AN-VR-BE. A Randomized Controlled Trial for Reducing Fear of Gaining Weight and Other Eating Disorder Symptoms in Anorexia Nervosa through Virtual Reality-Based Body Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Sample
2.2. Measures
2.2.1. Pre-Post Assessment Measures
Visual Analog Scales (VAS)
- Self-reported FBI levels were assessed within the VR environment using a VAS to determine the extent to which the patients felt the virtual body was their own body on a scale ranging from 0 (not at all) to 100 (completely).
- Self-reported FGW levels were assessed within the VR environment using a VAS to determine the extent to which the patients were afraid of gaining weight while owning the virtual body on a scale ranging from 0 (not at all) to 100 (completely).
- Self-reported anxiety levels were assessed within the VR environment using a VAS to determine the extent to which the patients were anxious about their own body while owning the virtual body on a scale ranging from 0 (not at all) to 100 (completely).
AB Measures
2.2.2. Pre-Assessment, Post-Assessment, and Three Months Follow-Up Measures
- The BMI was used as a common indicator of body fatness to assess changes in weight in the pre-, post-, and follow-up assessments. The BMI was calculated by dividing the patient’s weight (in kilograms) by the square of their height (in meters).
- Eating Disorder Inventory 3 (EDI-3) [59]. The EDI-3 is a widely used self-report and standardized measure to assess symptomatology and psychological features relevant to the development and maintenance of EDs. The third version (EDI-3) includes 91 items classified into 12 scales, with a six-point Likert scale for each answer ranging from 0 (never) to 5 (always). The EDI-3 questionnaire and materials have been translated into Spanish and validated [60]. This Spanish version presents robust validity and reliability indices (like the original version), with a Cronbach’s alpha ranging from 0.74 to 0.96. In the current study, we only used the Body Dissatisfaction (EDI-BD) and Drive for Thinness (EDI-DT) scales. The EDI-BD scale, with 10 items, measures the negative subjective attitude or evaluation of one’s body or specific body areas, including their shape, weight, and fitness. The EDI-DT assesses the strong desire to have a thinner body and to lose weight, as reflected by the intense concerns about the body shape or weight, the diet and the FGW. The Cronbach’s alpha values in this study were 0.825 for the EDI-BD scale and 0.84 for the EDI-DT scale. The fourth item of the EDI-DT scale (i.e., “I am terrified about gaining weight”) was also used as an independent measure to determine the FGW in the pre-, post-, and follow-up assessments.
- Physical Appearance State and Trait Anxiety Scale (PASTAS) [58]. The PASTAS is a reliable and valid measure for the assessment of trait and state body image anxiety. Patients had to rate, on a five-point scale ranging from 0 (never) to 5 (always), if they felt anxious or nervous about their physical appearance, including any tension, negative thoughts, and physiological responses. Although the questionnaire comprises two scales (weight-related and non-weight-related scales) that measure anxiety for 16 body areas, only the eight-item Weight Scale was used in this study. The scale presents good reliability and reliability indices [61]. In the current study, Cronbach’s alpha was 0.817.
- The Body Image Assessment Scale-Body Dimensions (BIAS-BD) [62] was used to assess the perceptual and emotional components of BIDs. This test assesses the discrepancy between the perceived body size and the self-determined ideal body size (to measure body dissatisfaction). Furthermore, it also reveals the discrepancy between the perceived body size and the real body size (to measure body distortion). The scale presents a range of 17 silhouettes, with different versions for women and men. The two test–retest A–B versions were used in the pre-assessment (version A), post-assessment (version B), and follow-up assessment (version A again). Patients had to select the silhouette that they perceived to be the most similar to their own and the silhouette that they would like to have. Finally, the researchers also selected the real silhouette based on the patient’s BMI.
- The Body Appreciation Scale (BAS) [63] was used to assess positive attitudes towards one’s own body. This 12-item scale was translated into Spanish by Jáuregui-Lobera and Bolaños-Ríos [64]. The scale measures a single dimension of a positive body image, which includes having a positive opinion and accepting one’s physical features, the adoption of healthy behaviors with regards to one’s body, and the rejection of dangerous body ideals usually displayed in social media. The BAS items are rated on a five-point Likert scale that is averaged to obtain a final score of body appreciation, which ranges from 0 to 60, with higher values suggesting greater body appreciation.
