsche, H.M.; Gupta, A.; Matsumoto, K.; Kassouf, W.; Martignoni, G.; Walton, T.J.; Tritschler, S.; Baba, S.; et al. Concomitant carcinoma in situ as an independent prognostic parameter for recurrence and survival in upper tract urothelial carcinoma: A multicenter analysis of 772 patients. World J. Urol. 2011, 29, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.S.; Hong, S.J.; Cho, N.H.; Choi, Y.D. Grade of hydronephrosis and tumor diameter as preoperative prognostic factors in ureteral transitional cell carcinoma. Urology 2007, 70, 662–666. [Google Scholar] [CrossRef]
- Rink, M.; Robinson, B.D.; Green, D.A.; Cha, E.K.; Hansen, J.; Comploj, E.; Margulis, V.; Raman, J.D.; Ng, C.K.; Remzi, M. Impact of histological variants on clinical outcomes of patients with upper urinary tract urothelial carcinoma. J. Urol. 2012, 188, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-M.; Li, C.-C.; Ke, H.-L.; Wu, W.-J.; Huang, C.-N.; Huang, C.-H. The prognostic predictors of primary ureteral transitional cell carcinoma after radical nephroureterectomy. J. Urol. 2009, 182, 451–458. [Google Scholar] [CrossRef]
- Kuroda, K.; Asakuma, J.; Horiguchi, A.; Kawaguchi, M.; Shinchi, M.; Masunaga, A.; Tasaki, S.; Sato, A.; Ito, K. Chronic kidney disease and positive surgical margins as prognosticators for upper urinary tract urothelial carcinoma patients undergoing radical nephroureterectomy. Mol. Clin. Oncol. 2019, 10, 547–554. [Google Scholar] [CrossRef] [Green Version]
- Stewart, J.H.; Buccianti, G.; Agodoa, L.; Gellert, R.; McCredie, M.R.; Lowenfels, A.B.; Disney, A.P.; Wolfe, R.A.; Boyle, P.; Maisonneuve, P. Cancers of the kidney and urinary tract in patients on dialysis for end-stage renal disease: Analysis of data from the United States, Europe, and Australia and New Zealand. J. Am. Soc. Nephrol. 2003, 14, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Tonelli, M.; Wiebe, N.; Culleton, B.; House, A.; Rabbat, C.; Fok, M.; McAlister, F.; Garg, A.X. Chronic kidney disease and mortality risk: A systematic review. J. Am. Soc. Nephrol. 2006, 17, 2034–2047. [Google Scholar] [CrossRef] [Green Version]
- Fried, L.F.; Katz, R.; Sarnak, M.J.; Shlipak, M.G.; Chaves, P.H.; Jenny, N.S.; Stehman-Breen, C.; Gillen, D.; Bleyer, A.J.; Hirsch, C. Kidney function as a predictor of noncardiovascular mortality. J. Am. Soc. Nephrol. 2005, 16, 3728–3735. [Google Scholar] [CrossRef] [Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.S.; Hwang, J.E.; Chung, H.S.; Cho, Y.H.; Kim, M.S.; Hwang, E.C.; Oh, K.J.; Kim, S.-O.; Jung, S.I.; Kang, T.W. Is preoperative chronic kidney disease status associated with oncologic outcomes in upper urinary tract urothelial carcinoma? A multicenter propensity score-matched analysis. Oncotarget 2017, 8, 66540–66549. [Google Scholar] [CrossRef] [Green Version]