2.3. Technical Features
2.3.1. Hardware
2.3.2. Software
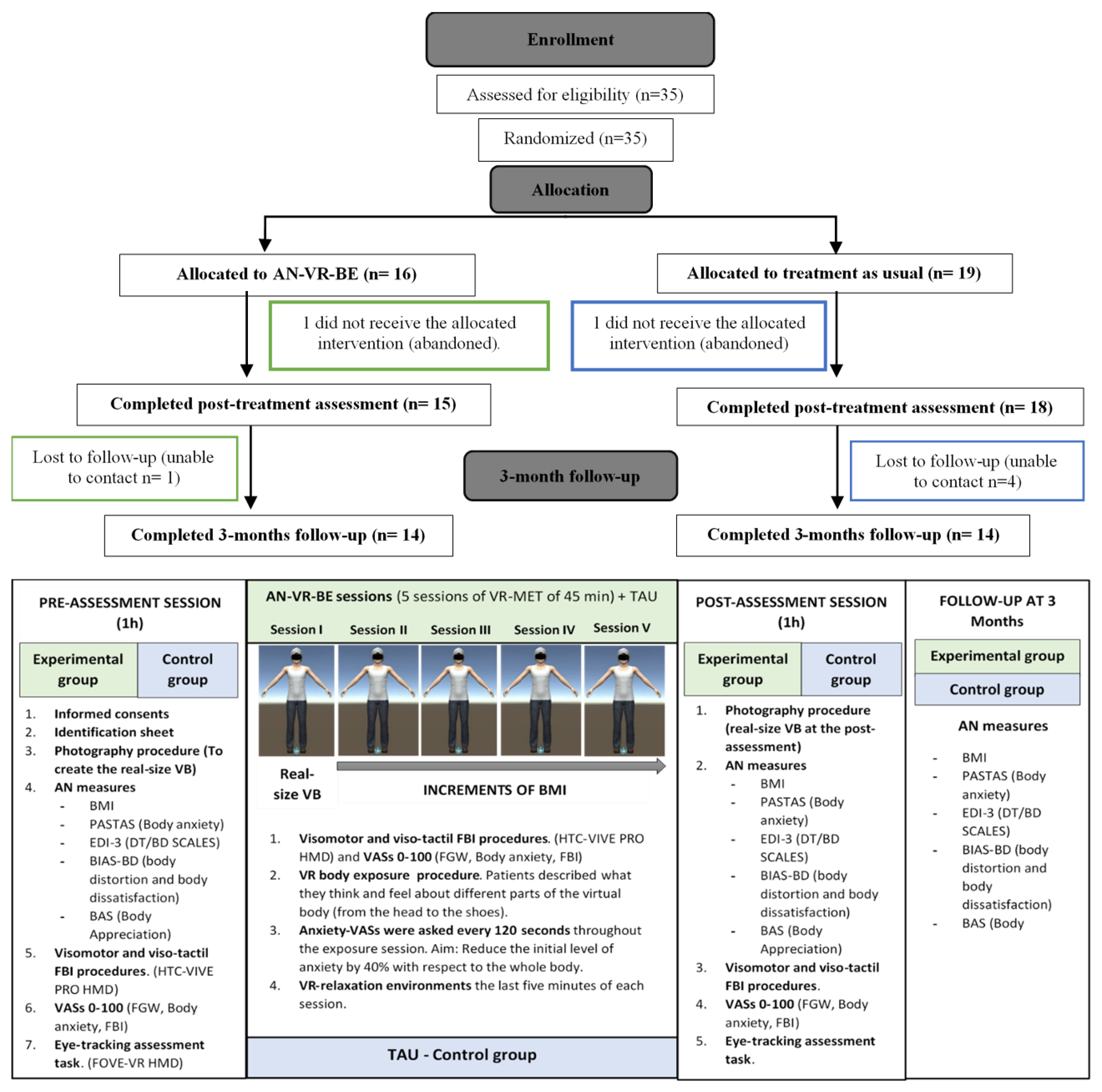
2.4. Procedure
2.4.1. Overview
2.4.2. Pre-Assessment, Post-Assessment, and Follow-Up Sessions
2.4.3. VR Body Exposure Sessions (Experimental Group)
2.5. Statistical Analyses
3. Results
3.1. Descriptive Results
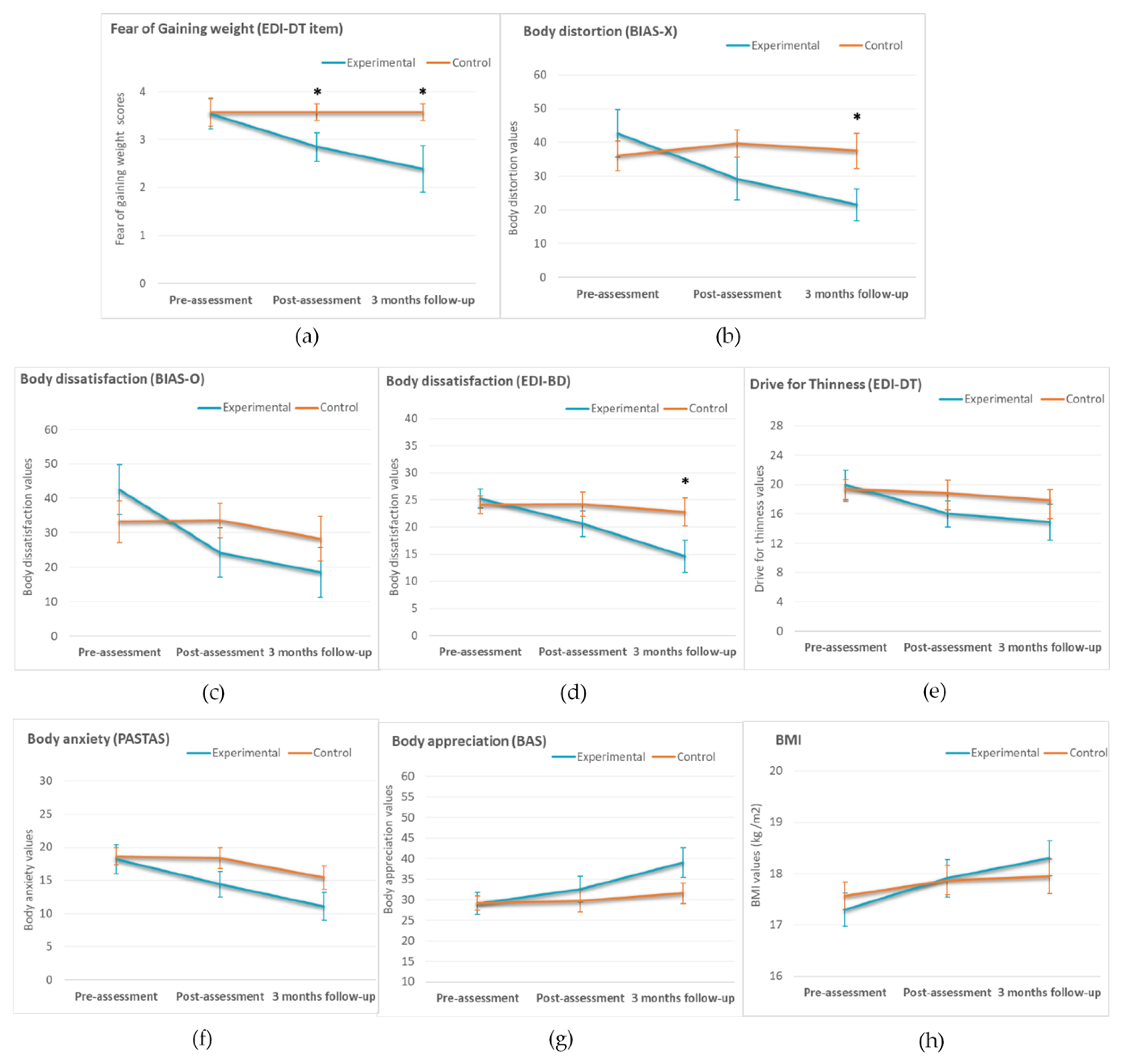
3.2. FBI, Body Anxiety and FGW VASs
3.3. Body-Related Attentional Bias
3.4. ED Measures (Pre-Assessment, Post-Assessment, and Three-Month Follow-Up)
3.5. Age as a Predictor of a Worse Treatment Outcome at Post-Assessment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; (DSM-5); American Psychiatric Association: Washington, DC, USA, 2013; p. 5. [Google Scholar]
- Keski-Rahkonen, A.; Mustelin, L. Epidemiology of eating disorders in Europe: Prevalence, incidence, comorbidity, course, consequences, and risk factors. Curr. Opin. Psychiatry 2016, 29, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Smink, F.R.E.; Van Hoeken, D.; Hoek, H.W. Epidemiology of Eating Disorders: Incidence, Prevalence and Mortality Rates. Curr. Psychiatry Rep. 2012, 14, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, S.; Giel, K.E.; Bulik, C.M.; Hay, P.; Schmidt, U. Anorexia nervosa: Aetiology, assessment, and treatment. Lancet Psychiatry 2015, 2, 1099–1111. [Google Scholar] [CrossRef]
- Favaro, A.; Caregaro, L.; Tenconi, E.; Bosello, R.; Santonastaso, P. Time trends in age at onset of anorexia nervosa and bulimia nervosa. J. Clin. Psychiatry 2009, 70, 1715–1721. [Google Scholar] [CrossRef]
- Nicholls, D.E.; Lynn, R.; Viner, R.M. Childhood eating disorders: British national surveillance study. Br. J. Psychiatry 2011, 198, 295–301. [Google Scholar] [CrossRef]
- Micali, N.; Hagberg, K.W.; Petersen, I.; Treasure, J.L. The incidence of eating disorders in the UK in 2000–2009: Findings from the General Practice Research Database. BMJ Open 2013, 3, e002646. [Google Scholar] [CrossRef]
- Herpertz-Dahlmann, B. Adolescent Eating Disorders: Update on Definitions, Symptomatology, Epidemiology, and Comorbidity. Child Adolesc. Psychiatr. Clin. N. Am. 2015, 24, 177–196. [Google Scholar] [CrossRef]
- Russell, G.F. The changing nature of anorexia nervosa: An introduction to the conference. J. Psychiatr. Res. 1985, 19, 101–109. [Google Scholar] [CrossRef]
- Murray, S.B.; Loeb, K.L.; Le Grange, D. Dissecting the core fear in anorexia nervosa: Can we optimize treatment mechanisms? JAMA Psychiatry 2016, 73, 891–892. [Google Scholar] [CrossRef] [PubMed]
- Levinson, C.A.; Williams, B.M. Eating disorder fear networks: Identification of central eating disorder fears. Int. J. Eat. Disord. 2020, 53, 1960–1973. [Google Scholar] [CrossRef]
- Carter, J.C.; Bewell-Weiss, C.V. Nonfat phobic anorexia nervosa: Clinical characteristics and response to inpatient treatment. Int. J. Eat. Disord. 2011, 44, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Calugi, S.; El Ghoch, M.; Conti, M.; Grave, R.D. Preoccupation with shape or weight, fear of weight gain, feeling fat and treatment outcomes in patients with anorexia nervosa: A longitudinal study. Behav. Res. Ther. 2018, 105, 63–68. [Google Scholar] [CrossRef]
- Rodgers, R.F.; Dubois, R.; Frumkin, M.R.; Robinaugh, D.J. A network approach to eating disorder symptomatology: Do desire for thinness and fear of gaining weight play unique roles in the network? Body Image 2018, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Linardon, J.; Phillipou, A.; Castle, D.; Newton, R.; Harrison, P.; Cistullo, L.L.; Griffiths, S.; Hindle, A.; Brennan, L. The relative associations of shape and weight over-evaluation, preoccupation, dissatisfaction, and fear of weight gain with measures of psychopathology: An extension study in individuals with anorexia nervosa. Eat. Behav. 2018, 29, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Legenbauer, T.; Thiemann, P.; Vocks, S. Body image disturbance in children and adolescents with eating disorders: Current evidence and future directions. Z. Kinder. Jugendpsychiatr. Psychother. 2014, 42, 51–59. [Google Scholar] [CrossRef]
- Cash, T.F.; Deagle, E.A. The nature and extent of body-image disturbances in anorexia nervosa and bulimia nervosa: A meta-analysis. Int. J. Eat. Disord. 1997, 22, 107–126. [Google Scholar] [CrossRef]
- Hagman, J.; Gardner, R.M.; Brown, D.L.; Gralla, J.; Fier, J.M.; Frank, G.K.W. Body size overestimation and its association with body mass index, body dissatisfaction, and drive for thinness in anorexia nervosa. Eat. Weight. Disord. Stud. Anorexia Bulim. Obes. 2015, 20, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Shafran, R. Information processing biases in eating disorders. Clin. Psychol. Rev. 2004, 24, 215–238. [Google Scholar] [CrossRef] [PubMed]
- Williamson, D.A.; White, M.A.; York-Crowe, E.; Stewart, T.M. Cognitive-Behavioral Theories of Eating Disorders. Behav. Modif. 2004, 28, 711–738. [Google Scholar] [CrossRef]
- Rodgers, R.F.; Dubois, R.H. Cognitive biases to appearance-related stimuli in body dissatisfaction: A systematic review. Clin. Psychol. Rev. 2016, 46, 1–11. [Google Scholar] [CrossRef]
- Tuschen-Caffier, B.; Bender, C.; Caffier, D.; Klenner, K.; Braks, K.; Svaldi, J. Selective Visual Attention during Mirror Exposure in Anorexia and Bulimia Nervosa. PLoS ONE 2015, 10, e0145886. [Google Scholar] [CrossRef]
- Bauer, S.; Schneider, S.; Waldorf, M.; Braks, K.; Huber, T.J.; Adolph, D.; Vocks, S. Selective Visual Attention Towards Oneself and Associated State Body Satisfaction: An Eye-Tracking Study in Adolescents with Different Types of Eating Disorders. J. Abnorm. Child Psychol. 2017, 45, 1647–1661. [Google Scholar] [CrossRef]
- Forrest, L.N.; Jones, P.J.; Ortiz, S.N.; Smith, A.R. Core psychopathology in anorexia nervosa and bulimia nervosa: A network analysis. Int. J. Eat. Disord. 2018, 51, 668–679. [Google Scholar] [CrossRef]
- Levinson, C.A.; Zerwas, S.; Calebs, B.; Forbush, K.; Kordy, H.; Watson, H.; Hofmeier, S.; Levine, M.; Crosby, R.D.; Peat, C.; et al. The core symptoms of bulimia nervosa, anxiety, and depression: A network analysis. J. Abnorm. Psychol. 2017, 126, 340–354. [Google Scholar] [CrossRef]
- Steinglass, J.E.; Sysko, R.; Glasofer, D.; Albano, A.M.; Simpson, H.B.; Walsh, B.T. Rationale for the application of Exposure and Response Prevention to the treatment of anorexia nervosa. Int. J. Eat. Disord. 2010, 44, 134–141. [Google Scholar] [CrossRef]