- Xylinas, E.; Rink, M.; Margulis, V.; Clozel, T.; Lee, R.K.; Comploj, E.; Novara, G.; Raman, J.D.; Lotan, Y.; Weizer, A.; et al. Impact of renal function on eligibility for chemotherapy and survival in patients who have undergone radical nephro-ureterectomy. BJU Int. 2013, 112, 453–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momota, M.; Hatakeyama, S.; Tokui, N.; Sato, T.; Yamamoto, H.; Tobisawa, Y.; Yoneyama, T.; Yoneyama, T.; Hashimoto, Y.; Koie, T.; et al. The Impact of Preoperative Severe Renal Insufficiency on Poor Postsurgical Oncological Prognosis in Patients with Urothelial Carcinoma. Eur. Urol. Focus 2019, 5, 1066–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jan, H.C.; Yang, W.H.; Ou, C.H. Combination of the Preoperative Systemic Immune-Inflammation Index and Monocyte-Lymphocyte Ratio as a Novel Prognostic Factor in Patients with Upper-Tract Urothelial Carcinoma. Ann. Surg. Oncol. 2019, 26, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Freifeld, Y.; Ghandour, R.; Singla, N.; Woldu, S.; Clinton, T.; Kulangara, R.; Bagrodia, A.; Matin, S.F.; Petros, F.G.; Raman, J.D.; et al. Preoperative predictive model and nomogram for disease recurrence following radical nephroureterectomy for high grade upper tract urothelial carcinoma. Urol. Oncol. 2019, 37, 758–764. [Google Scholar] [CrossRef]
- Koguchi, D.; Matsumoto, K.; Ikeda, M.; Taoka, Y.; Hirayama, T.; Murakami, Y.; Utsunomiya, T.; Matsuda, D.; Okuno, N.; Irie, A.; et al. Investigation of estimated glomerular filtration rate and its perioperative change in patients with upper urinary tract urothelial carcinoma: A multi-institutional retrospective study. Asia Pac. J. Clin. Oncol. 2018, 14, e420–e427. [Google Scholar] [CrossRef]
- Xing, Y.; Xiong, G.; Fang, D.; Yang, X.; Li, X.; Zhou, L. Prognostic Value of Gene Methylation and Clinical Factors in Non-Muscle-Invasive Upper Tract Urothelial Carcinoma After Radical Nephroureterectomy. Clin. Genitourin. Cancer 2016, 14, e371–e378. [Google Scholar] [CrossRef]
- Huang, Y.C.; Chang, Y.H.; Chiu, K.H.; Shindel, A.W.; Lai, C.H. Adjuvant radiotherapy for locally advanced upper tract urothelial carcinoma. Sci. Rep. 2016, 6, 38175. [Google Scholar] [CrossRef] [Green Version]
- Yeh, H.C.; Jan, H.C.; Wu, W.J.; Li, C.C.; Li, W.M.; Ke, H.L.; Huang, S.P.; Liu, C.C.; Lee, Y.C.; Yang, S.F.; et al. Concurrent Preoperative Presence of Hydronephrosis and Flank Pain Independently Predicts Worse Outcome of Upper Tract Urothelial Carcinoma. PLoS ONE 2015, 10, e0139624. [Google Scholar] [CrossRef]
- Morizane, S.; Yumioka, T.; Yamaguchi, N.; Masago, T.; Honda, M.; Sejima, T.; Takenaka, A. Risk stratification model, including preoperative serum C-reactive protein and estimated glomerular filtration rate levels, in patients with upper urinary tract urothelial carcinoma undergoing radical nephroureterectomy. Int. Urol. Nephrol. 2015, 47, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehdaie, B.; Shariat, S.F.; Savage, C.; Coleman, J.; Dalbagni, G. Postoperative nomogram for disease recurrence and cancer-specific death for upper tract urothelial carcinoma: Comparison to American Joint Committee on Cancer staging classification. Urol. J. 2014, 11, 1435–1441. [Google Scholar] [PubMed]
- Lee, K.-H.; Chen, Y.-T.; Chung, H.-J.; Liu, J.-S.; Hsu, C.-C.; Tarng, D.-C. Kidney disease progression in patients of upper tract urothelial carcinoma following unilateral radical nephroureterectomy. Ren. Fail. 2016, 38, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Leppert, J.T.; Lamberts, R.W.; Thomas, I.-C.; Chung, B.I.; Sonn, G.A.; Skinner, E.C.; Wagner, T.H.; Chertow, G.M.; Brooks, J.D. Incident CKD after radical or partial nephrectomy. J. Am. Soc. Nephrol. 2018, 29, 207–216. [Google Scholar] [CrossRef]