- Reilly, E.E.; Anderson, L.M.; Gorrell, S.; Schaumberg, K.; Anderson, D.A. Expanding exposure-based interventions for eating disorders. Int. J. Eat. Disord. 2017, 50, 1137–1141. [Google Scholar] [CrossRef]
- Brockmeyer, T.; Friederich, H.-C.; Schmidt, U. Advances in the treatment of anorexia nervosa: A review of established and emerging interventions. Psychol. Med. 2017, 48, 1228–1256. [Google Scholar] [CrossRef] [PubMed]
- Koskina, A.; Campbell, I.C.; Schmidt, U. Exposure therapy in eating disorders revisited. Neurosci. Biobehav. Rev. 2013, 37, 193–208. [Google Scholar] [CrossRef]
- Farrell, N.R.; Brosof, L.C.; Vanzhula, I.A.; Christian, C.; Bowie, O.R.; Levinson, C.A. Exploring Mechanisms of Action in Exposure-Based Cognitive Behavioral Therapy for Eating Disorders: The Role of Eating-Related Fears and Body-Related Safety Behaviors. Behav. Ther. 2019, 50, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Vocks, S.; Legenbauer, T.; Wächter, A.; Wucherer, M.; Kosfelder, J. What happens in the course of body exposure? Emotional, cognitive, and physiological reactions to mirror confrontation in eating disorders. J. Psychosom. Res. 2007, 62, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Delinsky, S.S.; Wilson, G.T. Mirror exposure for the treatment of body image disturbance. Int. J. Eat. Disord. 2005, 39, 108–116. [Google Scholar] [CrossRef]
- Hilbert, A.; Tuschen-Caffier, B.; Vögele, C. Effects of prolonged and repeated body image exposure in binge-eating disorder. J. Psychosom. Res. 2002, 52, 137–144. [Google Scholar] [CrossRef]
- Luethcke, C.A.; McDaniel, L.; Becker, C.B. A comparison of mindfulness, nonjudgmental, and cognitive dissonance-based approaches to mirror exposure. Body Image 2011, 8, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Domínguez, S.; Rodríguez-Ruiz, S.; Fernández-Santaella, M.C.; Jansen, A.; Tuschen-Caffier, B. Pure versus guided mirror exposure to reduce body dissatisfaction: A preliminary study with university women. Body Image 2012, 9, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Key, A.; George, C.L.; Beattie, D.; Stammers, K.; Lacey, H.; Waller, G. Body image treatment within an inpatient program for anorexia nervosa: The role of mirror exposure in the desensitization process. Int. J. Eat. Disord. 2002, 31, 185–190. [Google Scholar] [CrossRef]
- Vocks, S.; Wächter, A.; Wucherer, M.; Kosfelder, J. Look at yourself: Can body image therapy affect the cognitive and emotional response to seeing oneself in the mirror in eating disorders? Eur. Eat. Disord. Rev. 2008, 16, 147–154. [Google Scholar] [CrossRef]
- Morgan, J.F.; Lazarova, S.; Schelhase, M.; Saeidi, S. Ten Session Body Image Therapy: Efficacy of a Manualised Body Image Therapy. Eur. Eat. Disord. Rev. 2013, 22, 66–71. [Google Scholar] [CrossRef]
- Mountford, V.A.; Brown, A.; Bamford, B.; Saeidi, S.; Morgan, J.F.; Lacey, H. BodyWise: Evaluating a Pilot Body Image Group for Patients with Anorexia Nervosa. Eur. Eat. Disord. Rev. 2014, 23, 62–67. [Google Scholar] [CrossRef]
- Marzola, E.; Panero, M.; Cavallo, F.; Delsedime, N.; Abbate-Daga, G. Body shape in inpatients with severe anorexia nervosa. Eur. Psychiatry 2020, 63, e2. [Google Scholar] [CrossRef]
- García-Palacios, A.; Botella, C.; Hoffman, H.; Fabregat, S. Comparing Acceptance and Refusal Rates of Virtual Reality Exposure vs. In Vivo Exposure by Patients with Specific Phobias. Cyberpsychol. Behav. 2007, 10, 722–724. [Google Scholar] [CrossRef]
- Gutiérrez-Maldonado, J.; Wiederhold, B.K.; Riva, G. Future Directions: How Virtual Reality Can Further Improve the Assessment and Treatment of Eating Disorders and Obesity. Cyberpsychol. Behav. Soc. Netw. 2016, 19, 148–153. [Google Scholar] [CrossRef]
- Gutiérrez-Maldonado, J.; Ferrer-García, M.; Dakanalis, A.; Riva, G. Virtual Reality: Applications to Eating Disorders. In The Oxford Handbook of Eating Disorders; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Piryankova, I.V.; Wong, H.Y.; Linkenauger, S.A.; Stinson, C.; Longo, M.R.; Bülthoff, H.H.; Mohler, B.J. Owning an Overweight or Underweight Body: Distinguishing the Physical, Experienced and Virtual Body. PLoS ONE 2014, 9, e103428. [Google Scholar] [CrossRef]
- Maselli, A.; Slater, M. The building blocks of the full body ownership illusion. Front. Hum. Neurosci. 2013, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Serino, S.; Pedroli, E.; Keizer, A.; Triberti, S.; Dakanalis, A.; Pallavicini, F.; Chirico, A.; Riva, G. Virtual Reality Body Swapping: A Tool for Modifying the Allocentric Memory of the Body. Cyberpsychol. Behav. Soc. Netw. 2016, 19, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Botvinick, M.; Cohen, J. Rubber hands ‘feel’ touch that eyes see. Nature 1998, 391, 756. [Google Scholar] [CrossRef] [PubMed]
- Riva, G.; Serino, S.; Di Lernia, D.; Pavone, E.F.; Dakanalis, A. Embodied Medicine: Mens Sana in Corpore Virtuale Sano. Front. Hum. Neurosci. 2017, 11, 120. [Google Scholar] [CrossRef]
- Riva, G.; Wiederhold, B.K.; Mantovani, F. Neuroscience of Virtual Reality: From Virtual Exposure to Embodied Medicine. Cyberpsychol. Behav. Soc. Netw. 2019, 22, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Preston, C.; Ehrsson, H.H. Illusory Changes in Body Size Modulate Body Satisfaction in a Way That Is Related to Non-Clinical Eating Disorder Psychopathology. PLoS ONE 2014, 9, e85773. [Google Scholar] [CrossRef] [PubMed]
- Porras Garcia, B.; Ferrer Garcia, M.; Olszewska, A.; Yilmaz, L.; González Ibañez, C.; Gracia Blanes, M.; Gültekin, G.; Serrano Troncoso, E.; Gutiérrez Maldonado, J. Is this my own body? Changing the perceptual and affective body image experience among college students using a new virtual reality embodiment-based technique. J. Clin. Med. 2019, 8, 925. [Google Scholar] [CrossRef]
- Keizer, A.; Van Elburg, A.; Helms, R.; Dijkerman, H.C. A Virtual Reality Full Body Illusion Improves Body Image Disturbance in Anorexia Nervosa. PLoS ONE 2016, 11, e0163921. [Google Scholar] [CrossRef]
- Serino, S.; Polli, N.; Riva, G. From avatars to body swapping: The use of virtual reality for assessing and treating body-size distortion in individuals with anorexia. J. Clin. Psychol. 2019, 75, 313–322. [Google Scholar] [CrossRef]