- Clark, M.A.; Shikanov, S.; Raman, J.D.; Smith, B.; Kaag, M.; Russo, P.; Wheat, J.C.; Wolf, J.S.; Matin, S.F.; Huang, W.C. Chronic kidney disease before and after partial nephrectomy. J. Urol. 2011, 185, 43–48. [Google Scholar] [CrossRef]
- Yoneyama, T.; Tobisawa, Y.; Yoneyama, T.; Yamamoto, H.; Imai, A.; Mori, K.; Hatakeyama, S.; Hashimoto, Y.; Koie, T.; Ohyama, C. Sequential chemotherapy with gemcitabine plus carboplatin, followed by additional docetaxel for aged patients with advanced upper-tract urothelial cancer. J. Clin. Oncol. 2015, 33, 344. [Google Scholar] [CrossRef]
- Birtle, A.; Johnson, M.; Chester, J.; Jones, R.; Dolling, D.; Bryan, R.T.; Harris, C.; Winterbottom, A.; Blacker, A.; Catto, J.W. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): A phase 3, open-label, randomised controlled trial. Lancet 2020, 395, 1268–1277. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Schilsky, R.; Reichert, C.M.; Reddick, R.L.; Rozencweig, M.; Young, R.C.; Muggia, F.M. Toxic effects of cis-dichlorodiammineplatinum (II) in man. Cancer Treat. Rep. 1979, 63, 1527–1531. [Google Scholar] [PubMed]
- Arany, I.; Safirstein, R.L. Cisplatin nephrotoxicity. Semin. Nephrol. 2003, 23, 460–464. [Google Scholar] [CrossRef]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef] [Green Version]
| Study | Year | Country | Recruitment Period | No. of Patients | Prospective Data Collection | Inclusion/Exclusion Criteria | Consecutive Patients | Definition of Survival | Definition of eGFR |
|---|---|---|---|---|---|---|---|---|---|
| Xylinas | 2013 | Multination | 1994–2007 | 666 | No | Yes | NA | Yes | Yes |
| Ito | 2014 | Japan | 1999–2012 | 70 | No | Yes | NA | No | Yes |
| Raman | 2014 | Multination | 2003–2012 | 414 | No | No | NA | No | Yes |
| Morizane | 2015 | Japan | 2000–2012 | 345 | No | Yes | NA | Yes | No |
| Yeh | 2015 | Taiwan | 1991–2013 | 472 | No | Yes | NA | No | Yes |
| Huang | 2016 | Taiwan | 2001–2016 | 198 | No | Yes | NA | No | Yes |
| 2016 | China | 2000–2013 | 192 | No | Yes | NA | No | Yes | |
| Yu | 2017 | Korea | 2004–2014 | 566 | No | Yes | NA | No | Yes |
| Koguchi | 2018 | Japan | 1990–2015 | 433 | No | Yes | NA | No | Yes |
| Freifeld | 2019 | USA | 1993–2016 | 245 | No | Yes | NA | No | No |
| Jan | 2019 | Taiwan | 2007–2017 | 424 | No | Yes | NA | No | Yes |
| Kuroda | 2019 | Japan | 1999–2017 | 187 | No | No | NA | No | Yes |
| Momota | 2019 | Japan | 1995–2017 | 456 | No | Yes | NA | No | Yes |
| Study | Median Age, Range (Years) | Gender (Male/Female) | Median BMI, Range (kg/m2) | ECOG Performance Status (0/1/2/3) | Smoking | Surgical Approach (Open/Laparoscopic) | Median Follow-Up, Range (Months) |
|---|---|---|---|---|---|---|---|
| Xylinas | 69.6, 54–76 | 441/225 | 28.2, 24–32 (IQR) | 445/221 (1–3) | NA | 519/147 | 45.5, 24–67 (IQR) |
| Ito | NA | 47/23 | NA | NA | NA | 49/21 | 29.2, 1–157 |
| Raman | 70, 27–96 | 257/157 | NA | 82/165/159/8 | NA | NA | 16, 2–120 |
| Morizane | 74, 38–95 | 234/111 | 22.1, 13–34.2 | 241/103 (1–3) | 175 | 244/101 | 39.9, 6.1–160 |