- Porras-Garcia, B.; Serrano-Troncoso, E.; Carulla-Roig, M.; Soto-Usera, P.; Ferrer-Garcia, M.; Figueras-Puigderrajols, N.; Yilmaz, L.; Sen, Y.O.; Shojaeian, N.; Gutiérrez-Maldonado, J. Virtual Reality Body Exposure Therapy for Anorexia Nervosa. A Case Report with Follow-Up Results. Front. Psychol. 2020, 11, 956. [Google Scholar] [CrossRef] [PubMed]
- Levinson, C.A.; Rapp, J.; Riley, E.N. Addressing the fear of fat: Extending imaginal exposure therapy for anxiety disorders to anorexia nervosa. Eat. Weight. Disord. Stud. Anorexia Bulim. Obes. 2014, 19, 521–524. [Google Scholar] [CrossRef]
- Levinson, C.A.; Christian, C.; Ram, S.S.; Vanzhula, I.; Brosof, L.C.; Michelson, L.P.; Williams, B.M. Eating disorder symptoms and core eating disorder fears decrease during online imaginal exposure therapy for eating disorders. J. Affect. Disord. 2020, 276, 585–591. [Google Scholar] [CrossRef]
- Jacob, R.J.K.; Karn, K.S. Eye Tracking in Human-Computer Interaction and Usability Research. Ready to Deliver the Promises. In The Mind’s Eye: Cognitive and Applied Aspects of Eye Movement Research; Elsevier: Amsterdam, The Netherlands, 2003; pp. 573–605. [Google Scholar]
- Kerr-Gaffney, J.; Harrison, A.; Tchanturia, K. Eye-tracking research in eating disorders: A systematic review. Int. J. Eat. Disord. 2019, 52, 3–27. [Google Scholar] [CrossRef]
- Garner, D. Eating Disorder Inventory-3: Professional Manual; Psychological Assessment Resources: Lutz, FL, USA, 2004. [Google Scholar]
- Elosua, P.; López-Jaúregui, A.; Sánchez-Sánchez, F. EDI-3, Inventario de Trastornos de la Conducta Alimentaria-3, Manual; Tea Ediciones: Madrid, Spain, 2010. [Google Scholar]
- Reed, D.L.; Thompson, J.; Brannick, M.T.; Sacco, W.P. Development and validation of the Physical Appearance State and Trait Anxiety Scale (PASTAS). J. Anxiety Disord. 1991, 5, 323–332. [Google Scholar] [CrossRef]
- Gardner, R.M.; Jappe, L.M.; Gardner, L. Development and validation of a new figural drawing scale for body-image assessment: The BIAS-BD. J. Clin. Psychol. 2009, 65, 113–122. [Google Scholar] [CrossRef]
- Avalos, L.; Tylka, T.L.; Wood-Barcalow, N. The Body Appreciation Scale: Development and psychometric evaluation. Body Image 2005, 2, 285–297. [Google Scholar] [CrossRef]
- Lobera, I.J.; Ríos, P.B. Spanish version of the Body Appreciation Scale (BAS) for adolescents. Span. J. Psychol. 2011, 14, 411–420. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 2012, 10, 28–55. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59, 1736. [Google Scholar]
- Serrano-Troncoso, E.; Fàbrega-Ribera, M.; Coll-Pla, N.; Godrid-García, M.; Carulla-Roig, M.; Cecilia-Costa, R.; Soto-Usera, P.; Sánchez-Fernández, B.; Matalí-Costa, J.; Dolz-Abadia, M. Alternatives to inpatient treatment in adolescents with anorexia nervosa: Effectiveness and characteristics of a new intensive model of day patient treatment. Actas Esp. Psiquiatr 2020, 48, 19–27. [Google Scholar]
- Ramos-Álvarez, M. Programa Informático Para la Aleatorización por Ciclos. 2005. Available online: http://www4.ujaen.es/~mramos/EPIP/DescribeAleatorMetod.pdf (accessed on 30 June 2019).
- Porras-Garcia, B.; Ferrer-Garcia, M.; Serrano-Troncoso, E.; Carulla-Roig, M.; Soto-Usera, P.; Miquel-Nabau, H.; Shojaeian, N.; Santos-Carrasco, I.D.L.M.; Borszewski, B.; Díaz-Marsá, M.; et al. Validity of Virtual Reality Body Exposure to Elicit Fear of Gaining Weight, Body Anxiety and Body-Related Attentional Bias in Patients with Anorexia Nervosa. J. Clin. Med. 2020, 9, 3210. [Google Scholar] [CrossRef] [PubMed]
- Waltemate, T.; Gall, D.; Roth, D.; Botsch, M.; Latoschik, M.E. The Impact of Avatar Personalization and Immersion on Virtual Body Ownership, Presence, and Emotional Response. IEEE Trans. Vis. Comput. Graph. 2018, 24, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Porras-Garcia, B.; Ferrer-Garcia, M.; Ghita, A.; Moreno, M.; López-Jiménez, L.; Vallvé-Romeu, A.; Serrano-Troncoso, E.; Gutiérrez-Maldonado, J. The influence of gender and body dissatisfaction on body-related attentional bias: An eye-tracking and virtual reality study. Int. J. Eat. Disord. 2019, 52, 1181–1190. [Google Scholar] [CrossRef]
- Porras-Garcia, B.; Ferrer-Garcia, M.; Yilmaz, L.; Sen, Y.O.; Olszewska, A.; Ghita, A.; Serrano-Troncoso, E.; Treasure, J.; Gutiérrez-Maldonado, J. Body-related attentional bias as mediator of the relationship between body mass index and body dissatisfaction. Eur. Eat. Disord. Rev. 2020, 28, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.; Nederkoorn, C.; Mulkens, S. Selective visual attention for ugly and beautiful body parts in eating disorders. Behav. Res. Ther. 2005, 43, 183–196. [Google Scholar] [CrossRef]
- Roefs, A.; Jansen, A.; Moresi, S.; Willems, P.; Van Grootel, S.; Van Der Borgh, A. Looking good. BMI, attractiveness bias and visual attention. Appetite 2008, 51, 552–555. [Google Scholar] [CrossRef]
- Schmider, E.; Ziegler, M.; Danay, E.; Beyer, L.; Bühner, M. Is It Really Robust: Reinvestigating the robustness of ANOVA against violations of the normal distribution assumption. Methodology 2010, 6, 147–151. [Google Scholar] [CrossRef]
- Bulik, C.M.; Thornton, L.; Pinheiro, A.P.; Plotnicov, K.; Klump, K.L.; Brandt, H.; Crawford, S.; Fichter, M.M.; Halmi, K.A.; Johnson, C.; et al. Suicide Attempts in Anorexia Nervosa. Psychosom. Med. 2008, 70, 378–383. [Google Scholar] [CrossRef]
- Lock, J.; Le Grange, D.; Agras, W.S.; Moye, A.; Bryson, S.W.; Jo, B. Randomized Clinical Trial Comparing Family-Based Treatment With Adolescent-Focused Individual Therapy for Adolescents With Anorexia Nervosa. Arch. Gen. Psychiatry 2010, 67, 1025–1032. [Google Scholar] [CrossRef]
- Skrzypek, S.; Wehmeier, P.M.; Remschmidt, H. Body image assessment using body size estimation in recent studies on anorexia nervosa. A brief review. Eur. Child Adolesc. Psychiatry 2001, 10, 215–221. [Google Scholar] [CrossRef]
- Hildebrandt, T.; Loeb, K.; Troupe, S.; Delinsky, S. Adjunctive mirror exposure for eating disorders: A randomized controlled pilot study. Behav. Res. Ther. 2012, 50, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.; Bollen, D.; Tuschen-Caffier, B.; Roefs, A.; Tanghe, A.; Braet, C. Mirror exposure reduces body dissatisfaction and anxiety in obese adolescents: A pilot study. Appetite 2008, 51, 214–217. [Google Scholar] [CrossRef]
- Boehm, I.; Finke, B.; Tam, F.I.; Fittig, E.; Scholz, M.; Gantchev, K.; Roessner, V.; Ehrlich, S. Effects of perceptual body image distortion and early weight gain on long-term outcome of adolescent anorexia nervosa. Eur. Child Adolesc. Psychiatry 2016, 25, 1319–1326. [Google Scholar] [CrossRef]
- Dalhoff, A.W.; Frausto, H.R.; Romer, G.; Wessing, I. Perceptive Body Image Distortion in Adolescent Anorexia Nervosa: Changes After Treatment. Front. Psychiatry 2019, 10, 748. [Google Scholar] [CrossRef]
- Riva, G. The neuroscience of body memory: From the self through the space to the others. Cortex 2018, 104, 241–260. [Google Scholar] [CrossRef]
- Matamala-Gomez, M.; Maselli, A.; Malighetti, C.; Realdon, O.; Mantovani, F.; Riva, G. Virtual Body Ownership Illusions for Mental Health: A Narrative Review. J. Clin. Med. 2021, 10, 139. [Google Scholar] [CrossRef]
- Glashouwer, K.A.; Jonker, N.C.; Thomassen, K.; De Jong, P.J. Take a look at the bright side: Effects of positive body exposure on selective visual attention in women with high body dissatisfaction. Behav. Res. Ther. 2016, 83, 19–25. [Google Scholar] [CrossRef][Green Version]
- Jansen, A.; Voorwinde, V.; Hoebink, Y.; Rekkers, M.; Martijn, C.; Mulkens, S. Mirror exposure to increase body satisfaction: Should we guide the focus of attention towards positively or negatively evaluated body parts? J. Behav. Ther. Exp. Psychiatry 2016, 50, 90–96. [Google Scholar] [CrossRef]