| Yeh | 67, 24–95 | 204/268 | NA | NA | 99 | 269/203 | 33, 1–233 |
| Huang | 68.6, 23.6–91.6 | 103/95 | NA | NA | 26 | NA | 29.1, 6.4–164.9 |
| NA | 78/114 | NA | NA | NA | 84/145 | 65, 3–144 | |
| Yu | 72, 65–76 (IQR) | 165/401 | NA | 399/148/20/1 | NA | 142/424 | 31.1, 16.2–55.7 |
| Koguchi | 69, 62–75 (IQR) | 313/120 | NA | NA | 138 | 243/190 | 35.4, 13.8–74.5 (IQR) |
| Freifeld | 70 (mean) | 152/93 | 29 (mean) | 126/98 (1–3) | NA | NA | 27 |
| Jan | 70, 29–96 | 189/235 | NA | NA | 49 | NA | 35, 14–60 (IQR) |
| Kuroda | 71, 38–90 | 138/49 | NA | NA | NA | 104/83 | 49.2, 3.4–209.2 |
| Momota | NA | 309/147 | NA | 446 (0–1)/10 (2–3) | NA | 377/79 | 40 |
| Study | History of Bladder Cancer | Hydronephrosis | Tumor Size | Tumor Location (Pelvis/Ureter) | Tumor Multifocality | Adjuvant Chemotherapy |
|---|---|---|---|---|---|---|
| Xylinas | 244 | NA | NA | 420/246 | 164 | 62 |
| Ito | 17 | 26 | NA | 0/70 | 7 | 23 |
| Raman | NA | NA | NA | NA | NA | 55 |
| Morizane | 36 | 201 | NA | 140/205 | 51 | NA |
| Yeh | NA | NA | NA | 189/193 | 90 | 87 |
| Huang | 49 | NA | NA | NA | NA | 21 |
| NA | 119 | NA | 102/90 | 52 | NA | |
| Yu | 111 | 249 | NA | 258/308 | 49 | 205 |
| Koguchi | NA | NA | NA | 239/194 | NA | 99 |
| Freifeld | 80 | 71 | 3.4 (mean) | 116/85 | 35 | NA |
| Jan | 127 | 344 | NA | 191/138 | 116 | 40 |
| Kuroda | 47 | 112 | NA | NA | NA | NA |
| Momota | NA | 288 | NA | 175/254 | NA | 64 |
| Study | Tumor Grade (Low/High) | Pathologic T Stage (pT0/Is/a/1/2/3/4) | Pathologic N Stage (pNx/−/+) | Variant Form | LVI | Concomitant CIS | Positive Surgical Margin |
|---|---|---|---|---|---|---|---|
| Xylinas | 121/533 | 326 (≤T1)/118/182/40 | 291/291/84 | NA | 171 | 229 | NA |
| Ito | NA | NA | NA | NA | NA | NA | NA |
| Raman | 116/298 | 3/16/106/60/60/143/26 | 165/203/46 | NA | NA | NA | 25 |
| Morizane | 222/109 | 188 (≤T2)/152 (≥T3) | 205/119/21 | 29 | 102 | 43 | 22 |
| Yeh | 112/360 | 0/60(Tis/a)/130/112/142/28 | 261/170/41 | 8 | NA | NA | NA |
| Huang | 11/147 | 198 (T3) | 198 (N0) | NA | 31 | 20 | 5 |
| 170/22 | 30 (Ta)/162 (T1) | 192 (N0) | NA | NA | NA | NA | |
| Yu | 182/388 | 0/84 (Tis/a)/128/134/200/20 | NA | NA | 119 | 54 | NA |
| Koguchi | 300/127 | 0/18/66/91/81/153/24 | 181/221/31 | NA | 151 | NA | 30 |
| Freifeld | NA | NA | NA | NA | NA | NA | NA |
| Jan | 22/402 | 0/0/161 (Ta/1)/83/180 (T3/4) | 399 (Nx/−)/25 | NA | 115 | NA | NA |
| Kuroda | 55/132 | 96 (≤T2)/91 (≥T3) | 0/172/15 | NA | 65 | 22 | 21 |
| Momota | 24/432 | 260 (≤T2)/196 (≥T3) | 431 (Nx/−)/25 | NA | 160 | NA | 15 |
| Study | Survival Analysis | Threshold of eGFR (mL/min/1.73 m2) | Co-Factors | Analysis Results |
|---|---|---|---|---|
| Xylinas | OS | 60 | Standard clinico-pathological features | Not significant |
| Ito | PFS | 60 | cT stage (T3), length of cancer (3 cm), maximal diameter of cancer (1.6 cm), NLR (3) | Significant |
| Raman | CSS | 60 | Gender, race, age (70 years), ECOG performance status (0,1/2,3), pT stage (T3/T4), LN status, surgical margin status, adjuvant chemotherapy | Not significant |
| Morizane | CSS | 50 | ECOG performance status (0/≥1), number of tumor (1/>1), CRP (0.5) | Significant |