- Smeets, E.; Jansen, A.; Roefs, A. Bias for the (un)attractive self: On the role of attention in causing body (dis)satisfaction. Heal. Psychol. 2011, 30, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Renwick, B.; Campbell, I.C.; Schmidt, U. Attention bias modification: A new approach to the treatment of eating disorders? Int. J. Eat. Disord. 2013, 46, 496–500. [Google Scholar] [CrossRef]
- Graham, K.T.; Martin-Iverson, M.T.; Holmes, N.P.; Waters†, F.; Graham, K.T. The projected hand illusion: Component structure in a community sample and association with demographics, cognition, and psychotic-like experiences. Atten. Percept. Psychophys. 2014, 77, 207–219. [Google Scholar] [CrossRef]
- Serino, S.; Scarpina, F.; Dakanalis, A.; Keizer, A.; Pedroli, E.; Castelnuovo, G.; Chirico, A.; Catallo, V.; Di Lernia, D.; Riva, G. The Role of Age on Multisensory Bodily Experience: An Experimental Study with a Virtual Reality Full-Body Illusion. Cyberpsychol. Behav. Soc. Netw. 2018, 21, 304–310. [Google Scholar] [CrossRef] [PubMed]



| Variable | Experimental Group (n = 16) | Control Group (n = 19) | X2(df) | t-Test (df) | p Value |
|---|---|---|---|---|---|
| Age. Mean years (SD) | 18.25 (1.30) | 19.21 (1.78) | N/A | 0.419 | 0.68 |
| Group age, n (%) | 0.551 (1) | N/A | 0.5 | ||
| Adolescents | 9 (56.25) | 12 (63.16) | |||
| Adults | 7 (43.75) | 7 (36.84) | |||
| BMI a, mean kg/m2 (SD) | 17.30 (1.06) | 17.54 (1.27) | N/A | 0.513 | 0.44 |
| Sex, n (%) | 0.033 (1) | N/A | >0.99 | ||
| Women | 14 (87.5) | 17 (89.47) | |||
| Men | 2 (12.5) | 2 (10.52) | |||
| Main diagnosis, n (%) | 0.285 (2) | N/A | 0.7 | ||
| AN-R type b | 13 (81.3) | 14 (73.7) | |||
| AN-P type c | 3 (18.8) | 5 (26.3) | |||
| Comorbid diagnosis, n | N/A | N/A | N/A | ||
| GAD d | 1 | 0 | |||
| MDD e | 1 | 1 | |||
| PDD f | 1 | 0 | |||
| BPD g | 2 | 2 | |||
| PTSD h | 1 | 0 | |||
| Anxiety NOS i | 1 | 1 | |||
| Depression NOS | 0 | 2 | |||
| Number of comorbid diagnosis, n (%) | 0.810 (2) | N/A | 0.84 | ||
| 0 | 10 (68.6) | 14 (77.4) | |||
| 1 | 5 (31.3) | 4 (21.1) | |||
| 2 | 1 (6.3) | 1 (5.3) | |||
| Medication | 2.789 (4) | N/A | 0.69 | ||
| None | 7 | 12 | |||
| Antidepressant | 2 | 3 | |||
| Anxiolytic | 2 | 1 | |||
| Antidepressant and Anxiolytic | 2 | 2 | |||
| Antipsychotic + Antidepressant/Anxiolytic | 3 | 1 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porras-Garcia, B.; Ferrer-Garcia, M.; Serrano-Troncoso, E.; Carulla-Roig, M.; Soto-Usera, P.; Miquel-Nabau, H.; Fernández-Del castillo Olivares, L.; Marnet-Fiol, R.; de la Montaña Santos-Carrasco, I.; Borszewski, B.; et al. AN-VR-BE. A Randomized Controlled Trial for Reducing Fear of Gaining Weight and Other Eating Disorder Symptoms in Anorexia Nervosa through Virtual Reality-Based Body Exposure. J. Clin. Med. 2021, 10, 682. https://doi.org/10.3390/jcm10040682
Porras-Garcia B, Ferrer-Garcia M, Serrano-Troncoso E, Carulla-Roig M, Soto-Usera P, Miquel-Nabau H, Fernández-Del castillo Olivares L, Marnet-Fiol R, de la Montaña Santos-Carrasco I, Borszewski B, et al. AN-VR-BE. A Randomized Controlled Trial for Reducing Fear of Gaining Weight and Other Eating Disorder Symptoms in Anorexia Nervosa through Virtual Reality-Based Body Exposure. Journal of Clinical Medicine. 2021; 10(4):682. https://doi.org/10.3390/jcm10040682
Chicago/Turabian StylePorras-Garcia, Bruno, Marta Ferrer-Garcia, Eduardo Serrano-Troncoso, Marta Carulla-Roig, Pau Soto-Usera, Helena Miquel-Nabau, Laura Fernández-Del castillo Olivares, Rosa Marnet-Fiol, Isabel de la Montaña Santos-Carrasco, Bianca Borszewski, and et al. 2021. "AN-VR-BE. A Randomized Controlled Trial for Reducing Fear of Gaining Weight and Other Eating Disorder Symptoms in Anorexia Nervosa through Virtual Reality-Based Body Exposure" Journal of Clinical Medicine 10, no. 4: 682. https://doi.org/10.3390/jcm10040682
APA StylePorras-Garcia, B., Ferrer-Garcia, M., Serrano-Troncoso, E., Carulla-Roig, M., Soto-Usera, P., Miquel-Nabau, H., Fernández-Del castillo Olivares, L., Marnet-Fiol, R., de la Montaña Santos-Carrasco, I., Borszewski, B., Díaz-Marsá, M., Sánchez-Díaz, I., Fernández-Aranda, F., & Gutiérrez-Maldonado, J. (2021). AN-VR-BE. A Randomized Controlled Trial for Reducing Fear of Gaining Weight and Other Eating Disorder Symptoms in Anorexia Nervosa through Virtual Reality-Based Body Exposure. Journal of Clinical Medicine, 10(4), 682. https://doi.org/10.3390/jcm10040682