| Yeh | CSS/OS | 60 | Gender, age (67 years), smoking, surgery method (laparoscopic/open), tumor location, pT stage, pN stage, tumor grade, adjuvant chemotherapy, hematuria, hydronephrosis and flank pain | Significant/Significant |
| Huang | PFS/CSS/OS | 60 | Gender, age (68.6 years), current smoking, ASA score, recurrent bladder tumor, recurrent contralateral UTUC, tumor grade, LVI, CIS, surgical margin status, adjuvant radiotherapy, adjuvant chemotherapy | Significant/Significant/Significant |
| CSS | 30 | ABCC6 methylation, GDF15 methylation, tumor multifocality, surgery method (laparoscopic/open) | Significant | |
| Yu | PFS | 60 | BMI, pT stage (≤T2/≥T3), tumor grade, LVI | Significant |
| CSS | 60 | DM, pT stage (≤T2/≥T3), tumor grade, LVI, adjuvant chemotherapy | Significant | |
| OS | 60 | Age, BMI, ECOG performance status(0,1/2,3), tumor size, tumor multifocality, pT stage (≤T2/≥T3), tumor grade, LVI, adjuvant chemotherapy | Significant | |
| Koguchi | PFS/OS | 60 | Change rate of eGFR, age, gender, tumor location, tumor grade, pT stage, pN stage, LVI, surgical margin status | Not significant/Not significant |
| Freifeld | PFS | 50 | Age (66 years), ECOG performance status (0/≥1), hemoglobin, hydronephrosis, pT stage (≤T2/≥T3), tumor architecture | Not significant |
| Jan | PFS/CSS/OS | 60 | Gender, blood type, age (69 years), smoking, hemodialysis, DM or hypertension, previous or concomitant bladder cancer, hydronephrosis, hematuria, pT stage, pN stage, tumor grade, LVI, tumor location, tumor multifocality, tumor size (3 cm), tumor architecture, tumor necrosis, adjuvant chemotherapy, NLR (4), PLR (150), MLR (0.4), SII (580) | Not significant/Not significant/Not significant |
| Kuroda | PFS | 60 | Tumor histology, pT stage (≤T2/≥T3), tumor grade, pN stage, surgical margin status, LVI, CAR (0.079, NLR (2.035), PLR (165), GPS (1), fibrinogen (337) | Significant |
| CSS | 60 | Urine cytology, tumor histology, pT stage (≤T2/≥T3), tumor grade, pN stage, surgical margin status, LVI, CAR (0.079, NLR (2.035), PLR (165), GPS (1), fibrinogen (337) | Significant | |
| Momota | PFS/CSS/OS | 60 | age, gender, ECOG performance status, hypertension, CVD, DM, neoadjuvant chemotherapy, hydronephrosis, tumour location, tumor grade, pT stage (≤T2/≥T3), pN stage, LVI | Significant/Not significant/Not significant |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.H.; Yuk, H.D.; Jeong, C.W.; Kwak, C.; Kim, H.H.; Ku, J.H. Estimated Glomerular Filtration Rate as a Prognostic Factor in Urothelial Carcinoma of the Upper Urinary Tract: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 4155. https://doi.org/10.3390/jcm10184155
Kim MH, Yuk HD, Jeong CW, Kwak C, Kim HH, Ku JH. Estimated Glomerular Filtration Rate as a Prognostic Factor in Urothelial Carcinoma of the Upper Urinary Tract: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021; 10(18):4155. https://doi.org/10.3390/jcm10184155
Chicago/Turabian StyleKim, Min Hyuk, Hyeong Dong Yuk, Chang Wook Jeong, Cheol Kwak, Hyeon Hoe Kim, and Ja Hyeon Ku. 2021. "Estimated Glomerular Filtration Rate as a Prognostic Factor in Urothelial Carcinoma of the Upper Urinary Tract: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 10, no. 18: 4155. https://doi.org/10.3390/jcm10184155






